Abstract
Context
Mechanisms underlying pituitary corticotroph adenoma adrenocorticotropin (ACTH) production are poorly understood, yet circulating ACTH levels closely correlate with adenoma phenotype and clinical outcomes.
Objective
We characterized the 5′ ends of proopiomelanocortin (POMC) gene transcripts, which encode the precursor polypeptide for ACTH, in order to investigate additional regulatory mechanisms of POMC gene transcription and ACTH production.
Methods
We examined 11 normal human pituitary tissues, 32 ACTH-secreting tumors, as well as 6 silent corticotroph adenomas (SCAs) that immunostain for but do not secrete ACTH.
Results
We identified a novel regulatory region located near the intron 2/exon 3 junction in the human POMC gene, which functions as a second promoter and an enhancer. In vitro experiments demonstrated that CREB binds the second promoter and regulates its transcriptional activity. The second promoter is highly methylated in SCAs, partially demethylated in normal pituitary tissue, and highly demethylated in pituitary and ectopic ACTH-secreting tumors. In contrast, the first promoter is demethylated in all POMC-expressing cells and is highly demethylated only in pituitary ACTH-secreting tumors harboring the ubiquitin-specific protease 8 (USP8) mutation. Demethylation patterns of the second promoter correlate with clinical phenotypes of Cushing disease.
Conclusion
We identified a second POMC promoter regulated by methylation status in ACTH-secreting pituitary tumors. Our findings open new avenues for elucidating subcellular regulation of the hypothalamic-pituitary-adrenal axis and suggest the second POMC promoter may be a target for therapeutic intervention to suppress excess ACTH production.
Keywords: ACTH, POMC, Cushing disease, pituitary adenoma
Pituitary adenomas arise from differentiated anterior pituitary cell types, each exhibiting unique clinical features (1). Proopiomelanocortin (POMC), the precursor protein cleaved to form adrenocorticotropin (ACTH), is abundantly expressed in corticotroph ACTH-secreting adenomas in patients with Cushing disease (2). The POMC protein is also expressed in several nonpituitary tissues, and autonomous ACTH secretion may be encountered with ectopic tumors, including those in the breast, lung, colon, pancreas, and thymus (3). In contrast to these neoplastic sources of excess ACTH, silent corticotroph adenomas (SCAs) in the pituitary immunostain positively for but do not secrete ACTH (4). Although several mechanisms have been proposed to enhance autonomous adenoma ACTH production (5-7), the pathogenesis of these tumors and mechanisms driving dysregulated POMC gene expression remain largely unclear. Corticotroph adenomas overexpress epidermal growth factor receptor (EGFR) (8), and EGFR signaling induces POMC transcription. In a subset of Cushing tumors, a mutant ubiquitin-specific protease 8 (USP8) leads to attenuated EGFR ubiquitination and degradation (9, 10). These gain-of-function somatic USP8 mutations are consistent with reports that EGFR signaling drives the cell cycle regulator E2F1, which also binds the human POMC promoter (11). Patients not exhibiting mutations in the USP8 gene present with heterogeneous tumor phenotypes and clinical outcomes, suggesting that additional mechanisms distinct from the USP8-EGFR pathway are likely involved in overexpression of POMC and Cushing disease development (12, 13).
Activity of the Pomc promoter, located within the –480/+34-bp region of the murine Pomc (mPomc) gene, was largely characterized using mouse corticotroph AtT20 cells (14). Several transcription factors, including TPIT (15, 16), PITX1 (17), STAT3 (18), NEUROD1 (19), and TR4 (20), bind this region and regulate gene expression. In particular, TPIT and PITX1 binding sites are located in close proximity, and both transcription factors cooperate with NEUROD1 to regulate corticotroph-specific Pomc gene expression (15, 19, 21, 22). Thus, the proximal Pomc gene promoter regulates corticotroph-specific expression.
In analyzing 5′ ends of POMC messenger RNA (mRNA) derived from normal pituitary tissues, pituitary and ectopic ACTH-secreting tumors, and SCAs, we identified a second POMC promoter, located near the intron 2/exon 3 junction of the human POMC gene. This region contains a binding site for CREB, a methylation-sensitive transcription factor (23), and our results indicate that CREB binding plays a key role in POMC transcription from this region. We identified a dinucleotide 5′-CG-3′ (CpG) island in this locus and show that DNA demethylation of the CpG island induced second promoter activity. The second promoter is partially methylated in normal pituitary tissue and highly demethylated in Cushing disease tumors, and methylation levels of the second promoter in the different ACTH-expressing tumor types correlate with their respective clinical phenotypes. Our findings suggest that the second POMC promoter may be a potential target for therapeutic intervention to suppress excess ACTH production.
Materials and Methods
Study Approval
Postsurgical sample collection and tissue processing was approved by the institutional review board (IRB) at Cedars-Sinai Medical Center (Protocol No. 2873) and Toranomon Hospital (Protocol No. 20171222). Informed consent was obtained prior to sample collection at each recruitment site. Human autopsy pituitary tissue collection was approved by the Cedars-Sinai IRB (Protocol No. 000375). Receiving and handling of samples was approved by the University of Minnesota IRB (Protocol No. 00005168). Experiments were performed at Cedars-Sinai Medical Center and University of Minnesota under the same IRB approvals.
Patient Recruitment and Sample Collection
Male and female patients of any age were eligible for inclusion at either recruiting site after being diagnosed with Cushing disease according to standard guidelines. Specifically, patients had clinical signs of symptoms consistent with Cushing’s syndrome, biochemical evidence of hypercortisolism as determined by 24-hour urinary free cortisol, midnight salivary cortisol, and/or low-dose dexamethasone suppression test, as well as confirmed sellar lesion visualized on brain/pituitary magnetic resonance imaging prior to surgery. Diagnosis of Cushing disease was confirmed by histopathology.
SCA was diagnosed at Cedars-Sinai Medical Center using histopathology of pituitary tumor specimens that stained positive for ACTH retrieved from patients exhibiting neither clinical nor biochemical evidence of hypercortisolism. Ectopic ACTH-secreting tumors were diagnosed with histopathology of nonpituitary tumor specimens that stained positive for ACTH retrieved from patients exhibiting clinical and/or biochemical evidence of hypercortisolism who do not meet diagnostic criteria for pituitary Cushing disease.
Fresh tumor tissue was collected during surgical tumor resection at Cedars-Sinai Medical Center or Toranomon Hospital. Formalin-fixed, paraffin-embedded (FFPE) specimens used for USP8 hotspot mutation analysis (described later) were also obtained from both sites.
For ACTH assays, electrochemiluminescence immunoassay was used at Cedars-Sinai Medical Center (Quest Diagnostic) and Toranomon Hospital (Roche Diagnostics). For cortisol assays, chemiluminescent microparticle immunoassay (Abbott Architect i2000SR) was used at Cedars-Sinai Medical Center and electrochemiluminescence immunoassay (Roche Elecsys Cortisol II) was used at Toranomon Hospital.
Human autopsy pituitary tissue collection was approved by the Cedars-Sinai IRB (Protocol No. 000375). Specimens were collected and processed for formalin fixation within 24 hours of the patient’s death.
Cell Culture
DMS79 cells (human small cell lung carcinoma), and COLO320 cells (human carcinoid-like colorectal carcinoma) were cultured in RPMI 1640 with L-glutamine (catalog No. 11875-093, Thermo Fisher) and 10% fetal bovine serum. AtT20 cells were cultured in low glucose Dulbecco’s modified Eagle medium (catalog No. 11885076, Thermo Fisher) with 10% fetal bovine serum.
Analysis of Human Proopiomelanocortin Transcription Start Sites
We performed 5′-rapid amplification of complementary DNA [cDNA] ends (5′-RACE) (24) using RNA isolated from 4 pituitary ACTH-secreting tumors, 3 ectopic ACTH-secreting tumors, and POMC-producing DMS79 and COLO320 cell lines. Briefly, cDNA was prepared using a primer (CAGTCAGCTCCCTCTTGAACTCCA) that anneals to 3′ untranslated region of POMC transcripts. The G-tail was added at the 5′ end of the resulting cDNA by T4 DNA ligase, and 5′-ends were polymerase chain reaction (PCR) amplified using a poly(C) primer (CCCCCCCCCCCCCCC) and an antisense POMC primer (ACTCCAGGGGGAAGGCCTCGGCCGA). To concentrate the human POMC cDNA fragments, a second PCR was performed using an antisense POMC primer (TTGCCCTCGCGCGGGCCCGGCT) and the poly(C) primer. Resulting PCR products were cloned into pCR Blunt II TOPO and 5′ ends determined by Sanger sequencing. To avoid PCR artifacts, 3 independent PCR products were analyzed.
Luciferase Reporter Plasmids
A human POMC first promoter fragment (–428/+68) was cloned into the pGL3 basic vector (catalog No. E175-1, Promega) as previously described (11). A POMC intron 2/exon 3 gene fragment (+6417/+7136) was PCR amplified from human genomic DNA (Jurkat cells) and cloned into a pCR Blunt II TOPO vector. Deletion mutants of these fragments were generated, and Sal I and Hind III sites were introduced at the 5′ and 3′ ends, respectively, by PCR. The resulting fragments were then cloned into the Xho I and Hind III sites in the pGL3 basic vector. Mutations of STAT and CREB binding sequences in the second promoter fragment (+6657/+7136) were introduced by 2-step PCR assembly (25), and mutated fragments cloned into the Xho I and Hind III sites in the pGL3 basic vector.
To analyze enhancer activity, the second promoter region (+6657/+7136) was cloned downstream of the luciferase gene in the pGL3 basic with the first promoter (–480/+68). The CpG-free luciferase reporter plasmid (pCpGL-basic vector) was kindly provided by Dr Michael Rehli, University Hospital Regensburg, Germany (26), and used to analyze the methylation-dependent promoter activity as described later. Human first promoter (–480/+68), human second promoter (+6657/+7136), mouse first promoter (–459/+80), and mouse second promoter (+4841/+5208) fragments were amplified, and Nhe I and Hind II sites were added by PCR. These fragments were cloned into the Spe I and Hind III sites in the CpGL-basic vector. Final constructs were confirmed by Sanger sequencing.
In Vitro Methylation
A total of 10 µg of CpG free plasmids were treated by methyltransferase (SssI) with or without 160 µM S-adenosylmethionine (SAM; catalog No. B9003S, New England Biolabs) for 4 hours at 37 °C. Methylation levels in the promoter region were digested with Hpa II and Msp I (2.5 U/µg DNA; New England Biolabs) for 1 hour at 37 °C. SssI-treated plasmid DNA was purified by phenol/chloroform, and digested DNA was analyzed by agarose gel electrophoresis. Purified plasmids were also used for the luciferase assay.
Luciferase Assays
Luciferase reporter assays were performed using 2.5 × 105 DMS79 cells or COLO320 cells with 0.7 μg of luciferase reporter plasmids, or using 0.8 × 104 AtT20 cells with 0.7 μg of luciferase reporter plasmids; 50-ng pRL-Renilla was used as an internal control plasmid. Cells were transfected using lipofectamine 2000 (Invitrogen) and cultured in 0.5 mL of medium in 24-well plates. Forty-eight hours post transfection, cells were harvested and luciferase activity analyzed by a Dual-Luciferase Reporter Assay System (Promega). Luciferase assays were repeated at least 3 times.
DNA Pull-Down Assay
DNA pull-down assay was performed as described (23). A 120-bp DNA fragment (+6847/+6966) containing the CREB binding sequence or the mutated CREB sequence fragment was amplified by PCR using biotinylated primers. A 30-μL nuclear extract (100 μg of protein) was added to 180 μL of 5 mM Tris (pH 8.0)-14% glycerol buffer and preincubated on ice for 30 minutes. Then, 15 μL of poly(dI-dC)(dI-dC) (7.5 μg) and 18 μL of 5-fold concentrated binding buffer (300 mM KCl, 60 mM N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid [HEPES; pH 7.9], 20 mM Tris-HCl [pH 8.0], 0.5 mM EDTA, 25% glycerol, 5 mM DTT) and 6 μL of probe DNA (1 μg) were added, and the reaction incubated at room temperature for 15 minutes. DNA-protein complexes were collected with 10 μL Dynabeads M-280 Streptavidin magnetic beads (catalog No. 11205D, Life Technologies). Bound protein was eluted from the DNA-Dynabeads complex, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and analyzed by immunoblotting using anti-CREB (Santa Cruz Biotechnology, catalog No. sc-186, dilution 1:1000). Antibody binding was detected using horseradish peroxidase–conjugated antirabbit with ECL (catalog No. 934-1ML, Amersham).
Construction of Full and Truncated Proopiomelanocortin Expression Plasmids and Detection of Products by Enzyme-Linked Immunosorbent Assay
Full-length (+3799/+3940 and +6828/+7499), Del-A (+6929/+7499), and Del-B (+6996/+7499) POMC cDNA fragments were PCR amplified and cloned into Hind III and Xho I sites in the pMF neo expression vector. A total of 1 µg of plasmids was transfected into 5 × 105 COLO320 cells by lipofectamine 2000 (Invitrogen) and cultured for 48 hours, and the cultured media was collected for ELISA assays. Ninety-six–well plates were coated with anti-ACTH antibody (1 ng/well, Abcam, catalog No. ab20358) overnight at 4 °Ϲ and then blocked with 1% bovine serum albumin/phosphate-buffered saline for 1 hour at room temperature. Next, 50 µL of cultured supernatant was added into 2 wells and incubated for 1 hour at room temperature. After washing with phosphate-buffered saline, biotin-conjugate monoclonal antibody to hemagglutinin (HA) (1 µg, Sigma-Aldrich, catalog No. B9183) was added and incubated for 1 hour at room temperature. Antibody binding was detected with Streptavidin–horseradish peroxidase conjugate (Life Technologies, catalog No. SNN1004), TMB-ELISA substrate (Thermo Fisher Scientific, catalog No. 34028) and Stop Solution TMB (Thermo Fisher Scientific, catalog No. N600). Absorbance at 450 nm was measured by an ELISA plate reader, and a calibration curve used to calculate ACTH concentrations.
DNA Extraction and Methylation-Specific Polymerase Chain Reaction Assay
Methylation-specific PCR assay for the POMC promoter was performed as previously described (27). Genomic DNA was purified from paraffin-embedded tumor tissues and DNA bisulfite modification using the EpiTect Fast Bisulfite Conversion Kit from FFPE tissues (Qiagen). Methylation-specific primers for PCR analysis were designed using MethPrimer (http://www.urogene.org/methprimer/) and used for methylation-specific PCR analysis (28). PCR was performed using an EpitScope MSP kit (Clontech Laboratories) and amplified using a PCR program with 45 cycles at 95 °C for 5 seconds, 55 °C for 30 seconds, and 72 °C for 1 minute. Primers used for bisulfate DNA sequencing are as follows: human first promoter: (methylated-specific) 5′-TAGTTTTTAAATAATGGGGAAATCG, 5′-CGAA AATAAAATTACCTACGTACGTA, (demethylated-specific) 5′-TAGTTTTTAAATAATGGGGAAATTGG, 5′-CAAAAATAAAATTACCTACATACATA; and human second promoter: (methylated-specific) 5′-GGTACGGGTTCT TTTTATGTTTTC, 5′-ATAACGTACTTCCGAAAAT TCTCG, (demethylated specific) 5′-GGTATGGGTTGTTTTT ATGTTTTTG, 5′-TAACATACTAAAAATTCTCAAT; mouse first promoter: (methylated-specific) 5′-TTAAATGTTAGGAAGGTAGATGGAC, 5′-CAAAAACTTAACGTCCCTATACGAA, (demethylated-specific) 5′-TTTAAATGTTAGGAAGGTAGATGGAT, 5′-CAAAAACTTAACATCCCTATACAAA; mouse second promoter: (methylated-specific) 5′- TGGAGTATTTTCGTTGGAGTAATTC, 5′-ACCCT CACTAACCCTTCTTATACG, (demethylated specific) 5′-TGGAGTATTTTTGTTGGAGTAATTTG, 5′-ACCCTCACTAACCCTTCTTATACAC.
Bisulfite Sequencing
Bisulfite sequencing of the POMC promoter region was performed as previously described (29). Briefly, each bisulfite-converted DNA was amplified by PCR, PCR products were cloned into pCR2.1-TOPO (Invitrogen), and 6 clones from each tumor sample were sequenced. Primers used for bisulfite sequencing were: 5′-CGAGTGTTAAGAAAGTAG, 5′-TCGGCTAACCAATCAACTC.
Sequencing of Ubiquitin-Specific Protease 8 Hotspot Mutations
Genomic DNA was extracted from fresh samples using DNeasy Blood and Tissue Kit (Qiagen) or from FFPE samples using AllPrep DNA/RNA FFPE kit (Qiagen). The presence of USP8 hotspot mutations was determined by Sanger sequencing. The following primers were used for PCR amplification and sequencing: 5′-TCCAACTCATAAAGCCAAGCCACAGAT and 5′-TGGCTTGTTTTCCCGATTAACTGTTGG.
Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation assay was performed using AtT20 cells as previously described (30). Cells were fixed in 1% formaldehyde, 4.5 mM HEPES (pH 8.0), 9 mM NaCl, 0.09 mM EDTA, and 0.045 mM EGTA for 10 minutes at room temperature, and sonicated using a Bioruptor (Diagenode) in lysis buffer (1% sodium dodecyl sulfate, 10 mM EDTA, and 50 mM Tris-HCl pH 8.0) with proteinase inhibitor (Sigma-Aldrich, catalog No. P8340). Precleared lysates were incubated overnight at 4 °C with 2 µg of anti-STAT3 (Santa Cruz Biotechnology, catalog No. sc-482); anti-STAT5 (Santa Cruz Biotechnology, catalog No. sc-836); anti-CREB (Santa Cruz Biotechnology, catalog No. sc-186); or normal rabbit immunoglobulin G (Santa Cruz Biotechnology; catalog No. sc-2027). DNA fragments were isolated from immunoprecipitated chromatin and analyzed by quantitative PCR with SYBR Green PCR Master Mix (Applied Biosystems). PCR primers used were mPomc 5′-GCCGAGACTCCCATGTT, 5′-GTGGCCCATGACGTACT.
Statistics
Differences in methylation status between groups in each parameter were analyzed using t test with the Welch correction. Probability of P less than .05 was considered significant. Correlation of methylation status and plasma ACTH/cortisol levels was assessed by Spearman correlation. Results of luciferase assays and ELISA were analyzed by t test, and P less than .05 is considered significant.
Results
Identification of a Second Human Proopiomelanocortin Promoter Both in Corticotroph Adenomas and Ectopic Adrenocorticotropin-Secreting Tumors
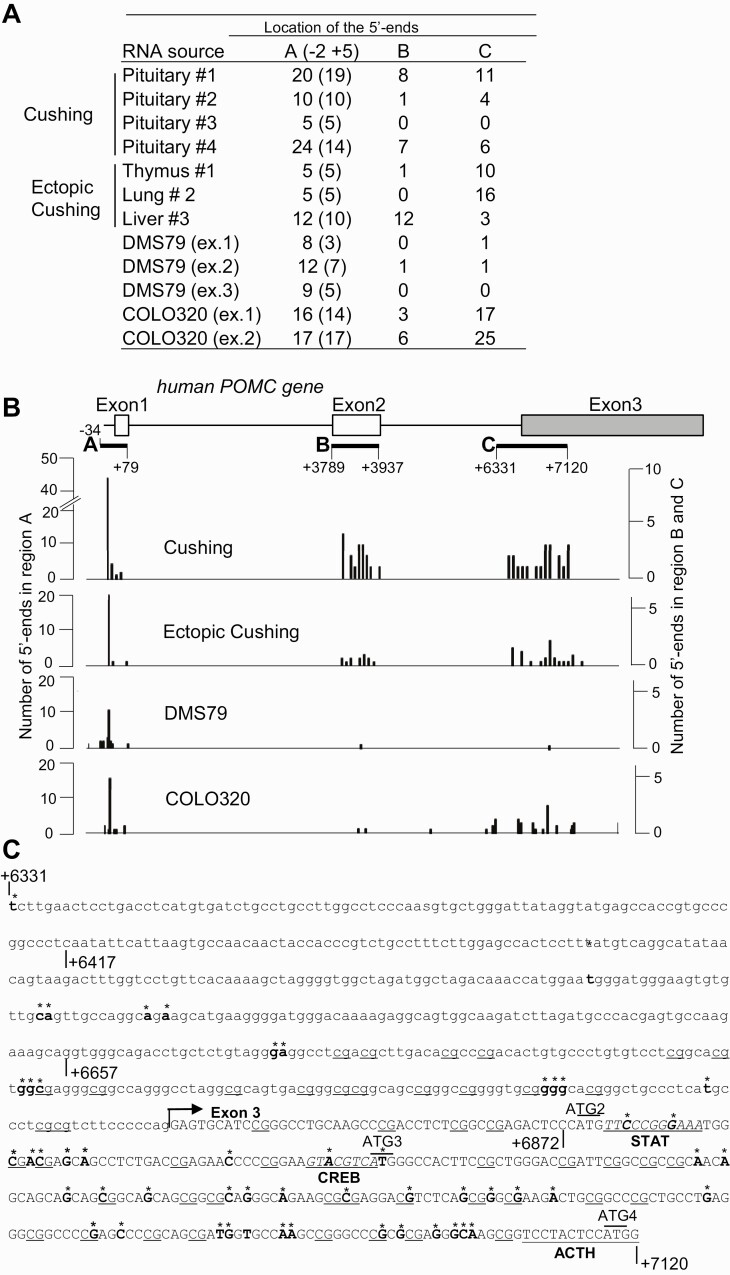
We analyzed transcription start sites of the POMC promoter in 7 human pituitary tumor samples. We performed 5′-RACE using RNA obtained from 4 ACTH-secreting pituitary tumors and 3 ectopic ACTH-secreting tumors resected from thymus, lung, and liver, as well as from 2 human cell lines that express POMC and ACTH (DMS79 [human small cell lung carcinoma] and COLO320 [human carcinoid-like colorectal carcinoma]) (Fig. 1A and Table 1). When mapping positions of the 5′ ends of POMC transcripts to the human POMC gene, we found start sites clustered in 3 regions (Fig. 1A and 1B). The first region at the 5′ end mapped just downstream of the previously characterized TATA-box in the POMC proximal promoter (region A, Fig. 1B), with the most abundant 5′ ends located approximately 30 bp downstream of the TATA box sequence (see Fig. 1A and 1B). The second region (region B) mapped to the coding sequence in the POMC exon 2 (see Fig. 1B). All the 5′ ends in region B were located within the POMC mRNA sequence and were mainly identified using RNA isolated from tumor tissue; as 5′ ends were rarely found in freshly isolated cell lines, they were likely derived from degraded RNA.
Figure 1.
Human proopiomelanocortin (hPOMC) transcription start sites in pituitary and ectopic adrenocorticotropin (ACTH)-secreting tumors and cell lines. A, 5′-rapid amplification of complementary DNA ends (5′-RACE) analysis of human POMC messenger RNA (mRNA) isolated from human pituitary corticotroph adenomas (Cushing) and ectopic Cushing tumors (ectopic Cushing) resected from thymus, lung, and liver (see Table 1), as well as ACTH-secreting DMS79 (3 independent experiments: ex. 1, ex. 2, ex. 3) and COLO320 cells (2 independent experiments: ex. 1, ex. 2). The number of identified 5′-ends of POMC mRNA located in regions A, B, and C (depicted in B) are noted, with the number of major 5′ ends in region A located approximately 30 bp downstream of the TATA box sequence shown in parentheses. B, Number of 5′-ends of POMC mRNA in pituitary ACTH-secreting (Cushing), ectopic ACTH-secreting tumors (ectopic Cushing), and DMS79 and COLO320 cells in each region. Top, relative position of each region, with the major mRNA start site defined as position +1; relative positions of exon 1, exon 2, and exon 3 are depicted in boxes. Left scale, number of 5′ ends located in region A; right scale, number of 5′ ends located in region B and region C. C, DNA sequence in region C. Identified 5′ ends of POMC mRNA are shown in bold and flagged with an asterisk. The intron 2 sequence is shown in lowercase letters and exon 3 sequence in capital letters. Arrow indicates 5′-end of exon 3. Potential STAT and CREB binding sequences are shown in italics and underlined. Dinucleotide 5′-CG-3′ (CpG) sequences identified as potential methylation sites are underlined. The ACTH coding sequence is also underlined.
Table 1.
Characteristics of adrenocorticotropin-secreting tumors used for 5′-rapid amplification of complementary DNA ends
| Tumor | Age, y, sex | ACTH, pg/mL | 24-hr UFC, µg/d | Maximum diameter, mm |
|---|---|---|---|---|
| Pituitary #1 | 29 F | 54 | 281 | 8 |
| Pituitary #2 | 73 M | 296 | 59 | 28 |
| Pituitary #3 | 33 F | 44 | 239 | 4 |
| Pituitary #4 | 16 F | 139 | 661 | 37 |
| Thymus #1 | 20 F | 42 | 1178 | 13 |
| Lung #2 | 23 F | 145 | 1030 | 100 |
| Liver #3 | 40 F | 1100 | NA | 20 |
ACTH and 24-hr UFC are preoperative values. Tumor with 10-mm or greater maximum diameter is defined as a macroadenoma, and less than 10 mm as a microadenoma.
Abbreviations: ACTH, adrenocorticotropin; F, female; M, male; NA, not available; UFC, urinary free cortisol.
In the third region detected, region C, located near the junction of intron 2 and exon 3 (see Fig. 1B), 5′ ends were identified in RNA from tumor tissue as well as isolated from COLO320 cells; 34% mapped to intron 2 (see Fig. 1B). As 5′ ends of partially degraded RNA would not contain sequences corresponding to intron 2 (31), these results suggest that POMC transcripts start from this region, while an additional POMC promoter is located upstream of these 5′ ends. Notably, unlike in region A, where 5′ ends of POMC mRNA were identified in all cell types tested, few 5′ ends mapped to region C in DMS79 cells, suggesting that POMC mRNA transcribed from region C may be cell-type specific.
CREB Regulates Activity of the Second Promoter
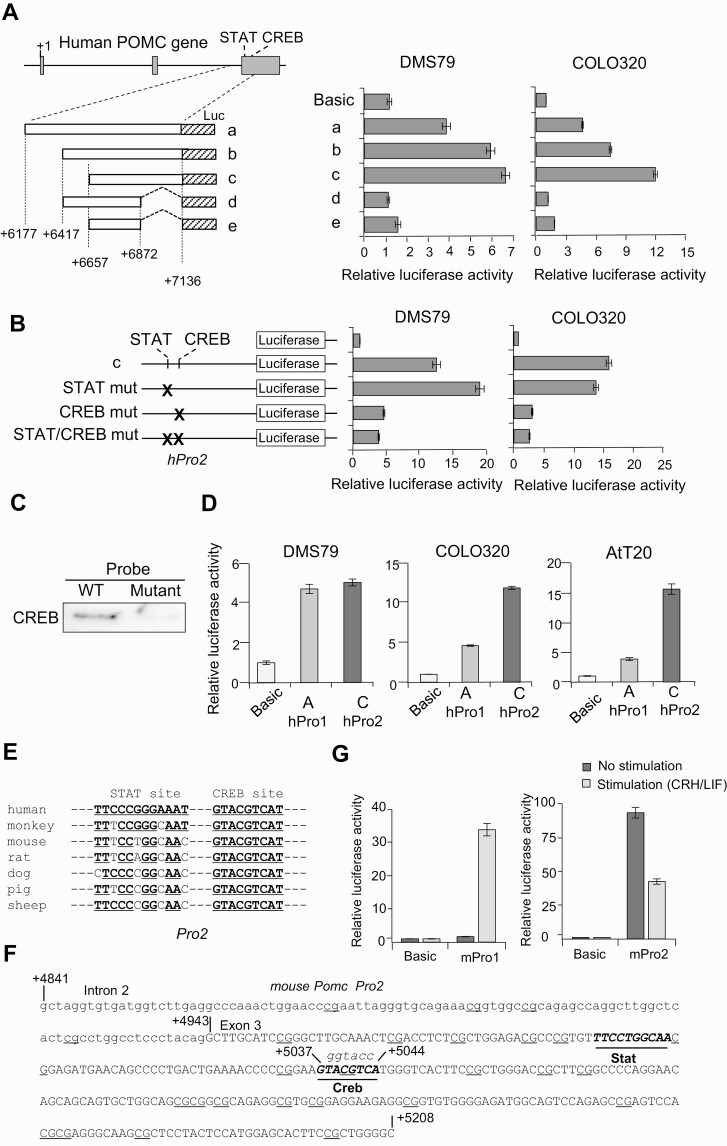
The 5′ ends of POMC mRNA in region C are located between +6331 and +7120 bp downstream of the previously identified POMC transcription start site (11) (Fig. 1C). This region does not contain a characteristic TATA box and is GC rich. To test whether this sequence possesses promoter activity, we cloned DNA fragment +6177 to +7136 and its deletion mutants into a luciferase reporter plasmid and promoter activities were analyzed by luciferase reporter assays in COLO320 and DMS79 cells (Fig. 2A). Promoter activities were detected in all 3 cloned fragments: +6177 to +7136, +6417 to +7136, and +6657 to +7136 (see Fig. 2A, fragments a-c). The strongest activity was detected in fragment c at +6657 to +7136 both in COLO320 and DMS79 cells, suggesting that negative regulatory elements may be present in +6177 to +6657, upstream of fragment c. As 3′ deletion constructs (see Fig. 2A, fragments d and e) exhibited reduced promoter activity in both cell lines, we concluded that the deleted 264-bp sequence likely contains critical regulatory promoter sequences. We therefore termed fragment c (+6657 to +7136) as the POMC second promoter. Analysis of transcription factor databases for this POMC second promoter identified potential consensus binding motifs for STAT (TTCCCGGGAAA, +6877 to +6887, see Fig. 1C) and CREB (GTACGTCA, +6924 to +6931, see Fig. 1C) transcription factors.
Figure 2.
Activity of the second proopiomelanocortin (POMC) promoter. A, Left, structure of 5 luciferase (Luc) reporter plasmids (a-e). The inserted POMC gene fragments are depicted as boxes, and positions of the 5′ and 3′ ends are noted. Right, luciferase assays were performed using indicated cells (DMS79 and COLO320) and generated luciferase activities with indicated plasmids compared with negative control plasmid (no promoter; Basic). B, Left, structure of luciferase reporter plasmids shown in A with mutated (mut) potential STAT, CREB, and STAT/CREB binding sequences (indicated by X) in fragment c in the second promoter (hPro2). Right, generated luciferase activities with indicated plasmids compared with negative control plasmid (no promoter; Basic) in DMS79 and COLO320 cells. C, Biotinylated probes from DNA binding assay with wild-type (WT) and mutated (mutant) CREB binding sites coprecipitated with proteins and analyzed by immunoblotting using anti-CREB. D, Luciferase assays using reporter plasmids containing the first promoter (hPro1) (–428 to +68) and second promoter (hPro2) (+6657 to +7136) compared with negative control plasmid (no promoter; Basic) using DMS79, COLO320, and AtT20 cell lines. E, Alignment of POMC second promoter STAT and CREB response elements by species. F, DNA sequence in mouse Pomc second promoter with the intron 2 sequence shown in lowercase letters and exon 3 sequence in capital letters. Potential STAT and CREB binding sequences are shown in italics and underlined, and dinucleotide 5′-CG-3′ (CpG) sequences identified as potential methylation sites are underlined. G, Luciferase assays using reporter plasmids mouse first promoter (mPro1) (–459 to +80) and mouse second promoter (mPro2) (+4841 to +5209) compared with negative control plasmid (no promoter; Basic) with CRH and LIF stimulation or without stimulation in AtT20 cells.
To assess the contribution of these transcription factors to the activity of the second POMC promoter, we introduced point mutations in potential STAT (TTCCCGGGAAA to TTGAATTCAAA) and CREB (GTACGTCA to GGGTACCA) binding sequences in the luciferase construct for fragment c (Fig. 2B). Point mutations in CREB, but not STAT, binding motifs reduced promoter activity in both cell lines (see Fig. 2B). To further validate this observation, we performed an in vitro DNA pulldown assay using nuclear extracts prepared from COLO320 (Fig. 2C). Immunoblotting with anti-CREB antibody showed that DNA probes with wild-type CREB sites, but not CREB site mutations, were bound by CREB protein (see Fig. 2C). Taken together, these results demonstrate that sequences between +6657 and +7136 (fragment c) may serve as a POMC promoter and transcription start site, regulated by CREB.
Regulation of the Second Promoter Is Distinct From the First Promoter
We next compared activity of the second promoter with the first promoter located upstream of exon 1 (–428 to +68 including region A) that we previously cloned (11). Both promoters were active in DMS79, COLO320, and mouse corticotroph AtT20 cell lines (Fig. 2D). Activity of the 2 promoters was similar in DMS79; however, the second promoter was more robust in COLO320 and AtT20 cells (see Fig. 2D), consistent with cell-type specificities.
Next, we examined species specificity of the second promoter. GC-rich and CREB and STAT binding sequences are conserved in the intron 2/exon 3 junction region in the mPomc gene as well as other species (Fig. 2E). We cloned this GC-rich fragment from the mPomc gene (Fig. 2F) into luciferase reporter plasmids, and activity was analyzed in mouse-derived AtT20 cells. We detected the mouse second promoter in this fragment, and its activity was stronger than that of the first promoter in nonstimulated AtT20 cells (Fig. 2G). As the first Pomc promoter is stimulated with corticotropin-releasing hormone (CRH) and leukemia inhibitory factor (LIF) (18, 32), we analyzed promoter activity after these treatments (see Fig. 2G). Activity of the first promoter was increased 24-fold with CRH/LIF stimulation, consistent with known increased POMC expression in response to CRH (33). By contrast, the second mouse promoter activity was downregulated in response to these stimuli (see Fig. 2G), indicating distinct responses to hypothalamic/pituitary signaling.
Adrenocorticotropin Is Produced From Proopiomelanocortin Messenger RNA Transcribed From the Second Promoter
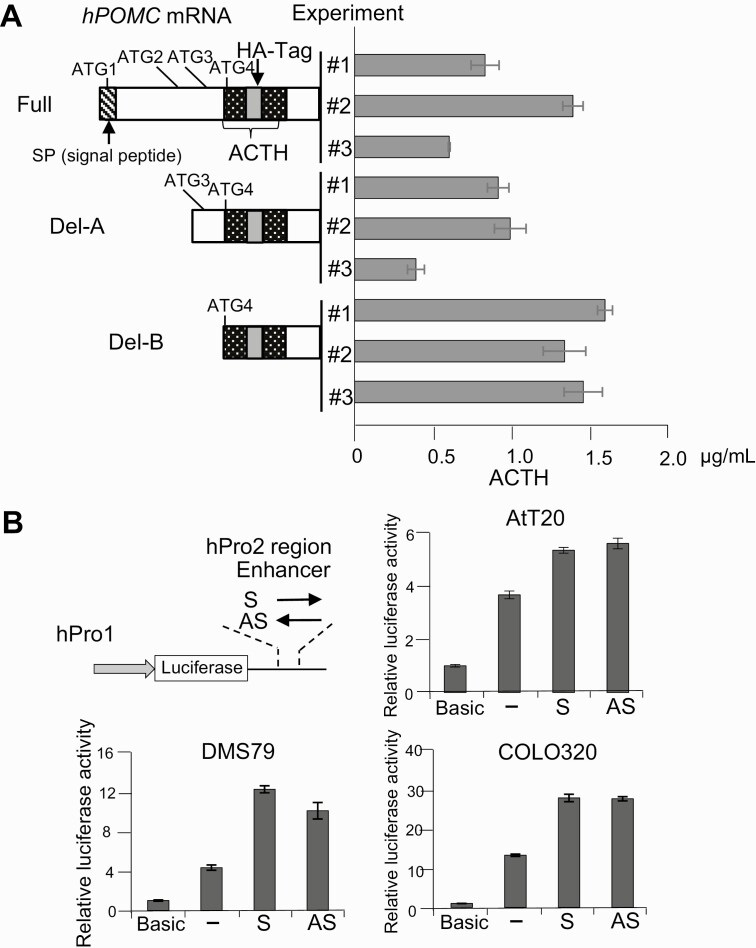
To determine whether second promoter–dependent transcripts are translated to ACTH, we sought ATG sequences in-frame with the ACTH sequence in human POMC mRNA and identified 4 ATG (ATG1 to ATG4) sequences upstream of and within the ACTH coding sequence (Fig. 3A). ATG1 is located nearest the 5′ end and is considered the putative translation start site for the full-length POMC polypeptide. ATG2, ATG3, and ATG4 are located in exon 3, within the previously defined region C; specifically, ATG2 and ATG3 are located upstream of the ACTH coding sequence, and ATG4 is within the ACTH sequence. Because POMC is a complex precursor peptide and is cleaved into several small peptide hormones with different roles, we considered that translation might start from ATG2-4. To test this possibility, we generated 3 POMC constructs with a HA-tag at the 3′ end of the ACTH coding sequence, cloned the sequences into the EF1α promoter–driven expression vector pMF (see Fig. 3A), and transfected the resulting constructs into COLO320 cells. Recombinant HA-tagged ACTH was detected in the culture supernatant of all transfectants (see Fig. 3A), indicating that second promoter–dependent POMC transcripts lead to production of secreted ACTH.
Figure 3.
Adrenocorticotropin (ACTH) production and enhancer activity of second promoter-dependent proopiomelanocortin (POMC) messenger RNA (mRNA) transcripts. A, Left, wild type (Full) and truncated (Del-A and Del-B) human POMC mRNA expression plasmids were constructed in the indicated complementary DNA encoding regions. Relative positions of 4 ATG sequences (ATG1-4) upstream of the ACTH coding sequence are shown. A HA-tag sequence (gray box) was inserted in the ACTH sequence (patterned box) in frame. Right, secretion of ACTH-HA tag fusion proteins analyzed by enzyme-linked immunosorbent assay using anti-ACTH as a capture antibody and anti-HA as a detection antibody in COLO320 cells transfected with these plasmids and cultured for 48 hours. Three independent experiments (#1, #2, and #3) using each plasmid were performed. B, Top left, structure of luciferase reporter plasmids with the 480-bp fragment (+6657 to +7136) inserted downstream of the human POMC (hPOMC) first promoter (hPro1; –428 to +68)/luciferase gene unit both in the sense (S) and antisense (AS) directions in the second (hPro2) promoter. Top right and bottom, luciferase assays performed using the resulting plasmids (S and AS) and control plasmids with no enhancer fragment (–) and compared with no promoter/no enhancer fragment (Basic) in the AtT20, DMS79, and COLO320 cell lines.
The Second Proopiomelanocortin Promoter Exhibits Enhancer Activity
Because the second promoter sequence is located within intron 2 and exon 3, we considered whether it may function as an enhancer for the first promoter. We therefore constructed luciferase reporter assays by cloning the 596-bp human POMC first promoter sequence (–428 to +68) upstream of luciferase and the 480-bp human POMC second promoter sequence (+6657 to +7136) downstream of the luciferase gene both in sense and antisense directions (Fig. 3B). Luciferase assays using DMS79, COLO320, and AtT20 cells showed that the second promoter fragment enhanced the first promoter activity approximately 2-fold in both directions (see Fig. 3B), suggesting that the second POMC promoter sequence also functions as an enhancer for human POMC gene expression.
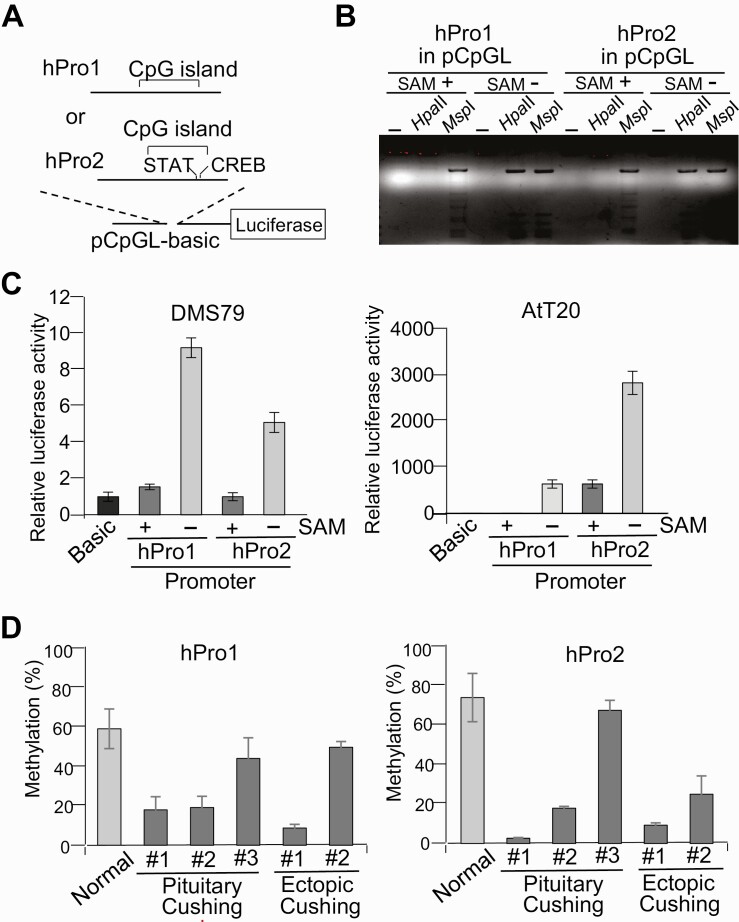
DNA Methylation Regulates Activities of the First and Second Promoters
Because both promoters contain CpG-rich sequences, we assessed whether promoter activities may be regulated by DNA methylation. We constructed 2 luciferase reporter plasmids, with the first and second promoters cloned into the CpG sequence-free pCpGL luciferase reporter plasmid (26) (Fig. 4A). To methylate CpG sequences in the inserted promoters, reporter plasmids were treated with SssI with or without SAM. We then examined promoter methylation levels by digestion of methylation-sensitive Hpa II and methylation-insensitive Msp I restriction enzymes, both of which recognize the 5′-CCGG sequence (Fig. 4B). Plasmid DNA treated by SssI with SAM were digested by Msp I but not Hpa II, indicating high methylation levels. Luciferase reporter assays using these plasmids showed that activity of both promoters was strongly inhibited by DNA methylation both in DMS79 and AtT20 cells (Fig. 4C), suggesting that activity of both promoters is regulated by demethylation and may be conserved in the mouse and human.
Figure 4.
First and second proopiomelanocortin (POMC) promoter activity with DNA methylation. A, Structure of luciferase reporter plasmids using the dinucleotide 5′-CG-3′ (CpG) sequence free luciferase vector CpG-free luciferase reporter plasmid (pCpGL) and first (hPro1; –428 to +68) and second promoter (hPro2; +6657 to +7136) fragments. The relative position of the CpG island and potential STAT and CREB binding sites on these fragments are indicated. B, First (hPro1) and second (hPro2) promoter reporter plasmids treated with methyltransferase with (+) or without (–) S-adenosylmethionine (SAM), digested with no enzyme (–), Hpa II, or Msp I, and analyzed by agarose gel electrophoresis. C, Luciferase assays using first (hPro1) and second (hPro2) promoter reporter plasmids treated with (+) and without (–) SAM, with generated luciferase activities compared to negative control plasmid CpG-free luciferase reporter plasmid (pCpGLbasic) (Basic). D, Methylation levels in first (hPro1) and second (hPro2) promoters analyzed by bisulfite-conversion–based methylation-specific polymerase chain reaction using DNA isolated from 3 pituitary adrenocorticotropin (ACTH)-secreting tumors (pituitary #1, #2, and #3; see Fig. 1A) and 2 ectopic ACTH-secreting tumors (thymus #1 and lung #2; see Fig. 1A), with normal pituitary obtained at autopsy (normal) as control. Tumor characteristics are shown in Tables 1 and 2.
Considering that varying POMC expression and ACTH levels in Cushing tumors may be reflective of promoter activities regulated by DNA methylation, we next analyzed DNA methylation levels of these promoter regions by a PCR-based method (28). We assessed 3 ACTH-secreting pituitary corticotroph tumors and 2 ectopic ACTH-secreting tumors, and compared their respective methylation levels with the average methylation level of 11 normal pituitary specimens obtained from autopsy processed within 24 hours of death and used as controls (Table 2). As expected, given the variable 5′-RACE results (see Fig. 1A), methylation levels of ACTH-secreted tumors varied widely (Fig. 4D). The first and second promoters in 2 pituitary ACTH-secreting tumors (pituitary #1 and #2) as well as in 1 of the ectopic tumors (ectopic #1) were highly demethylated. Interestingly, in these samples, 5′-RACE detected substantial numbers of transcription starts in the second promoter (see Fig. 1A). Methylation levels of the second promoter in pituitary #3 was high (see Fig. 4D), and no transcripts were detected from the second promoter site (see Fig. 1A), suggesting that methylation levels correspond to transcriptional activities. By contrast, in ectopic #2, the second promoter was more demethylated than the first promoter, and 3-fold more mRNA in this sample were transcribed from the second promoter. In summary, POMC gene expression levels in ACTH-secreting tumors seem to be regulated by DNA methylation both in first and second promoters.
Table 2.
Characteristics of tissue samples used for methylation analyses
| Sample No. | Tissue type | Sex | Age, y |
|---|---|---|---|
| 1 | N | M | 45 |
| 2 | N | M | 52 |
| 3 | N | F | 74 |
| 4 | N | F | 68 |
| 5 | N | F | 78 |
| 6 | N | M | 54 |
| 7 | N | F | 82 |
| 8 | N | F | 77 |
| 9 | N | M | 67 |
| 10 | N | M | 73 |
| 11 | N | M | 65 |
| 12 | SCA | F | 58 |
| 13 | SCA | F | 42 |
| 14 | SCA | M | 58 |
| 15 | SCA | M | 70 |
| 16 | SCA | M | 63 |
| 17 | SCA | F | 35 |
| 18 | EC lung | F | 23 |
| 19 | EC thymus | F | 20 |
Samples 1, 2, 3, 4, 12, 13, and18 were randomly selected for use in bisulfite methylation analysis sequencing.
Abbreviations: EC, ectopic Cushing tumor; F, female; M, male; N, normal pituitary; SCA, silent corticotroph adenoma.
DNA Methylation Regulates Proopiomelanocortin Production in Adrenocorticotropin-Secreting Tumors
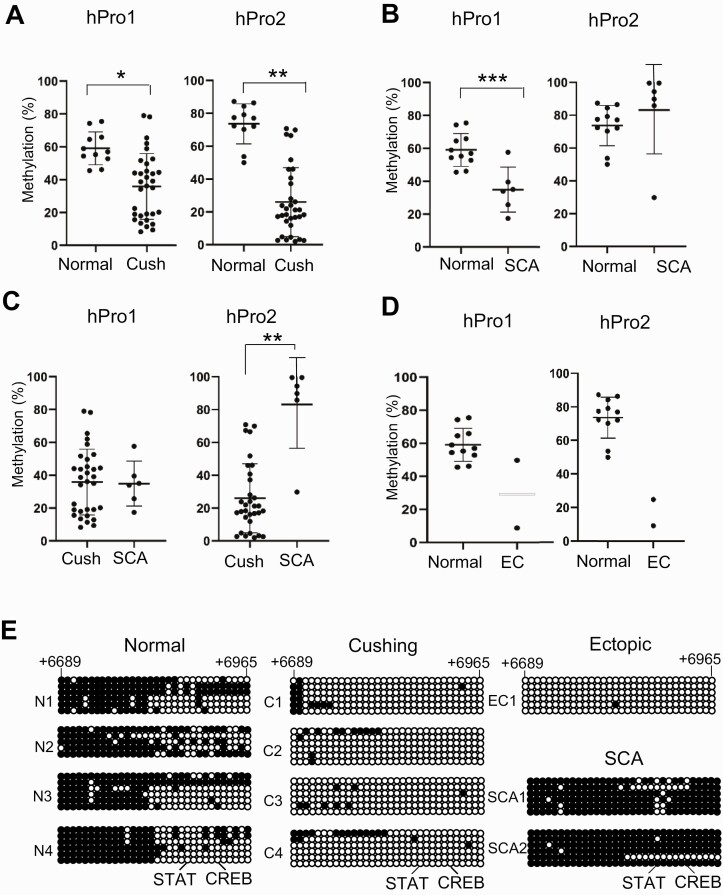
We then analyzed methylation/demethylation levels in first and secondary promoter regions in 11 normal pituitary autopsy specimens, 32 pituitary ACTH-secreting tumors, 2 ectopic ACTH-secreting tumors, and 6 SCAs (see Tables 2 and 3). In pituitary ACTH-secreting tumors, both the first and second promoters were significantly demethylated compared with normal pituitary tissue, and the degree of demethylation was greater in the second promoter (Fig. 5A). By contrast, in SCAs, which do not produce ACTH, although the first promoter was significantly demethylated, the second promoter was highly methylated compared with normal pituitary (Fig. 5B). Differences in methylation patterns between the first and second promoters were even more striking when comparing pituitary ACTH-secreting tumors and nonsecreting SCAs (Fig. 5C). Because only 2 ectopic ACTH-secreting tumor samples were available for study, statistical analysis was not possible. However, visual inspection showed that the first promoter was highly demethylated in one specimen, while the second promoter was highly demethylated in both (Fig. 5D).
Table 3.
Characteristics of adrenocorticotropin-secreting pituitary corticotroph tumors
| hPro1, % | hPro2, % | Micro/Macro | Recur | Crooke | USP8 mut | Sex | Age group, y | ACTH, pg/mL | Serum cortisol, µg/dL | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17.9 | 2.7 | Macro | N | Y | WT | M | ≥ 40 | NA | NA |
| 2 | 19.1 | 17.8 | Macro | Y | Y | WT | F | < 40 | 19.1 | NA |
| 3 | 44.0 | 67.3 | Micro | Y | N | WT | F | < 40 | 44.1 | NA |
| 4 | 15.6 | 21.3 | Macro | N | Y | WT | M | ≥ 40 | NA | NA |
| 5 | 35.1 | 24.0 | Micro | N | N | WT | M | < 40 | NA | NA |
| 6 | 9.4 | 16.7 | Micro | Y | N | WT | M | < 40 | 35.1 | NA |
| 7 | 8.3 | 46.1 | Micro | N | N | Mut | F | ≥ 40 | NA | NA |
| 8 | 15.3 | 11.8 | Macro | N | Y | WT | F | ≥ 40 | NA | NA |
| 9 | 45.2 | 16.0 | Macro | N | N | WT | F | < 40 | NA | NA |
| 10 | 39.1 | 70.8 | Micro | N | N | Mut | F | < 40 | 17.4 | 107.7 |
| 11 | 43.7 | 17.2 | Macro | Y | N | WT | M | < 40 | 179.2 | 37.9 |
| 12 | 36.1 | 14.4 | Macro | N | Y | WT | F | ≥ 40 | 96.0 | 16.5 |
| 13 | 34.2 | 69.9 | Macro | N | N | WT | M | ≥ 40 | 388.0 | 10.3 |
| 14 | 12.9 | 4.8 | Macro | N | N | Mut | F | < 40 | 88.6 | 23.7 |
| 15 | 22.4 | 17.1 | Macro | N | Y | Mut | F | ≥ 40 | 109.0 | 22.3 |
| 16 | 20.2 | 26.2 | Micro | N | N | WT | F | ≥ 40 | 115.9 | 18.3 |
| 17 | 13.5 | 3.2 | Macro | N | N | Mut | F | ≥ 40 | 136.9 | 45.9 |
| 18 | 11.4 | 66.5 | Macro | N | N | Mut | F | < 40 | 127.8 | 15.7 |
| 19 | 18.9 | 21.2 | Macro | Y | N | WT | F | ≥ 40 | 91.7 | 17.4 |
| 20 | 62.0 | 2.6 | Macro | N | N | WT | M | ≥ 40 | 140.9 | 17.3 |
| 21 | 44.4 | 22.0 | Micro | N | N | WT | M | < 40 | 86.2 | 16.3 |
| 22 | 38.7 | 4.6 | Micro | Y | Y | WT | F | ≥ 40 | 69.8 | 19.9 |
| 23 | 41.4 | 37.2 | Macro | Y | N | WT | F | < 40 | 68.2 | 25.4 |
| 24 | 51.7 | 40.7 | Micro | N | N | WT | F | ≥ 40 | 81.1 | 12.3 |
| 25 | 52.6 | 18.3 | Macro | N | N | WT | F | ≥ 40 | 59.8 | 16.7 |
| 26 | 58.9 | 46.0 | Micro | N | N | WT | F | ≥ 40 | 105.6 | 53.1 |
| 27 | 78.3 | 28.0 | Macro | N | N | WT | F | ≥ 40 | 242.7 | 25.9 |
| 28 | 49.7 | 2.9 | Macro | N | Y | WT | F | ≥ 40 | 72.1 | 10.0 |
| 29 | 79.0 | 18.3 | Micro | N | N | WT | M | ≥ 40 | 44.4 | 14.1 |
| 30 | 43.6 | 51.8 | Micro | N | N | WT | F | < 40 | 45.5 | 7.1 |
| 31 | 19.0 | 2.0 | Macro | Y | Y | WT | M | ≥ 40 | 500.1 | 17.2 |
| 32 | 65.5 | 23.9 | Macro | N | Y | WT | F | ≥ 40 | 68.6 | 17.8 |
ACTH and serum cortisol are preoperative levels. Samples 1, 6, 8, and 9 were randomly selected for use in bisulfite methylation analysis sequencing.
Abbreviations: ACTH, adrenocorticotropin; Crooke, Crooke cell changes; F, female; hPro1, first promoter; hPro2, second promoter; M, male; Macro, macroadenoma (defined as ≥10 mm); Micro, microadenoma (defined as < 10 mm); Mut, mutated; N, no; NA, not available; Recur, recurrent disease; USP8, ubiquitin-specific protease 8; WT, wild-type; Y, yes.
Figure 5.
DNA methylation of first and second promoters. A to D, DNA methylation in the proopiomelanocortin (POMC) first (hPro1) and second (hPro2) promoters analyzed by bisulfite-conversion–based methylation-specific polymerase chain reaction using DNA isolated from 11 normal autopsy-derived pituitaries (normal), 32 pituitary adrenocorticotropin (ACTH)-secreting tumors (Cush), 2 ectopic ACTH-secreting tumors (EC), and 6 silent corticotroph adenomas (SCA). Results were compared between A, normal and Cush; B, normal and SCA; C, Cush and SCA; and D, normal and EC. *P = .001; **P < .001; ***P = .008. E, CpG methylation sites in the POMC second promoter were analyzed by bisulfate sequencing using DNA isolated from 4 normal pituitary (N1-4), 4 pituitary ACTH-secreting tumors (C1-4), 1 ectopic ACTH-secreting tumor (EC1), and 2 SCAs (SC1,2). Circles indicate potential methylation sites in the dinucleotide 5′-CG-3′ (CpG) island at +6689 to +6965. The DNA sequence of the CpG island is shown in Fig. 1C. Positions of STAT and CREB binding sequences are indicated. Open and closed circles indicate demethylated and methylated sites, respectively. Tumor characteristics are shown in Tables 2 and 3.
To assess methylation/demethylation positions in the second promoter, we performed bisulfite-conversion–based DNA sequencing of the CpG islands (+6689 to +6965) in 4 normal pituitaries, 4 ACTH-secreting pituitary tumors, 1 ectopic ACTH-secreting tumor, and 2 SCAs, all randomly selected from the full set (see Tables 2 and 3). The 5′ half of the CpG islands in normal pituitary specimens were highly methylated, and nearly the entire region was highly methylated in SCAs that express POMC but do not secrete ACTH (Fig. 5E). By contrast, pituitary ACTH-secreting tumors and ectopic ACTH-secreting tumor specimens that express POMC and actively secrete ACTH were highly demethylated (see Fig. 5E). These results suggest that methylation status underlies regulation of POMC production from the second promoter in different POMC-expressing tissue types.
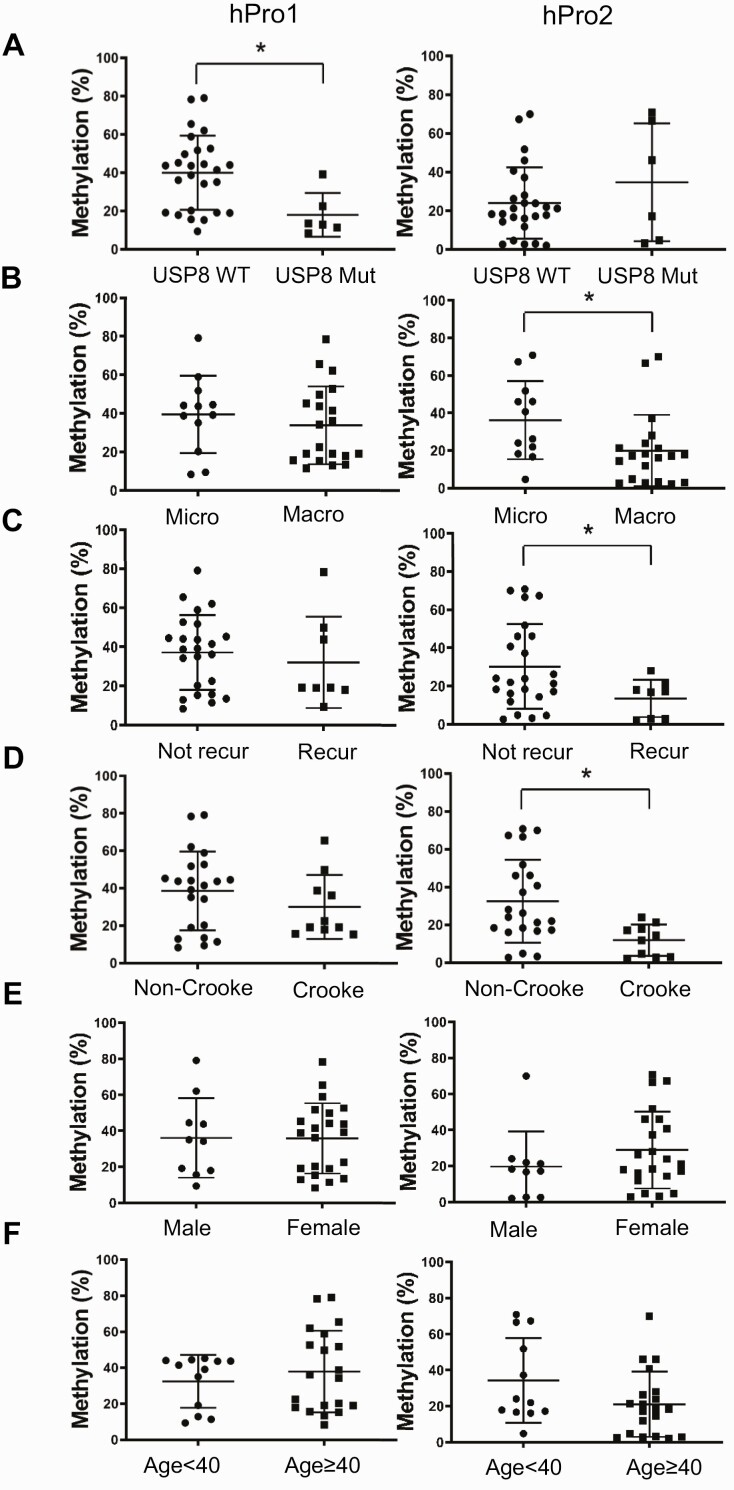
Demethylation of the Second Proopiomelanocortin Promoter Correlates With Aggressive Features of Cushing Disease
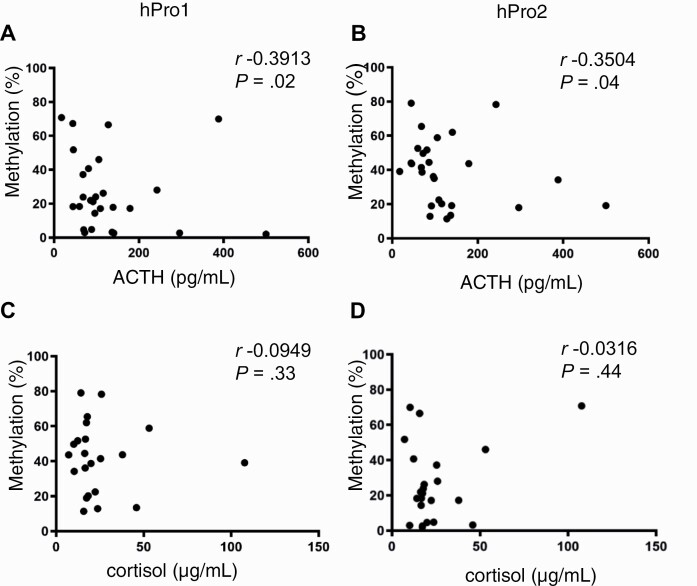
We next investigated whether demethylation of the second POMC promoter is associated with tumor-specific and clinical features of ACTH-secreting pituitary corticotroph tumors (see Table 3). The first promoter was markedly demethylated in USP8-mutated tumors (Fig. 6A), whereas the second promoter was demethylated in specimens derived from patients with more adverse phenotypic characteristics (34), including macroadenoma (Fig. 6B) and tumor recurrence (Fig. 6C), defined as biochemical evidence of hypercortisolism in patients who previously achieved resolution of hypercortisolism, as well as Crooke cell changes (Fig. 6D), all indicating an aggressive phenotype. Neither age nor sex was associated with methylation status of either promoter (Fig. 6E and 6F). These results suggest that second promoter demethylation is associated with aggressive features of Cushing disease unrelated to USP8 mutation and EGFR signaling. We also observed that methylation levels of both first and second promoters correlated with serum ACTH (Fig. 7A and 7B), but not serum cortisol levels (Fig. 7C and 7D).
Figure 6.
DNA methylation in first and second promoters correlates with pituitary adrenocorticotropin (ACTH)-secreting tumor characteristics. DNA methylation levels in first (hPro1) and second (hPro2) promoters were analyzed by bisulfite-conversion–based methylation-specific polymerase chain reaction based on A, ubiquitin-specific protease 8 (USP8) mutation status (USP8 wild-type [WT] (n = 26) vs USP8 mutated [mut] (n = 6), *P = .0029); B, tumor size (microadenoma [micro < 10 mm] (n = 12) vs macroadenoma [macro ≥ 10 mm] (n = 20), *P = .0383); C, recurrent disease (nonrecurrent [not recur] (n = 24) vs recurrent [recur] (n = 8), *P = .007); D, Crooke cell changes (absent [non-Crooke] (n = 22) vs present [Crooke] (n = 10), *P = .0006); E, sex (male [n = 10] vs female [n = 22]); and F, age (< 40 [n = 12] vs ≥ 40 years [n = 20]). Characteristics of all 32 ACTH-secreting pituitary tumors are given in Table 3.
Figure 7.
DNA methylation of first and second promoters correlate with adrenocorticotropin (ACTH) and cortisol levels. Correlations between preoperative serum ACTH levels and methylation status of A, first (hPro1), and B, second (hPro2) promoters, and between morning serum cortisol levels and methylation status of C, first (hPro1), and D, second (hPro2) promoters. ACTH and cortisol values are given in Table 3.
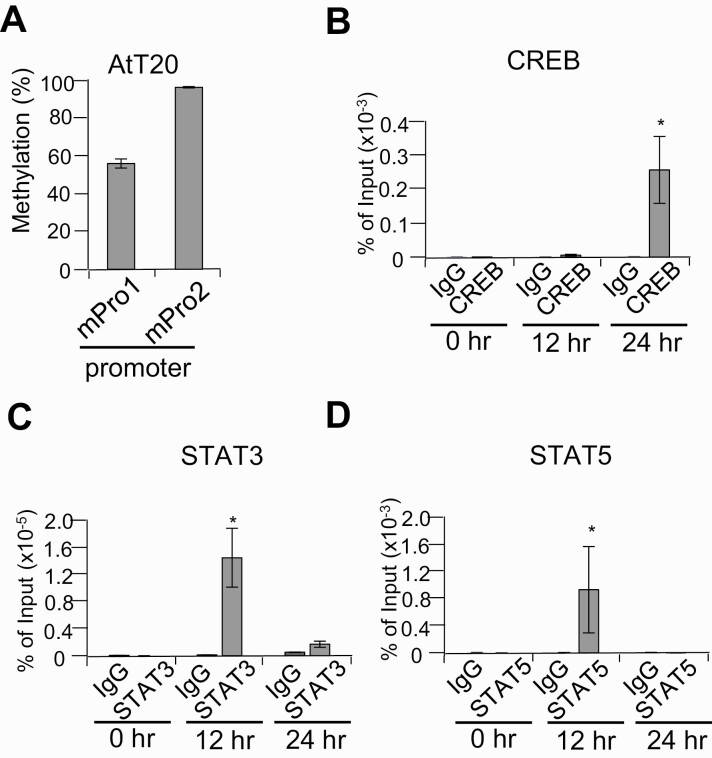
Leukemia Inhibitory Factor Signaling May Regulate CREB Binding to the Second Promoter by Activating STAT3
Although we identified potential binding sequences in the second promoter both for STAT and CREB (see Fig. 1C), luciferase reporter assays (see Fig. 2B) and DNA binding assays (see Fig. 2C) showed a regulatory role for CREB in the second promoter but did not reveal a contribution for STAT. We therefore performed in vivo chromatin immunoprecipitation assay using AtT20 cells, which are highly methylated (Fig. 8A), and treated cells with LIF to induce potential STAT activation. Methylation-sensitive CREB binding to this region was not detected until 24 hours later, whereas binding of STAT3 and STAT5 was detected after 12 hours (Fig. 8B-8D).
Figure 8.
In vivo binding of STAT3, STAT5, and CREB to the second promoter in leukemia inhibitory factor (LIF)-stimulated AtT20 cells. A, DNA methylation levels in first (mPro1) and second (mPro2) promoters analyzed by bisulfite-conversion–based methylation-specific polymerase chain reaction procedure in AtT20 cells. B to D, Chromatin immunoprecipitation assay of AtT20 cells stimulated with LIF for the indicated times using B, anti-CREB (CREB); C, anti-STAT3 (STAT3); and D, anti-STAT5 (STAT5), with control antibody (immunoglobulin G [IgG]). *P < .05 vs control.
Discussion
Analyzing 5′ ends of POMC transcripts in pituitary and ectopic ACTH-secreting tumors, we identified an additional regulatory region located near the intron 2/exon 3 junction of the POMC gene. This region appears to function as a second POMC promoter with activity regulated by DNA methylation and as an enhancer of the first POMC promoter situated upstream of exon 1.
DNA methylation regulates promoter activities, with hypermethylation leading to repression of gene expression and hypomethylation allowing for gene activation (35). Promoter methylation has been shown to be an epigenetic determinant of ectopic POMC expression in normal and tumorous tissues (36), and POMC promoter demethylation was reported with ectopic ACTH secretion by a pheochromocytoma (37). However, we report here that methylation patterns in the first and second POMC promoters were strikingly different, suggesting different roles for methylation in POMC-expressing ACTH-secreting tumors depending on which promotor is activated.
We found the first promoter demethylated in 6 pituitary ACTH-secreting tumors that harbor gain-of-function USP8 mutations. USP8-mutated pituitary ACTH-secreting tumors show enhanced EGFR signaling (9, 10), which we showed increases activity of the POMC first promoter (8). These findings suggest that the USP8-EGFR pathway regulates the first promoter and that methylation status of this promoter may contribute to driving the corticotroph adenoma phenotype. By contrast, the second promoter was demethylated in tumors harboring other markers of an aggressive phenotype (34), including size 10 mm or larger, recurrent disease, and Crooke cell changes, suggesting that methylation status of the second POMC promoter is involved in driving inherent corticotroph tumor behavior.
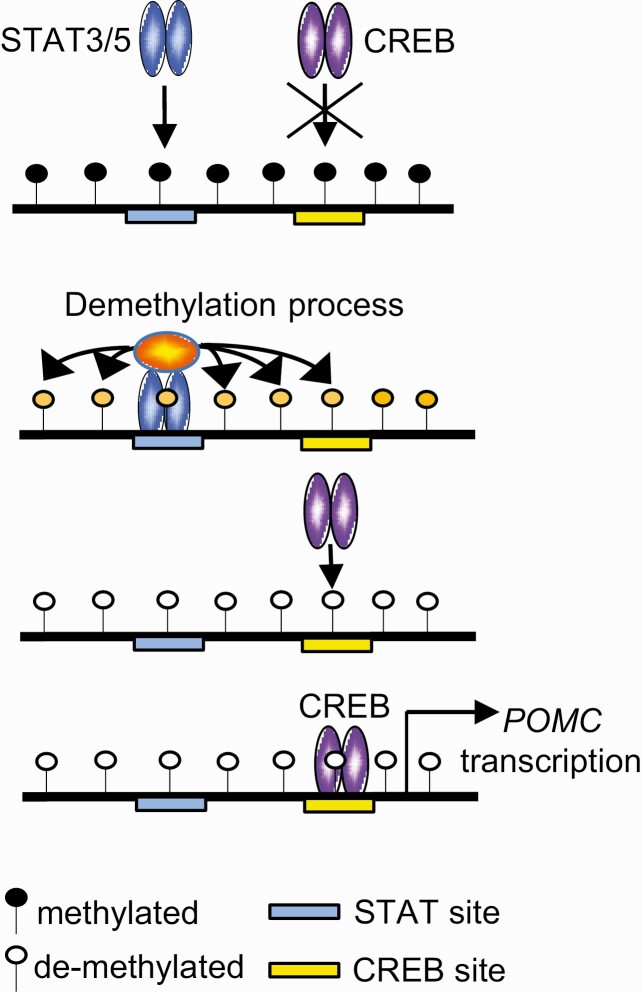
Of note, differences in methylation status were also apparent when comparing ACTH-secreting pituitary tumors and nonsecreting SCAs, suggesting that methylation of the second promoter may play a role in POMC gene silencing in SCAs. Several mechanisms have been proposed to explain the distinctive pathogenesis of SCAs vs pituitary ACTH-secreting tumors and how they might affect POMC activity (4), although promoter methylation has not been proposed. Phenotypic transition of SCA to active Cushing disease is uncommon (38, 39), and the potential contribution of sequential hypomethylation of the second promoter to activate POMC expression and ACTH secretion requires study. The second POMC promoter contains closely located binding sites for CREB and STAT, which are highly methylated in normal pituitary tissue. We show here that CREB activates POMC transcription by the second promoter, but CREB binding occurs later, after STAT3/5 binding, suggesting step-wise binding in second promoter transcriptional regulation (Fig. 9). We have shown step-wise regulation for the highly methylated Foxp3 enhancer (23), which also contains STAT and CREB binding sites in close proximity. In that setting, ten-eleven translocation methylcytosine dioxygenase (TET)1 and TET2 bind with STAT5, inducing demethylation of the Foxp3 enhancer, which allows methylation-sensitive CREB to bind and activate transcription in T cells (40). Our results suggest the possibility of a similar mechanism for the second POMC promoter, with STAT3/5 cooperating with TET or other proteins to demethylate the second promoter for CREB-dependent promoter activation.
Figure 9.
Illustration of possible step-wise binding in transcriptional regulation of the second promoter. STAT3/5 binds and demethylates the second proopiomelanocortin (POMC) promoter, which allows CREB to bind the promoter, activating transcription.
Robust constitutive baseline activity of the second promoter was downregulated by CRH and LIF stimulation, whereas the opposite reaction was seen with the first promoter. POMC gene expression is associated with a range of hypothalamic-pituitary-adrenal axis functions, including acute stress response, negative feedback response by glucocorticoid administration, and constitutive active expression maintaining circadian rhythms (2). Further study of second promoter activation could elucidate both physiologic and pathologic phenomena associated with Cushing disease.
We identified a second POMC promoter regulated by methylation status in ACTH-secreting pituitary tumors. Demethylation of this promoter is associated with characteristics of aggressive pituitary ACTH-secreting tumor phenotypes independent of USP8 mutations, and may be regulated by CREB and stimulated by STAT3/5 activation. Our findings open new avenues for elucidating subcellular regulation of the hypothalamic-pituitary-adrenal axis and suggest the second POMC promoter as a locus for targeted therapies in patients with Cushing disease phenotypes.
Acknowledgments
We thank Dr Adam Mamelak and Dr Daniel Luthringer for providing tissue samples and Ms Shira Berman for assistance with manuscript preparation.
Financial Support: This work was supported by the National Institutes of Health (NIH grant Nos. R01DK113998 and T32DK007770), the University of Minnesota (grant Nos. UMF0011528 and AHC Grant-in-Aid 212588), and the Doris Factor Molecular Endocrinology Laboratory at Cedars-Sinai. The funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Glossary
Abbreviations
- 5′-RACE
5′-rapid amplification of complementary DNA ends
- ACTH
adrenocorticotropin
- cDNA
complementary DNA
- CpG
dinucleotide 5′-CG-3′
- CRH
corticotropin-releasing hormone
- EGFR
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- FFPE
formalin-fixed paraffin-embedded
- Pro1
first promoter
- Pro2
second promoter
- IRB
institutional review board
- LIF
leukemia inhibitory factor
- mRNA
messenger RNA
- pCpGL
CpG-free luciferase reporter plasmid
- PCR
polymerase chain reaction
- POMC
proopiomelanocortin
- SAM
S-adenosylmethionine
- SCA
silent corticotroph adenoma
- SssI
methyltransferase
- TET
ten-eleven translocation methylcytosine dioxygenase
- USP8
ubiquitin-specific protease 8.
Additional Information
Disclosures: The authors have nothing to declare.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- 1. Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med. 2020;382(10):937-950. [DOI] [PubMed] [Google Scholar]
- 2. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374-381. [DOI] [PubMed] [Google Scholar]
- 3. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society . Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben-Shlomo A, Cooper O. Silent corticotroph adenomas. Pituitary. 2018;21(2):183-193. [DOI] [PubMed] [Google Scholar]
- 5. Hofland LJ, Feelders RA, de Herder WW, Lamberts SW. Pituitary tumours: the sst/D2 receptors as molecular targets. Mol Cell Endocrinol. 2010;326(1-2):89-98. [DOI] [PubMed] [Google Scholar]
- 6. Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing’s disease. Endocr Rev. 2015;36(4):385-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Aken MO, Pereira AM, van den Berg G, Romijn JA, Veldhuis JD, Roelfsema F. Profound amplification of secretory-burst mass and anomalous regularity of ACTH secretory process in patients with Nelson’s syndrome compared with Cushing’s disease. Clin Endocrinol (Oxf). 2004;60(6):765-772. [DOI] [PubMed] [Google Scholar]
- 8. Fukuoka H, Cooper O, Ben-Shlomo A, et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121(12):4712-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reincke M, Sbiera S, Hayakawa A, et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31-38. [DOI] [PubMed] [Google Scholar]
- 10. Ma ZY, Song ZJ, Chen JH, et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Araki T, Liu NA, Tone Y, et al. E2F1-mediated human POMC expression in ectopic Cushing’s syndrome. Endocr Relat Cancer. 2016;23(11):857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez-Rivas LG, Theodoropoulou M, Ferraù F, et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J Clin Endocrinol Metab. 2015;100(7):E997-E1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi K, Inoshita N, Kawaguchi K, et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur J Endocrinol. 2016;174(2):213-226. [DOI] [PubMed] [Google Scholar]
- 14. Jeannotte L, Trifiro MA, Plante RK, Chamberland M, Drouin J. Tissue-specific activity of the pro-opiomelanocortin gene promoter. Mol Cell Biol. 1987;7(11):4058-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamolet B, Pulichino AM, Lamonerie T, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104(6):849-859. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Lin C, Gleiberman A, et al. Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci U S A. 2001;98(15):8674-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamonerie T, Tremblay JJ, Lanctôt C, Therrien M, Gauthier Y, Drouin J. Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev. 1996;10(10):1284-1295. [DOI] [PubMed] [Google Scholar]
- 18. Bousquet C, Zatelli MC, Melmed S. Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immuno-neuroendocrine interfacing. J Clin Invest. 2000;106(11):1417-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulin G, Turgeon B, Drouin J. NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol Cell Biol. 1997;17(11):6673-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du L, Bergsneider M, Mirsadraei L, et al. Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc Natl Acad Sci U S A. 2013;110(21):8555-8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Araki T, Liu X, Kameda H, et al. EGFR induces E2F1-mediated corticotroph tumorigenesis. J Endocr Soc. 2017;1(2):127-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamolet B, Poulin G, Chu K, Guillemot F, Tsai MJ, Drouin J. Tpit-independent function of NeuroD1(BETA2) in pituitary corticotroph differentiation. Mol Endocrinol. 2004;18(4):995-1003. [DOI] [PubMed] [Google Scholar]
- 23. Ogawa C, Tone Y, Tsuda M, Peter C, Waldmann H, Tone M. TGF-β-mediated Foxp3 gene expression is cooperatively regulated by Stat5, Creb, and AP-1 through CNS2. J Immunol. 2014;192(1):475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988;85(23):8998-9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rydzanicz R, Zhao XS, Johnson PE. Assembly PCR oligo maker: a tool for designing oligodeoxynucleotides for constructing long DNA molecules for RNA production. Nucleic Acids Res. 2005;33(Web Server issue):W521-W525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klug M, Rehli M. Functional analysis of promoter CpG methylation using a CpG-free luciferase reporter vector. Epigenetics. 2006;1(3):127-130. [DOI] [PubMed] [Google Scholar]
- 27. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93(18):9821-9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18(11):1427-1431. [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Tollefsbol TO. DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol. 2011;791:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tone Y, Kojima Y, Furuuchi K, et al. OX40 gene expression is up-regulated by chromatin remodeling in its promoter region containing Sp1/Sp3, YY1, and NF-κB binding sites. J Immunol. 2007;179(3):1760-1767. [DOI] [PubMed] [Google Scholar]
- 31. Leenen FA, Vernocchi S, Hunewald OE, et al. Where does transcription start? 5′-RACE adapted to next-generation sequencing. Nucleic Acids Res. 2016;44(6):2628-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Auernhammer CJ, Melmed S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev. 2000;21(3):313-345. [DOI] [PubMed] [Google Scholar]
- 33. Boutillier AL, Monnier D, Lorang D, Lundblad JR, Roberts JL, Loeffler JP. Corticotropin-releasing hormone stimulates proopiomelanocortin transcription by cFos-dependent and -independent pathways: characterization of an AP1 site in exon 1. Mol Endocrinol. 1995;9(6):745-755. [DOI] [PubMed] [Google Scholar]
- 34. Raverot G, Burman P, McCormack A, et al. ; European Society of Endocrinology . European Society of Endocrinology clinical practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1-G24. [DOI] [PubMed] [Google Scholar]
- 35. Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20(10):590-607. [DOI] [PubMed] [Google Scholar]
- 36. Newell-Price J, King P, Clark AJ. The CpG island promoter of the human proopiomelanocortin gene is methylated in nonexpressing normal tissue and tumors and represses expression. Mol Endocrinol. 2001;15(2):338-348. [DOI] [PubMed] [Google Scholar]
- 37. Sakuma I, Higuchi S, Fujimoto M, et al. Cushing syndrome due to ACTH-secreting pheochromocytoma, aggravated by glucocorticoid-driven positive-feedback loop. J Clin Endocrinol Metab. 2016;101(3):841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Psaras T, Honegger J, Buslei R, et al. Atypical type II silent corticotrophic adenoma developing into Cushing’s disease upon second recurrence. Exp Clin Endocrinol Diabetes. 2007;115(9):610-615. [DOI] [PubMed] [Google Scholar]
- 39. Yokoyama S, Kawahara Y, Sano T, Nakayama M, Kitajima S, Kuratsu J. A case of non-functioning pituitary adenoma with Cushing’s syndrome upon recurrence. Neuropathology. 2001;21(4):288-293. [DOI] [PubMed] [Google Scholar]
- 40. Yang R, Qu C, Zhou Y, et al. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity. 2015;43(2):251-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.