Abstract
The first case of coronavirus disease 2019 (COVID-19) was reported in December 2019 in China. World Health Organization declared it a pandemic on March 11, 2020. It has caused significant morbidity and mortality worldwide. Persistent symptoms and serious complications are being reported in patients who survived COVID-19 infection, but long-term sequelae are still unknown. Several vaccines against COVID-19 have been approved for emergency use around the globe. These vaccines have excellent safety profiles with few reported side effects. Drug-induced hepatotoxicity is mainly seen with different drugs or chemicals. There are only a few reported cases of hepatotoxicity with vaccines. We present a case of liver injury after administration of the vaccine against the COVID-19 infection.
Keywords: covid-19 vaccine, liver dysfunctions, covid-19 pandemic, covid-19, drug-induced liver injury
Introduction
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) causing coronavirus disease 2019 (COVID-19) emerged in December 2019 in Wuhan, China, resulting in an ongoing pandemic [1]. To date, it has caused more than 173 million cases and over 3.7 million death worldwide as per World Health Organization [2]. Although the respiratory system is the most common system affected by this disease, it affects multiple organ manifestations [3]. Despite international efforts to develop treatments for this disease, there are still limited therapeutic options available with remdesivir as the only Food and Drug Administration-approved drug [4]. Given the rapid spread, high morbidity, and mortality worldwide, a coordinated effort led to developing the vaccine in a year of first diagnosed case. Multiple COVID vaccines have been developed at an unprecedented rate. These vaccines have excelled safety and efficacy profiles [5-7]. The most common adverse effects reported with these vaccines included mild effects like pain at the vaccine site, fever, fatigue, headache, arthralgia, myalgia, lymphadenopathy, and severe effects like anaphylactic reaction [8]. Drug-induced hepatotoxicity is a common adverse event seen with prescription and nonprescription drugs [9]. There are few reported hepatotoxicity cases due to vaccines, namely anti-rabies vaccination-induced hepatotoxicity and autoimmune hepatitis due to influenza virus and hepatitis A and B vaccines [10-17]. We report a case of liver injury after receiving the COVID vaccine.
Case presentation
A 61-year-old female with a known history of irritable bowel disease and cholecystectomy presented to the emergency department with generalized weakness, body aches, dry heaving, and a low-grade temperature of 99.9 Fahrenheit. The patient received a second dose of the Pfizer COVID-19 vaccine nine days before the start of symptoms. She was noted to have conjunctival icterus, mild generalized abdominal tenderness without guarding, or rigidity on physical examination. On admission, the patient's vitals were stable except for tachycardia with a heart rate between 90 and 110 beats/min.
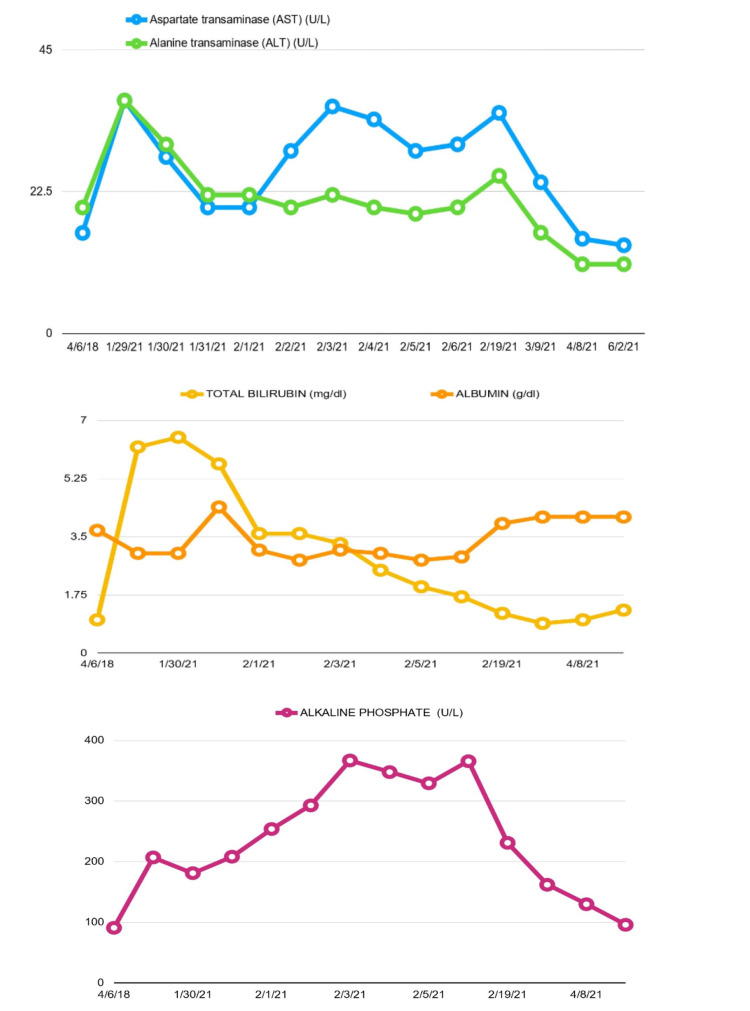
Laboratory analysis was remarkable for elevated alkaline phosphatase (ALP) of 207 U/L, total bilirubin of 6.2 mg/dL, direct bilirubin of 3.9 mg/dL, white blood cell (WBC) count of 17.2 x 109/L, and mildly elevated aspartate transaminase of 37 U/L (Table 1 and Figure 1). Abdominal ultrasound showed increased echogenicity within the liver compatible with fatty infiltrates, and common duct diameter was measured to be 6 mm. At the same time, CT of the abdomen with contrast showed no acute abnormalities. The patient was admitted to the hospital and started on empiric antibiotics for presumed cholangitis. Gastroenterology consultation was obtained. Magnetic resonance cholangiopancreatography without contrast showed no filling defect within the biliary duct, status post cholecystectomy, bile duct diameter within a normal range, and unremarkable liver. The patient remained afebrile, WBC trended down, and abdominal pain improved over the course of the hospital stay. Given these findings, infectious disease specialist recommended discontinuing antibiotics. Antibodies to liver/kidney microsomal type 1, smooth muscle, anti-mitochondrial, alpha-1 antitrypsin came back negative, and, additionally, ceruloplasmin, antinuclear antibody, alpha-fetoprotein, and viral serologies for hepatitis A, B, and C came back negative (Table 2). Liver biopsy showed minimal pallor suggesting slight edema along with scattered inflammatory cells consisting of small lymphocytes, scattered polymorphonuclear leukocytes, and few eosinophils, no evidence of florid duct lesion on interface hepatitis, and no evidence of fibrosis on trichrome and reticulin stain.
Table 1. Liver function tests trend.
| Date | Aspartate transaminase (U/L) | Alanine transaminase (U/L) | Total bilirubin (mg/dL) | Alkaline phosphate (U/L) | Albumin (g/dL) |
| 4/6/2018 | 16 | 20 | 1 | 91 | 3.7 |
| 1/29/2021 | 37 | 37 | 6.2 | 207 | 3 |
| 1/30/2021 | 28 | 30 | 6.5 | 181 | 3 |
| 1/31/2021 | 20 | 22 | 5.7 | 208 | 4.4 |
| 2/1/2021 | 20 | 22 | 3.6 | 254 | 3.1 |
| 2/2/2021 | 29 | 20 | 3.6 | 293 | 2.8 |
| 2/3/2021 | 36 | 22 | 3.3 | 367 | 3.1 |
| 2/4/2021 | 34 | 20 | 2.5 | 348 | 3 |
| 2/5/2021 | 29 | 19 | 2 | 329 | 2.8 |
| 2/6/2021 | 30 | 20 | 1.7 | 366 | 2.9 |
| 2/19/2021 | 35 | 25 | 1.2 | 231 | 3.9 |
| 3/9/2021 | 24 | 16 | 0.9 | 162 | 4.1 |
| 4/8/2021 | 15 | 11 | 1 | 130 | 4.1 |
| 6/2/2021 | 14 | 11 | 1.3 | 96 | 4.1 |
Table 2. Immunologic and infectious work-up for liver disease.
| Test | Results |
| Gamma-glutamyl transpeptidase | 103 U/L (1-24 U/L reference range) |
| Hepatitis A IgM antibody | Negative |
| Hepatitis B surface antigen | Negative |
| Hepatitis B core IgM antibody | Negative |
| Hepatitis C antibody | Negative |
| Anti-liver/kidney microsomal antibody | Negative (≤20 = negative, reference range) |
| Ferritin | 975.2 ng/mL (10.0-291.0 reference range) |
| Antinuclear antibody reflex | Negative |
| Smooth muscle antibody | Negative |
| Anti-mitochondrial antibody | Negative (≤20 = negative, reference range) |
| Ceruloplasmin | 38 mg/dl (18-53 mg/dL reference range) |
Figure 1. Graphs showing liver function test trends.
Given that all work-up for infection, autoimmune diseases, and any obstruction came back negative, the patient's clinical picture and laboratory findings were attributed as a liver injury due to the COVID-19 vaccine. Her liver function levels continued to trend down, and she was discharged from the hospital after a week of hospitalization. On the patient's follow-up with a gastroenterologist, abdominal pain was resolved, and her liver function test values normalized (Table 1 and Figure 1).
Discussion
Drug-induced hepatotoxicity leads to nearly 10% of all cases of acute hepatitis and more than 50% cases of liver failure [18]. It is one of the common reasons for the withdrawal of medications from the market and modification of use [19]. It can be either type A (predictable), dose-related and short latent period in days, or type B (idiosyncratic), dose-independent, unpredictable, and variable latency [20,21]. Based on population-based studies, drug-induced liver injury incidence varies between 13.9 and 19.1 cases per 100,000 people per year [22,23]. Patients have either hepatocellular injury (three times upper limit of transaminase in comparison to ALP), cholestatic injury (three times increase in ALP comparison to transaminase), or mixed pattern (where both ALP and aminotransferase are three times upper limit) [24-26]. Most patients improve spontaneously after the removal of the offending drug. If acute liver failure (ALF) is suspected, early liver transplant referral is important due to high ALF mortality [25,27]. From the spontaneous reports from patients who received Pfizer/BioNTech BNT162b2 mRNA in the UK between 9/12/20 and 26/05/2021, there are reports of 45 patients having abnormal liver function analysis and three patients having drug-induced liver injury [28].
In this case, the review of medications and history did not reveal any other reason for hepatotoxicity. She also denied the use of any over-the-counter medications or supplements. Although it is rare with vaccination, the COVID-19 vaccine is likely the cause of hepatotoxicity in our patient based on a diagnosis of exclusion. In this case, the patient had a cholestatic pattern with elevated ALP and bilirubin with mild elevation in the transaminases.
Pfizer/BioNTech BNT162b2 mRNA trial included only 0.6% (217/37,706) patients with liver disease. Among patients with liver disease, 214 were with mild liver disease and only three with moderate to severe liver disease. This patient has underlying fatty liver disease. It is unclear if that was a likely risk factor for hepatotoxicity in this case [5]. Although only a small number were included in trials for Pfizer/BioNTech BNT162b2 mRNA, Moderna mRNA-1273, and the AstraZeneca/University of Oxford ChAdOx1-nCoV-19 chimpanzee adenovirus vector vaccine, both the American Association for Study of Liver Diseases and European Association for the Study of Liver recommend vaccination against SARS-COV-2 with these highly effective and safe vaccines, given a greater risk of health consequences from SARS-COV-2 infection in these patients [29,30].
Hepatotoxicity can occur with vaccines, even though it is more common with prescription and nonprescription drugs. So, the clinician should be watchful in patients showing clinical signs and symptoms after a vaccine.
Conclusions
In summary, we presented a case of liver injury after the COVID-19 vaccine. We attributed the cause of liver injury to the COVID-19 vaccine, given no other cause in our patient after extensive work-up. There are reports of drug-induced liver injury and abnormal liver function analysis from the spontaneous reports from patients who received Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine in the UK. The purpose of this manuscript is to raise awareness of potential side effects; it should not alter the recommendation of healthcare providers regarding vaccinations.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lu R, Zhao X, Li J, et al. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard. [Jun;2021 ];https://covid19.who.int/ 2021
- 3.Coronavirus disease (COVID-19): comprehensive review of clinical presentation. Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Front Public Health. 2020;8:582932. doi: 10.3389/fpubh.2020.582932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH: COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [Jun;2021 ];https://www.covid19treatmentguidelines.nih.gov/ 2021 [PubMed]
- 5.Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. Polack FP, Thomas SJ, Kitchin N, et al. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Voysey M, Clemens SAC, Madhi SA, et al. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. Baden LR, El Sahly HM, Essink B, et al. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. Eur Rev Med Pharmacol Sci. 2021;25:1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 9.Drug-induced liver injury: a review. Kosanam S, Boyina R. Int J Pharmacol Res. 2014;5 [Google Scholar]
- 10.The elevation of liver enzymes due to Hepatitis B vaccine. Önlen Y, Savaş L, Özer B, İris NE. Eur J Gen Med. 2006;3:197–200. [Google Scholar]
- 11.Autoimmune hepatitis following influenza virus vaccination: two case reports. Sasaki T, Suzuki Y, Ishida K, Kakisaka K, Abe H, Sugai T, Takikawa Y. Medicine (Baltimore) 2018;97:0. doi: 10.1097/MD.0000000000011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaccine-related autoimmune hepatitis: the same disease as idiopathic autoimmune hepatitis? Two clinical reports and review. van Gemeren MA, van Wijngaarden P, Doukas M, de Man RA. Scand J Gastroenterol. 2017;52:18–22. doi: 10.1080/00365521.2016.1224379. [DOI] [PubMed] [Google Scholar]
- 13.Hepatitis A vaccine associated with autoimmune hepatitis. Berry PA, Smith-Laing G. World J Gastroenterol. 2007;13:2238–2239. doi: 10.3748/wjg.v13.i15.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaccination as a triggering event for autoimmune hepatitis. Perumalswami P, Peng L, Odin JA. Semin Liver Dis. 2009;29:331–334. doi: 10.1055/s-0029-1233537. [DOI] [PubMed] [Google Scholar]
- 15.Vaccination-induced autoimmune hepatitis. Veerappan GR, Mulhall BP, Holtzmuller KC. Dig Dis Sci. 2005;50:212–213. doi: 10.1007/s10620-005-1303-z. [DOI] [PubMed] [Google Scholar]
- 16.Acute exacerbation of autoimmune hepatitis induced by Twinrix. Csepregi A, Treiber G, Röcken C, Malfertheiner P. World J Gastroenterol. 2005;11:4114–4116. doi: 10.3748/wjg.v11.i26.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anti-rabies vaccination induced hepatotoxicity - a case report. Rajegowda RY, Nanjappa NB, Muthahanumai NK. Int J Basic Clin Pharmacol. 2016;5:2280–2282. [Google Scholar]
- 18.Drug-induced liver injury caused by adalimumab: a case report and review of the bibliography. Frider B, Bruno A, Ponte M, Amante M. Case Reports Hepatol. 2013;2013:406901. doi: 10.1155/2013/406901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Idiosyncratic drug hepatotoxicity. Kaplowitz N. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 20.Drug-induced liver injury. Kaplowitz N. Clin Infect Dis. 2004;38 Suppl 2:0–8. doi: 10.1086/381446. [DOI] [PubMed] [Google Scholar]
- 21.Diagnosis, management and prevention of drug-induced liver injury. Verma S, Kaplowitz N. Gut. 2009;58:1555–1564. doi: 10.1136/gut.2008.163675. [DOI] [PubMed] [Google Scholar]
- 22.Incidence of drug-induced hepatic injuries: a French population-based study. Sgro C, Clinard F, Ouazir K, et al. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 23.Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Gastroenterology. 2013;144:1419-25, 1425.e1-3; quiz e19-20. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Drug-induced liver injury. Fisher K, Vuppalanchi R, Saxena R. Arch Pathol Lab Med. 2015;139:876–887. doi: 10.5858/arpa.2014-0214-RA. [DOI] [PubMed] [Google Scholar]
- 25.Drug-induced liver injury. Katarey D, Verma S. Clin Med (Lond) 2016;16:0–9. doi: 10.7861/clinmedicine.16-6s-s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idiosyncratic DILI: analysis of 46,266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Teschke R. Front Pharmacol. 2019;10:730. doi: 10.3389/fphar.2019.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acute liver failure induced by idiosyncratic reaction to drugs: challenges in diagnosis and therapy. Tujios SR, Lee WM. Liver Int. 2018;38:6–14. doi: 10.1111/liv.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.print C-mPBva. 2021. All UK Spontaneous Reports Received Between 9/12/20 and 26/05/21 for mRNA Pfizer/BioNTech Vaccine Analysis Print. [Google Scholar]
- 29.AASLD Expert Panel consensus statement: vaccines to prevent COVID-19 infection in patients with liver disease. Fix OK, Blumberg EA, Chang KM, et al. Hepatology. 2021 doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]