Abstract
Context
The decision whether to treat a child with delayed puberty with sex steroids is primarily based on patient, family, and provider preference. Knowing when children with constitutional delay eventually enter puberty would inform this decision.
Objective, Design, Setting, Participants, and Outcome Measures
To estimate and compare rates of pubertal entry, we conducted a retrospective cohort study by reviewing medical records of children evaluated for delayed puberty at a large academic medical center between 2000 and 2015, extracting data on pubertal status for all clinical visits, then conducting time-to-event analyses.
Results
Of 392 girls and 683 boys with delayed puberty, constitutional delay was the most common cause, found in 32% of girls and 70% of boys. In a subcohort of 97 girls and 243 boys who were prepubertal at one or more visits, we observed a broad age range for pubertal entry, up to >16 years for girls and >17 years for boys. The probability of entering puberty within the next year for 12- to 15.5-year-old girls and 13.5- to 16.5-year-old boys with delayed puberty ranged between 38% and 74%. No differences in the rates of pubertal entry were seen between girls and boys after data harmonization.
Conclusion
The broad range of ages at pubertal entry for children with constitutional delay challenges the concept that constitutional delay is merely an extreme of normal variation. Discussions with patients and families about management should consider the possibility that some children may need to wait years after presentation until puberty starts.
Keywords: delayed puberty, constitutional delay, hypogonadotropic hypogonadism
Delayed puberty is a common reason to consult a pediatric endocrinologist, and the management of delayed puberty can be fraught with uncertainty (1-3). In some patients, a specific cause of delayed puberty can be identified; such causes include (1) primary gonadal insufficiency, readily recognizable from a pattern of hypergonadotropic hypogonadism, which arises from loss of negative-feedback restraint from gonadal hormones on the hypothalamic and pituitary levels of the reproductive endocrine axis; (2) functional hypogonadotropic hypogonadism (FHH), a normal physiological response of the reproductive endocrine system to a stressor such as chronic illness, undernutrition, or overexercise; (3) hypogonadotropic hypogonadism (HH) resulting from endocrine conditions such hyperprolactinemia, hypothyroidism, and cortisol excess, which can suppress reproductive endocrine function; and (4) HH as part of global impairment of hypothalamic and/or pituitary function, which can be congenital (eg, congenital hypopituitarism) or acquired (eg, from neurosurgery affecting the hypothalamic/pituitary region). After such identifiable causes of delayed puberty are ruled out, most children with unexplained delayed puberty are presumed to have constitutional delay, a condition in which puberty is late to start but, once started, generally proceeds normally. However, some children with unexplained delayed puberty have idiopathic hypogonadotropic hypogonadism (IHH), a pathologic defect in the hypothalamic and/or pituitary levels of the reproductive endocrine axis that results in failure to achieve full reproductive endocrine function by an adult age.
There is considerable variation in clinical practice around the management of delayed puberty. Because most children with unexplained delayed puberty have self-limited constitutional delay, the typical options are treatment with sex steroids to induce secondary sex characteristics and pubertal growth acceleration or reassurance without intervention while waiting for puberty to start (though the possibility of IHH looms until puberty eventually starts and progresses). Textbooks and review articles largely leave the decision whether to treat with sex steroids to the preference of patients and parents/caregivers (4, 5), and there are no guidelines on the management of delayed puberty, though some authors have advocated for treatment with sex steroids (2, 6). In a survey of pediatric endocrine providers, we found that some providers are willing to provide sex-steroid treatment to girls and boys as young as 13 years old or even younger, whereas others are reluctant to treat girls and boys as old as 15 years (7). Of note, providers on average tend to wait longer to treat girls than boys; it is unclear whether this difference in practice is justified by differences in biology.
Counseling of patients with delayed puberty and decision-making around management would benefit from data on the natural progression of constitutional delay. Specifically, providing estimates for how long a patient may need to wait for puberty to begin spontaneously would set realistic expectations and would allow patients and families to decide whether they are willing to wait that long if they decide against sex-steroid treatment. Furthermore, basing these estimates on age would allow these expectations and decisions to be updated over time. To provide these data, we retrospectively analyzed clinical records of children evaluated for delayed puberty to identify the causes of delayed puberty, to estimate rates of pubertal entry for children with delayed puberty, and to estimate whether a child who is still prepubertal at a given age will fail to enter puberty by age 18 years.
Methods
Ethical approval
This research study was approved by the Boston Children’s Hospital (BCH) institutional review board (protocol number IRB-P00017406) before data collection and met criteria for a waiver of informed consent.
Review of medical records
The BCH clinical data repository was queried to identify patients seen at BCH at age <18 years old between January 1, 2000, and March 31, 2015, and given a diagnosis code for delayed puberty (International Classification of Diseases-9 code 259.0, International Classification of Diseases-10 code E30.0). We reviewed medical records from this initial patient list to identify individuals who met criteria for delayed puberty, defined for this study as: (1) absence of breast development in girls 12 years or older, as ~97.7% of girls exhibit breast development by this age (8-11); (2) testicular volume <4 mL in boys 13 years and 6 months or older (12-14), as ~97.7% of boys exhibit pubertal testicular volumes by this age; (3) for girls who were already pubertal at first presentation, breast development that was delayed by 2.25 SD or more beyond the mean based on the Copenhagen Puberty Study nomograms (ie, Tanner 2 breast development at age 14 years and 3 months or older) (15); or (4) for boys who were already pubertal at first presentation, testicular development that was delayed by 2.25 SD or more beyond the mean based on the Copenhagen Puberty Study nomograms (ie, testes ≤4 mL at 14 years and 3 months or older, ≤6 mL at 15 years and 0 months or older, and/or ≤8 mL at 16 years and 3 months or older) (16). We also extracted potential causes of delayed puberty as described by providers in clinical notes.
A more extensive chart review was then performed for individuals with unexplained delayed puberty, ie, those who did not have any identified underlying conditions that could have caused their delayed puberty, including but not limited to underweight (defined as body mass index <5th percentile for bone age or, if bone age was not available, for chronological age), cancer, celiac disease, disordered eating, thyroid issues, hypopituitarism (congenital or acquired), hyperprolactinemia, cystic fibrosis, and primary gonadal insufficiency. Data were extracted and stored in a Research Electronic Data Capture (REDCap) database (17). The information recorded included medical history, medications, including hormonal treatments for delayed puberty, and all recorded assessments of height, weight, pubertal status, and bone age. Height, weight, and laboratory data that had been collected at BCH were checked against the medical record for accuracy then imported from the BCH clinical data repository into REDCap.
Quality assurance and data cleaning
To ensure standardization in data abstraction, a comprehensive standard operating procedure for data abstraction was developed and followed. Double data entry into REDCap was performed for the first 5 records entered by any new member of the research staff, and then additionally completed for 15% of records subsequently entered. Any inconsistencies between the 2 sets of data entries were reviewed by a senior member of the research team, compared against source documents, and corrected accordingly. Any data points open to clinical interpretation were reviewed and completed by the principal investigator. Data imported from clinical databases were first reviewed for accuracy by comparing the pulled values with clinic notes and medical records and corrected if necessary; common corrections included incorrect units and missing or incorrect values. Subsets of the data, such as trajectories of testicular volume and Tanner stages, were also examined for accuracy and consistency. Upon discovery of a single anomalous value in a string of otherwise consistent measurements, the value that did not conform with the other measurements was omitted from analysis; when there were multiple conflicting values, we relied on ancillary data to adjudicate (eg, laboratory measurements, timing of growth spurt); and when adjudication was not possible, anomalous values were omitted.
Classification of causes of delayed puberty
Individuals were classified as having IHH if no other cause of delayed puberty was identified and (1) there was no spontaneous pubertal development by age 18 years or (2) a serum sex-steroid measurement at age 18 years or older was below the adult reference range and serum gonadotropins were not elevated, or both. Remaining individuals with unexplained delayed puberty were classified as having constitutional delay; these individuals were either observed to enter puberty or were lost to clinical follow-up, and analytical methods to account for loss to clinical follow-up are described in the following section.
Statistical analysis
We constructed curves describing the time course of onset of puberty using methods for interval-censored data, which account for the fact that our database did not provide a precise date for pubertal entry, but rather a lower limit (last verified prepubertal clinic visit) and, in some cases but not all, an upper limit (first verified postpubertal clinic visit). For maximum flexibility we used a nonparametric method (18), which rendered the time course as an ascending series of plateaus. At age x, the height Px of the plateau represents the probability that a child has entered puberty by that age, conditional on having been prepubertal at the age specified for inclusion (12 years for girls, 13.5 years for boys). The method provides a standard error for this probability, SE(Px), and estimates for the quartile boundaries of age at pubertal onset with 95% confidence limits. We derived 2 additional parameters conditional on prepubertal status at age x. The probability of puberty starting within the next time interval Δt was calculated as (Px + Δt– Px) / (1 – Px) (interpolating Px when necessary), and the probability of not entering puberty by 18 years as (1 – P18y)/ (1 – Px), with asymptotic standard errors derived from SE(Px). Comparison between 2 onset curves was made with a weighted log-rank test (19).
We tested several models to account for children who were not observed to enter puberty (ie, who were still prepubertal at their last recorded clinical visit and were subsequently lost to clinical follow-up). One model used uninformative right-censoring, which assumes that the reason for not returning to clinic was completely unrelated to timing of pubertal onset. However, we reasoned that the most likely reason for not returning to clinic was that puberty had started, and thus that this assumption underlying uninformative censoring was incorrect, such that uninformative censoring would underestimate the probability of pubertal entry by a given age. We therefore tested several informative right-censoring models to compensate for this presumed bias. These models designated that the onset of puberty fell within 1 year, 6 months, or 1 day of the last clinic visit, assuming that a child who did not enter puberty within this timeframe would have returned to clinic (with the 1-day model as an extreme case that would systematically overestimate the probability of pubertal entry by a given age). Thus, for the interval for pubertal entry, we assigned the date of the last clinic visit as the earlier (left) boundary and 1 year/6 months/1 day beyond the last clinic visit, or age 18 years if earlier, as the later (right) boundary.
Because we interpreted breast development as the sign for pubertal initiation in girls, and treatment with exogenous estrogen induces breast development directly, thereby confounding this interpretation, girls treated with estrogen were right-censored at the time of starting treatment. Boys were not censored for starting testosterone treatment, with one exception: to compare pubertal onset between girls and boys, we harmonized the girls’ and boys’ onset curves by right-censoring testosterone-treated boys at the time of treatment initiation and additionally by using age relative to the age cutoff for inclusion in the cohort, as this cutoff differed between girls and boys.
We used SAS software (version 9.4; Cary, NC) for statistical analyses.
Results
Causes of delayed puberty
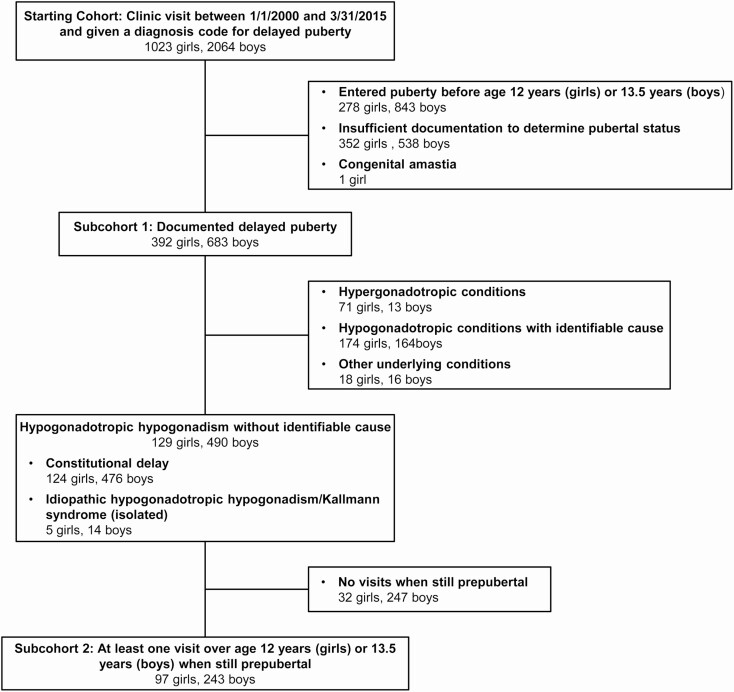
A search of medical records at BCH for patients younger than 18 years of age seen between January 2000 and March 2015 and given a diagnosis code for delayed puberty identified 3087 individuals, 1023 girls (33%) and 2064 boys (67%; Fig. 1). An initial chart review revealed that 278 of the girls (27% of the 1023 girls) had pubertal onset before 12 years, 352 (34%) did not have enough information recorded to determine if puberty was truly delayed, and 1 girl (0.1%) had congenital absence of the breasts (amastia), such that pubertal status could not be readily determined based on physical examination. For boys, 843 (41% of the 2064 boys) entered puberty before 13.5 years, and 538 (26%) did not have enough information to confirm a diagnosis of delayed puberty. These individuals were excluded from further analysis.
Figure 1.
Patient records analyzed in this study. Detailed categories are provided in Supplemental Tables 1 and 2 (20).
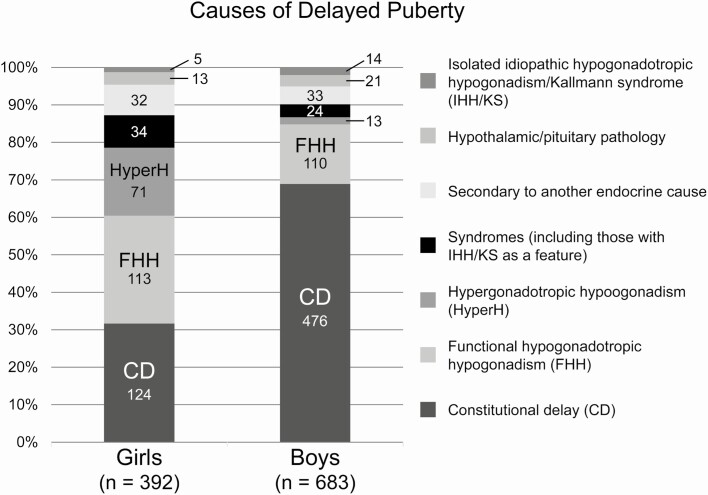
For the remaining 392 girls (38% of the initial girls’ charts reviewed) and 683 boys (33% of boys’ charts reviewed) with documented evidence of delayed puberty, we performed a more extensive chart review to identify the underlying causes of delayed puberty (Fig. 2, Supplemental Table 1; (20)). In girls, the most common causes of delayed puberty were constitutional delay (124 girls, 32%), functional hypogonadotropic hypogonadism (113 girls, 29%), and hypergonadotropic hypogonadism (ie, primary gonadal insufficiency; 71 girls, 18%; Fig. 2). IHH and Kallmann syndrome (IHH in combination with anosmia) were rare and were observed as part of a broader identified syndrome (ie, coloboma, heart anomaly, choanal atresia, retardation, and genital and ear anomalies syndrome; Prader-Willi syndrome) in 16 girls (4%) and as an isolated condition in 5 girls (1.3%). Of the individuals with isolated IHH/Kallmann syndrome, all 5 had no spontaneous physical signs of puberty by 18 years (ie, none had pubertal development that started spontaneously and then stalled). Other causes of delayed puberty included endocrine conditions such as hypothyroidism and hyperprolactinemia (32 girls, 8%) and acquired and congenital conditions disrupting function of the hypothalamus and/or pituitary gland (7 girls, 1.8%, and 6 girls, 1.5%, respectively).
Figure 2.
Causes of delayed puberty in girls and boys. Figure modeled after ref. (21).
In boys with confirmed delayed puberty, constitutional delay was by far the most common cause (476 boys, 70%), followed by functional hypogonadotropic hypogonadism (110 boys, 16%; Fig. 2, Supplemental Table 2; (20)). Primary gonadal insufficiency was found in 13 boys (2%), IHH/Kallmann syndrome in the context of a broader syndrome in 8 boys (1.2%), IHH/Kallmann syndrome in isolation in 14 boys (2.0%), endocrine conditions secondarily affecting reproductive endocrine function in 33 boys (4.8%), and acquired and congenital conditions disrupting hypothalamic/pituitary function in 11 boys (1.6%) and 2 boys (0.3%), respectively. Of the boys with isolated IHH/Kallmann syndrome, 9 had no pubertal development by age 18 years, and 5 had pubertal development that started and then stalled.
Timing of pubertal entry in children with constitutional delay and IHH
To assess rates of pubertal entry in children who presented with delayed puberty and whose initial evaluation did not reveal an underlying cause, we reviewed medical records in detail for children who (1) were eventually diagnosed with constitutional delay or IHH/Kallmann syndrome and (2) had at least 1 visit at which they were noted to be prepubertal at an age by which puberty was expected to have started. This identified 97 girls and 243 boys for further analysis (Fig. 1).
We then performed time-to-event analyses to determine when these children eventually entered puberty. We tested several models to account for children who were still prepubertal at their last clinical visit and were subsequently lost to clinical follow-up (see Methods). These models bracket the plausible range of outcomes for these children, ranging from the possibility that they entered puberty the day immediately after their last clinical visit, to the possibility that their loss to follow-up was completely unrelated to their timing of pubertal entry (even though we reasoned that the most likely reason not to return for follow-up was that the child entered puberty). The estimated chances of entering puberty were comparable across these various models (Supplemental Figure 1; (20)); for our primary model, we selected the one in which individuals lost to clinical follow-up did not return because puberty started within 1 year of their last clinical visit.
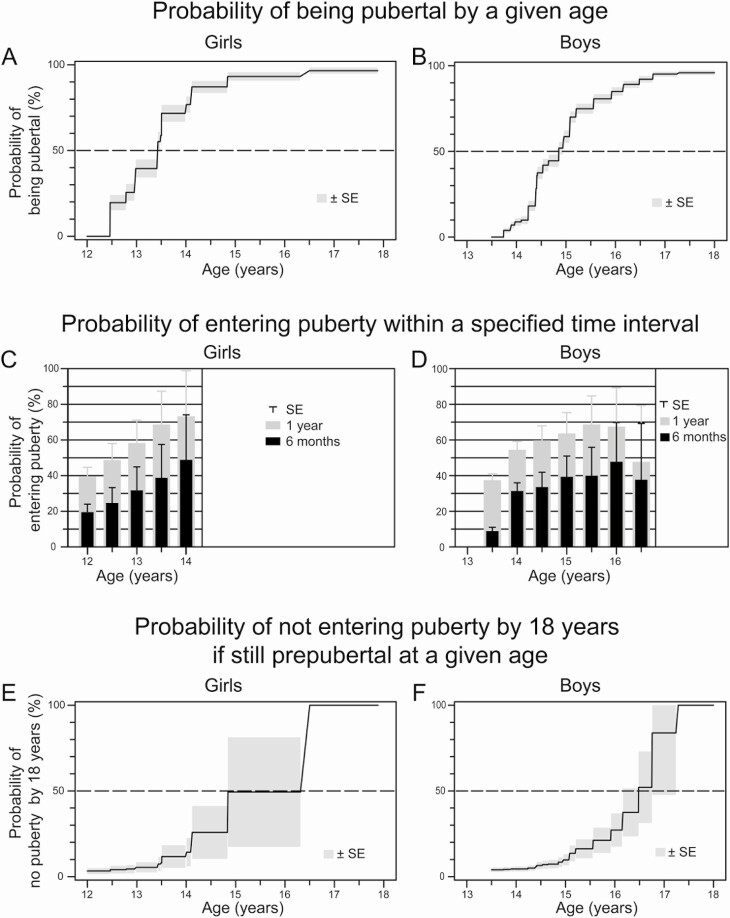
Girls entered puberty across a broad range of ages, with 25% entering puberty by age 12.8 years (95% CI, 12.5-13.0), 50% by age 13.4 years (13.0-13.5), and 75% by age 14.0 years (13.5-14.1 y; Fig. 3A). Boys similarly entered puberty across a broad age range, with 25% entering puberty by age 14.4 years (14.4–14.4), 50% by age 14.9 years (14.7–15.0), and 75% by age 15.6 years (15.1–15.6; Fig. 3B).
Figure 3.
Probability of pubertal entry with age in (A) girls and (B) boys with unexplained delayed puberty because of constitutional delay or idiopathic hypogonadotropic hypogonadism; probability of entering puberty within the next 6 to 12 months by age for (C) girls and (D) boys of a given age; and probability of not entering puberty by age 18 years if still prepubertal at a given age for (E) girls and (F) boys.
This analysis allowed us to estimate the probability that a child with delayed puberty at a given age will enter puberty within a given time interval. In our cohort, the probability of entering puberty in the next year was 40 ± 5% (estimate ± SE) for a 12-year-old girl, 62 ± 13% for a 13-year-old girl, and 74 ± 36% for a 15.5-year-old girl (Fig. 3C). For boys, the probability of entering puberty in the next year was 38 ± 4% for a 13.5-year-old boy in our cohort, 55 ± 5% for a 14-year-old boy, 64 ± 12% for a 15-year-old boy, and 68 ± 22% for a 16-year-old boy, but fell to 48 ± 32% for a not-yet-pubertal 16.5-year-old boy (Fig. 3D).
Estimated probability of not entering puberty by 18 years
Most children with unexplained delayed puberty have constitutional delay, but some will not enter puberty by age 18 years. In a cohort of children with delayed puberty, the children with constitutional delay will enter puberty with increasing age; as a result, over time the remaining children who have not entered puberty have a higher likelihood of not entering puberty by adulthood. We analyzed our time-to-event results to quantify this increasing probability over time of failing to enter puberty by age 18 years.
For a 12-year-old girl who has not yet entered puberty, the estimated probability of not entering puberty by 18 years was 3 ± 2% (Fig. 3E). By age 13 years, this probability increased to 5 ± 3%, and the probability increased further to 14 ± 8% by age 14 years and 49 ± 32% by age 15 years (Fig. 3E). The estimated probability of not entering puberty by 18 years was 4 ± 1% for a 13.5-year-old boy who had not yet entered puberty and remained essentially unchanged at age 14 years. The probability increased to 10 ± 3% by age 15 years, 27 ± 10% by age 16 years, and 84 ± 36% by age 17 years (Fig. 3F).
Comparison of pubertal timing between girls and boys
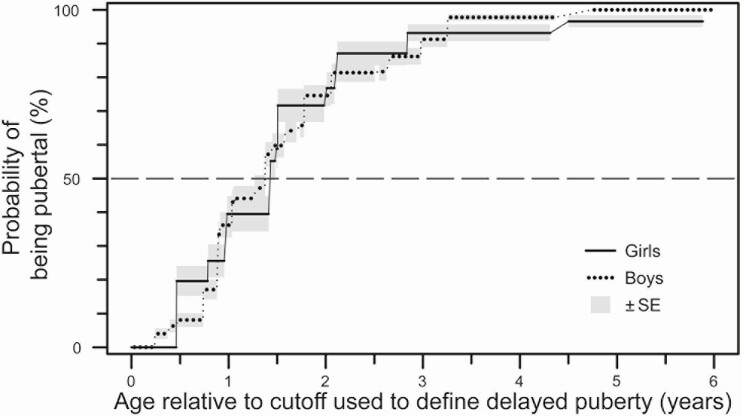
To compare rates of pubertal entry between girls and boys, we first harmonized data from girls and boys in 2 ways. First, because different age cutoffs were used to define delayed puberty in girls and boys, we converted chronological age to “years beyond cutoff age” (with the age cutoffs used in this study being 12 years for girls and 13.5 years for boys). Second, because girls were removed from analysis (right-censored) at the time of starting estrogen treatment (because treatment induces breast development, thereby obscuring the primary physical marker of pubertal entry in girls), boys were similarly right-censored at the time of starting androgen treatment (no boys in the cohort were treated with aromatase inhibitors). After these harmonization steps, no differences were seen between rates of pubertal entry between girls and boys (χ 2 = 0.001, P = 0.97; Fig. 4).
Figure 4.
Comparison between girls and boys of the probability of pubertal entry with age (expressed relative to age cutoffs used to define delayed puberty in this study [ie, 12 years for girls and 13.5 years for boys]).
Discussion
We reviewed the medical records of 392 girls and 683 boys with delayed puberty seen at BCH between 2000 and 2015 and found that the most common causes of delayed puberty in both girls and boys were self-limited constitutional delay and FHH, similar to what has been observed in other cohorts (16, 21-23). For a subset of patients with delayed puberty without a clear cause at presentation, most of whom had constitutional delay, we observed a markedly broad range of ages for entry into puberty, a finding that has implications both for our understanding of the causes of constitutional delay and for clinical practice.
Constitutional delay has been described as a benign developmental variant that represents the extreme end of the normal (Gaussian) distribution for pubertal timing (1, 24). If this were the case, according to a normal distribution, a child who has not entered puberty by 2 SD beyond the population mean (approximately 12-13 years for girls (8-11) and 13.5-14 years for boys (12-14)) would have a 94% chance of entering puberty within the next 1 SD (approximately 1 year). In contrast, we observed in our cohort that the chance of entering puberty within the next year for 12- to 13-year-old girls with confirmed or presumed constitutional delay was 40% to 62% and for 13.5- to 14-year-old boys was 38% to 55%, much lower than what is predicted by a normal distribution. Although we may have misclassified some individuals in our cohort as having constitutional delay because of the limitations of a retrospective analysis, we would have to have misclassified more than one-half of the cases for pubertal timing to conform to a normal distribution.
If constitutional delay does not represent the tail end of the normal distribution for age at pubertal entry, then there must be causes of delayed puberty beyond the factors that cause normal variation in pubertal timing. Although not all factors that determine normal pubertal timing are known, in developed countries ~75% of variation in pubertal timing is attributable to genetics (25). Genome-wide association studies on the timing of puberty (using markers such as menarche and voice-breaking) have identified hundreds of common variants that contribute to variation in normal pubertal timing (25, 26), and these genetic variants also appear to contribute to delayed puberty (25). However, our findings raise the possibility that common genetic variation is not the sole cause of constitutional delay; indeed, rare genetic variation (which may be reflected by family history) has been suggested to contribute to delayed puberty, and other factors may be involved as well (27-31). Identifying these factors and in turn assessing these factors in the clinical setting may allow for improved prediction of the timing of pubertal onset for patients affected by constitutional delay.
Our results also allowed us to compare delayed puberty between girls and boys. We previously found that clinicians tend to treat boys with delayed puberty with sex steroids more readily than girls (7), potentially because of assumptions about differences in underlying biology. Such assumptions may arise from the fact that boys are seen for delayed puberty more often than girls (21, 32), a difference observed in our cohort as well. However, this may be largely because of referral bias rather than true differences in the prevalence of (and physiologic disposition to) delayed puberty (21), particularly as reasons for consulting a specialist (such as concerns about height) may differ between girls and boys. Providers may also assume that the well-known differences in normal pubertal timing between girls and boys extend to delayed puberty. However, we found no difference in the rates of pubertal entry between girls and boys after accounting for the different age cutoffs used to define delayed puberty. Though our results do not preclude other differences between girls and boys with delayed puberty, if differences in management of girls and boys with delayed puberty are based on the assumption that the eventual timing of puberty differs between girls and boys with delayed puberty, our data show this assumption to be incorrect.
Our findings may help to inform discussions about whether to treat delayed puberty with sex steroids. The most frequently cited goal for sex-steroid treatment is to address psychosocial distress (1-3), and our data describe the length of time such distress may need to be endured if treatment is not started. Furthermore, our data allowed us to predict based on a child’s age the likelihood that the child will not enter puberty by 18 years, which could further inform decisions about treatment. (Of note, although most sources define IHH as failure to achieve normal adult reproductive endocrine function by age 18 years [eg, ref. (1)], some sources propose an age cutoff of 17 years for girls and 18 years for boys [eg, ref. (33)]; our results support the use of different cutoffs for girls and boys.) These data on rates of pubertal entry and the likelihood of not entering puberty by age 18 years can be integrated with other factors such as the degree of psychosocial distress and the values of the patient and family to aid in decision-making regarding sex-steroid treatment.
One limitation of our study is that BCH is a single major referral center with an active neurosurgery program, and our findings may not be generalizable to most pediatric endocrinology practices, much less the general population. Specifically, our cohort is likely to be enriched for individuals with underlying medical conditions, including neurosurgical conditions, and this is likely to have resulted in higher rates of functional HH and HH because of structural lesions of the hypothalamus/pituitary. Indeed, studies of causes of delayed puberty at other centers have generally reported lower rates of FHH (Table 1; 16, 21-23)). Even a previous study at our medical center found a lower proportion of FHH in girls than observed in the current study (21), likely reflecting increasing complexity of our patient population over time. Furthermore, it is likely that children with mildly delayed puberty often do not seek specialist care and therefore are underrepresented in our cohort, and thus that rates of pubertal entry are higher for the general population than for those presenting for specialist care, particularly for individuals with milder pubertal delay.
Table 1.
Causes of delayed puberty reported in the literature and in this study
| Boston 1996-1999 (21) | Copenhagen (16) | Toronto (22)a | Helsinki (23) | Boston 2000-2015 | |
|---|---|---|---|---|---|
| Girls | n = 74 | n = 20 | n = 70 | n = 392 | |
| Hypergonadotropic hypogonadism | 26% | 10%b | 14% | 18% | |
| Functional hypogonadotropic hypogonadism | 16% | 50% | 15% | 29% | |
| Delayed puberty secondary to another endocrine condition | 1% | b | 0% | 8% | |
| Hypogonadotropic hypogonadism because of generalized hypothalamic/pituitary pathology | 15% | b | 1% | 3% | |
| Idiopathic hypogonadotropic hypogonadism/Kallmann syndrome | 4% | 0% | 8% | 0.5% | |
| Other syndromes and other causes | 8% | 10% | 6% | 9% | |
| Constitutional delay | 30% | 30% | 55% | 32% | |
| Boys | n = 158 | n = 391 | n = 28 | n = 174 | n = 683 |
| Hypergonadotropic hypogonadism | 7% | 0.5% | 0%b | 2% | 2% |
| Functional hypogonadotropic hypogonadism | 14% | 20% | 43% | 6% | 16% |
| Delayed puberty secondary to another endocrine condition | 2% | 2% | b | 0% | 5% |
| Hypogonadotropic hypogonadism due to generalized hypothalamic/pituitary pathology | 8% | 7%c | 4%b | 2% | 2% |
| Idiopathic hypogonadotropic hypogonadism/Kallmann syndrome | 3% | 4% | 8% | 2% | |
| Other syndromes and other causes | 4% | 0% | 0% | 0% | 4% |
| Constitutional delay | 63% | 71% | 50% | 82% | 70% |
a L. Abitbol and M. Palmert provided additional details through personal communication.
b These percentages do not account for 16 girls and boys with previously known hypogonadotropic and hypergonadotropic conditions (eg, Turner syndrome, panhypopituitarism) for whom the specific breakdown of diagnoses was not available.
c This reference did not distinguish between these 2 categories of hypogonadotropic hypogonadism. This percentage includes 14 patients with GH deficiency and 13 with hypogonadotropic hypogonadism.
Additional limitations of this study largely derive from its retrospective design. The intervals between clinic visits were variable and sometimes long, making it difficult to determine the exact timing of pubertal entry for some individuals. Furthermore, most individuals were not followed until age 18 years, and approximately one-third of the patients in our cohort were not observed to enter puberty during the course of their clinical care. To account for this, our model used an informative right-censoring model that assumed that any individual who had not entered puberty within one year after their last visit would have returned for care (ie, that most individuals with IHH/Kallmann syndrome would have remained in or returned to clinical care). This assumption potentially underestimates the true prevalence of IHH; however, given the rarity of IHH, we feel that this model produced reasonable estimates of rates of pubertal entry. Indeed, when we tested alternative models with different censoring criteria, we did not find markedly different results. Another limitation of our study was sample size, particularly at older ages, leading to limited accuracy for girls older than age 14 years and boys older than age 16 years of the estimates of the rates of pubertal entry and the probability of not entering puberty by age 18 years. Also, the limited number of patients with IHH/Kallmann syndrome in our cohort precluded us from examining factors other than age, such as family history of delayed puberty, in assessing the probability that a child with delayed puberty would not enter puberty by age 18 years. Furthermore, because the outcome analyzed in our study was pubertal entry (and many individuals were discharged from pediatric endocrine care once this hallmark was reached), we were able to definitively identify individuals with IHH/Kallmann syndrome who had no pubertal development by 18 years, but not those with IHH/Kallmann syndrome with partial pubertal development (ie, those who entered puberty but then stalled). Studies of adults with IHH/Kallmann syndrome demonstrate that up to one-half had partial pubertal development (34), so we expect that few individuals with IHH/Kallmann syndrome were misclassified as having constitutional delay. Finally, data on ancestry, race, and/or ethnicity were not reliably available, so we were unable to assess the effects of these factors. Because of these limitations, caution must be taken when attempting to generalize our findings to other populations.
In summary, our finding of a broad range of ages of pubertal entry for children with delayed puberty suggests that the causes of self-limited delayed puberty are heterogeneous and extend beyond the mechanisms that determine normal pubertal timing. Our results can be shared with patients and families to create appropriate expectations for when puberty may begin and to inform decisions about treatment with sex steroids. Future studies with larger and/or more diverse cohorts may further refine our understanding of the factors that influence the timing of puberty and the characteristics that distinguish constitutional delay from IHH/Kallmann syndrome so we can improve clinical management for patients with delayed puberty.
Acknowledgments
The authors thank Selby Knudsen, Sinead Christiansen, Kara McLaughlin, Alexandria Shirey, and Rebecca Shin for assistance with data abstraction and cleaning and Leah Abitbol and Mark Palmert for communicating unpublished data.
Financial Support: This work was conducted with support from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01 HD090071 and from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541), and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Glossary
Abbreviations
- BCH
Boston Children’s Hospital
- FHH
functional hypogonadotropic hypogonadism
- HH
hypogonadotropic hypogonadism
- IHH
idiopathic hypogonadotropic hypogonadism
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366(5):443-453. [DOI] [PubMed] [Google Scholar]
- 2. Soliman AT, De Sanctis V. An approach to constitutional delay of growth and puberty. Indian J Endocrinol Metab. 2012;16(5):698-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei C, Crowne EC. Recent advances in the understanding and management of delayed puberty. Arch Dis Child. 2016;101(5):481-488. [DOI] [PubMed] [Google Scholar]
- 4. Crowley WF, Pitteloud N.. Approach to the patient with delayed puberty. Waltham, MA. UpToDate: Wolters Kluwer; 2018. [Google Scholar]
- 5. Rosenfield RL, Cooke DW, Radovick S. Puberty in the female and its disorders. In: Sperling MA, Majzoub JA, Menon RK, Stratakis CA, eds. Sperling Pediatric Endocrinology. Philadelphia: Elsevier; 2020:528- 626. [Google Scholar]
- 6. Stempfel RS Jr. The question of sex hormone therapy in cases of delayed puberty. J Pediatr. 1967;70(6):1023-1024. [DOI] [PubMed] [Google Scholar]
- 7. Zhu J, Feldman HA, Eugster EA, et al. Practice variation in the management of girls and boys with delayed puberty. Endocr Pract. 2020;26(3):267-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505-512. [DOI] [PubMed] [Google Scholar]
- 9. Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84-88. [DOI] [PubMed] [Google Scholar]
- 10. Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123(5):e932-e939. [DOI] [PubMed] [Google Scholar]
- 11. Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sørensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metab. 2010;95(1):263-270. [DOI] [PubMed] [Google Scholar]
- 13. Ma HM, Chen SK, Chen RM, et al. ; Pubertal Study Group of the Society of Pediatric Endocrinology and Genetic Disease, Chinese Medical Association . Pubertal development timing in urban Chinese boys. Int J Androl. 2011;34(5 Pt 2):e435-e445. [DOI] [PubMed] [Google Scholar]
- 14. Herman-Giddens ME, Steffes J, Harris D, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130(5):e1058-e1068. [DOI] [PubMed] [Google Scholar]
- 15. van Buuren S, Ooms JC. Stage line diagram: an age-conditional reference diagram for tracking development. Stat Med. 2009;28(11):1569-1579. [DOI] [PubMed] [Google Scholar]
- 16. Lawaetz JG, Hagen CP, Mieritz MG, Blomberg Jensen M, Petersen JH, Juul A. Evaluation of 451 Danish boys with delayed puberty: diagnostic use of a new puberty nomogram and effects of oral testosterone therapy. J Clin Endocrinol Metab. 2015;100(4):1376-1385. [DOI] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wellner JA, Zhan Y.. A hybrid algorithm for computation of the nonparametric maximum likelihood estimator from censored data. J Am Stat Associ. 1997;92:945-959. [Google Scholar]
- 19. Sun J. A non-parametric test for interval-censored failure time data with application to AIDS studies. Stat Med. 1996;15(13):1387-1395. [DOI] [PubMed] [Google Scholar]
- 20.Chan YM. Supplementary figure and tables for “Timing of Pubertal Onset in Girls and Boys with Constitutional Delay.” Posted November 28, 2020. figshare. https://figshare.com/articles/SupplementalF000ure_and_Tables_for_Jonsdottir-Lewis_Feld_et_al_/13298510. [DOI] [PMC free article] [PubMed]
- 21. Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87(4):1613-1620. [DOI] [PubMed] [Google Scholar]
- 22. Abitbol L, Zborovski S, Palmert MR. Evaluation of delayed puberty: what diagnostic tests should be performed in the seemingly otherwise well adolescent? Arch Dis Child. 2016;101(8):767-771. [DOI] [PubMed] [Google Scholar]
- 23. Varimo T, Miettinen PJ, Känsäkoski J, Raivio T, Hero M. Congenital hypogonadotropic hypogonadism, functional hypogonadotropism or constitutional delay of growth and puberty? An analysis of a large patient series from a single tertiary center. Hum Reprod. 2017;32(1):147-153. [DOI] [PubMed] [Google Scholar]
- 24. Rosenfield RL. Clinical review 6: diagnosis and management of delayed puberty. J Clin Endocrinol Metab. 1990;70(3):559-562. [DOI] [PubMed] [Google Scholar]
- 25. Day FR, Thompson DJ, Helgason H, et al. ; LifeLines Cohort Study; InterAct Consortium; kConFab/AOCS Investigators; Endometrial Cancer Association Consortium; Ovarian Cancer Association Consortium; PRACTICAL consortium . Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49(6):834-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hollis B, Day FR, Busch AS, et al. ; PRACTICAL Consortium; 23andMe Research Team . Genomic analysis of male puberty timing highlights shared genetic basis with hair colour and lifespan. Nat Commun. 2020;11(1):1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu J, Choa RE, Guo MH, et al. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2015;100(4):E646-E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howard SR, Guasti L, Ruiz-Babot G, et al. IGSF10 mutations dysregulate gonadotropin-releasing hormone neuronal migration resulting in delayed puberty. EMBO Mol Med. 2016;8(6):626-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cassatella D, Howard SR, Acierno JS, et al. Congenital hypogonadotropic hypogonadism and constitutional delay of growth and puberty have distinct genetic architectures. Eur J Endocrinol. 2018;178(4):377-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howard SR, Oleari R, Poliandri A, et al. HS6ST1 insufficiency causes self-limited delayed puberty in contrast with other GnRH deficiency genes. J Clin Endocrinol Metab. 2018;103(9):3420-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mancini A, Howard SR, Marelli F, et al. LGR4 deficiency results in delayed puberty through impaired Wnt/beta-catenin signaling. JCI Insight. 2020;5(11):e133434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitchell AL, Dwyer A, Pitteloud N, Quinton R. Genetic basis and variable phenotypic expression of Kallmann syndrome: towards a unifying theory. Trends Endocrinol Metab. 2011;22(7):249-258. [DOI] [PubMed] [Google Scholar]
- 33. Kim HG, Bhagavath B, Layman LC. Clinical manifestations of impaired GnRH neuron development and function. Neurosignals. 2008;16(2-3):165-182. [DOI] [PubMed] [Google Scholar]
- 34. Pitteloud N, Hayes FJ, Boepple PA, et al. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87(1):152-160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.