Abstract
Introduction:
Hypopituitary patients are at risk for bone loss. Hypothalamic-posterior pituitary hormones oxytocin and vasopressin are anabolic and catabolic, respectively, to the skeleton. Patients with hypopituitarism may be at risk for oxytocin deficiency. Whether oxytocin and/or vasopressin contribute to impaired bone homeostasis in hypopituitarism is unknown.
Objectives:
To determine the relationship between plasma oxytocin and vasopressin levels and bone characteristics [bone mineral density (BMD) and hip structural analysis (HSA)] in patients who have anterior pituitary deficiencies with (CDI) or without (APD) central diabetes insipidus.
Methods:
Cross-sectional study. Thirty-seven men (17 CDI and 20 APD), age 20-60 years. Main outcome measures were fasting plasma oxytocin and vasopressin levels, and BMD and HSA using dual x-ray absorptiometry.
Results:
Mean BMD and HSA variables did not differ between the CDI and APD groups. Mean BMD Z-scores at most sites were lower in those participants who had fasting oxytocin levels below, than above, the median. There were positive associations between fasting oxytocin levels and (1) BMD Z-scores at the spine, femoral neck, total hip and subtotal body and (2) favorable hip geometry and strength variables at the intertrochanteric region in CDI, but not APD, participants. No associations between vasopressin levels and bone variables were observed within CDI or ADP groups.
Conclusions:
This study provides evidence for a relationship between oxytocin levels and BMD and estimated hip geometry and strength in hypopituitarism with CDI. Future studies will be important to determine whether oxytocin could be used therapeutically to optimize bone health in patients with hypopituitarism.
Keywords: Oxytocin, vasopressin, diabetes insipidus, hypopituitarism, bone mineral density
Introduction
Hypopituitarism is associated with bone loss and skeletal fragility leading to an increased risk for bone fractures [1-3]. Multiple factors play a role in bone impairment in patients with hypopituitarism, including endocrine hyperfunction related to underlying pituitary disease (e.g., in Cushing’s disease, acromegaly, prolactinomas), pituitary hormone deficiencies (e.g., gonadotropin or growth hormone (GH) deficiency), and/or suboptimal replacement of hormones (e.g., excessive glucocorticoid or thyroid hormone exposure) [4-6]. Pituitary hormones directly or indirectly modulate bone metabolism and remodeling and are essential for optimal bone homeostasis [7]. Management of pituitary hypersecretion through surgery, medical therapy and/or radiation therapy, and treatment of anterior pituitary deficiency of gonadotropins and in some cases growth hormone, are typically addressed as the main reversible causes of low bone mineral density (BMD) in these patients [8, 9]. Although appropriate hormone replacement results in a sustained increase in BMD at long-term follow-up [10, 3, 11, 12], some patients remain at high risk for fractures and require pharmacological therapies to treat low BMD [7].
The effects of hypothalamic-posterior pituitary hormones oxytocin (OT) and vasopressin (VP) on bone have received less attention. OT and VP are produced in the same areas of the hypothalamus and stored and secreted from the posterior pituitary gland. Recent studies have shown that OT and VP have important opposing effects on bone turnover via their respective receptors (OTR and AVPR1A) on osteoblasts and osteoclasts [13-15]. Ot−/− and Otr−/− mice have severe bone loss that is reversible in the former case with administration of OT, which has bone anabolic effects [16]. Human studies in females have consistently shown associations between low circulating OT levels and low BMD, impaired hip geometry and microarchitecture, and high estimated fracture risk [17-21]. Avpr1a−/− mice display a marked increase in bone mass [22] and VP administration in wild-type mice increases bone resorption and reduces osteoblastogenesis, suggesting that VP is catabolic to bone [15]. Further, inappropriately increased VP secretion (SIADH) induces hyponatremia, which is associated with bone loss and higher risk for fractures in both preclinical and human studies [23-25, 15, 26-29], but the pathophysiologic mechanism involved is not completely understood.
While there is increasing evidence showing the importance of hypothalamic-posterior pituitary hormones in bone metabolism, few studies have examined BMD in patients with central diabetes insipidus (CDI), who have deficient VP, and there are no data on the relationships between OT or VP and bone characteristics in patients with hypopituitarism. Despite the fact that patients with CDI do not have evidence of greater bone loss than those with anterior pituitary deficiencies (APD) only, men with CDI have been shown to have a higher fracture risk than those without CDI [3]. Therefore, identifying potential therapeutic targets to optimize bone health in this population is important.
We recently demonstrated lower levels of fasting 1-hour pool of plasma OT in hypopituitary men with CDI in comparison to men with APD only or healthy men without pituitary disease [30]. Furthermore, men with CDI had greater psychopathology on average than healthy participants, suggestive of a possible OT-deficient state, as OT has pro-social, antidepressant and anxiolytic properties [30]. To improve our understanding of the clinical implications of OT deficiency and the effects of hypothalamic-posterior pituitary hormones on bone health, we investigated the relationship between OT, VP and bone variables in men with hypopituitarism. We hypothesized that lower plasma OT and higher plasma VP levels would be associated with lower BMD and adverse bone characteristics assessed by hip structural analysis (HSA) in men with hypopituitarism.
Materials and Methods
We performed a cross-sectional study of 37 male patients with hypopituitarism, ages 20-60 years: 20 with APD only and 17 with CDI of similar age, body mass index (BMI) and number of adenohypophyseal deficiencies. The study was approved by the Institutional Review Board of Partners HealthCare. Informed consent was obtained from all participants by a study physician or nurse practitioner prior to any procedures. Participants were primarily recruited through the Neuroendocrine and Pituitary Tumor Clinical Center at Massachusetts General Hospital (MGH). Clinical characteristics of study participants including levels of hormones were previously reported; the current study did not include two patients who had a history of bisphosphonate use and one participant younger than 20 years old who were reported in the prior publication [30]. Bone variables and their relationship to OT and VP levels, the focus of this study, have not been published.
Diagnoses of pituitary deficiencies were established as per routine clinical practice based on clinical symptoms, sodium levels, and water deprivation tests, if needed for CDI, as well as a combination of basal and validated dynamic tests to assess anterior pituitary function. All CDI and no APD participants were receiving desmopressin for central diabetes insipidus. All subjects had been receiving stable hormone replacement for at least the prior three months. Normal free T4 and normal free testosterone levels were required for participation. Participants were excluded from the study if they were receiving human chorionic gonadotropin, which can increase levels of estradiol, a key modulator of OT secretion and receptor distribution; taking medication for osteoporosis; or if lab tests showed a creatinine greater than 1.5 mg/dL, ALT or AST levels greater than 2.5 times the upper limit of normal, or hematocrit less than 34%. Additional exclusion criteria for APD included the presence of CDI.
All participants were admitted to the Translational and Clinical Research Center (TCRC) at MGH for a screening visit (medical history, physical exam and blood work) and if eligible, a main study visit. For the main study visit, medical history and physical examination were updated, including body mass index (BMI) and waist-to-hip ratio (WHR; calculated by dividing the measurement of waist circumference at the level of iliac crest in cm by the broadest hip circumference in cm).
Patients presented to the TCRC after an 8-hour fast, and an intravenous catheter was placed at least 30 minutes prior to a fasting blood draw to minimize the impact of the stress of the venipuncture on hormone levels. A fasting blood draw for blood analytes, including hormones (OT, VP, total testosterone, free T4, IGF-1, prolactin) was performed, followed by frequent sampling of blood every 5 minutes over one hour (0800-0900 h) for assessment of pooled OT levels. Blood draws were performed by experienced nurses using identical procedures on patients and controls. Interactions and conversation were kept to a minimum to avoid effects of social stimulation on OT levels.
Whole body composition and BMD (g/cm2) at the posteroanterior (PA) lumbar spine, total hip, femoral neck, distal 1/3 radius, and subtotal body (total body minus head) were assessed by dual energy x-ray absorptiometry (DXA) scan (Hologic QDR-Horizon A, Hologic Inc., Waltham, MA). DXA has a precision < 0.01g/cm2 for total body BMD and 3% for fat mass [31]. Sex and race-specific BMD Z-scores were calculated from US Health and Nutritional Examination Survey-I reference data. Standard protocols for analysis and positioning were used by trained technicians. Hip geometry and femoral strength were assessed via hip structural analysis (HSA) of total hip DXA images. HSA variables strongly correlate with equivalent measurements assessed by high resolution quantitative computed tomography (QCT) [32]. HSA was performed at three femoral sites: narrow neck (NN; narrowest point of the femoral neck), intertrochanteric region (IT; along the bisector of the area produced by neck and shaft axes) and femoral shaft (FS; 1.5 cm from the NN to the intersection of the neck and shaft axes) [32]. Reported HSA variables include: section modulus (Z; index of maximum bending strength in cm3) and buckling ratio (BR; index of susceptibility to cortical buckling under compressive loads or index of cortical wall stability), which are associated with fracture risk [33, 34], and cortical thickness (in centimeters). Higher values are associated with more favorable hip geometry and strength, except for BR in which lower values are associated with greater femoral strength.
Biochemical analysis
Serum and plasma for hormones were stored at −80 °C and run in a single batch. Equal aliquots of plasma from each timepoint were combined into a 1-h pool for an integrated measure of OT for each participant. Radioimmunoassay (RIA) of extracted plasma OT was done in duplicate using antibody Pitt-Ab-2 (University of Pittsburgh, Pittsburgh, PA, USA) [35]. Minimum limit of detection was 0.4 pg/mL, intra-assay CV was 4.2 % and inter-assay CV was 12.4%. The antiserum displayed significant cross-reactivity with arginine vasotocin (AVT), but <1% cross-reactivity with VP, lysine vasopressin (LVP) and desmopressin [36]. RIA of plasma VP was performed in duplicate after acetone-ether extraction. Minimal detectable VP concentration in extracted plasma was 0.1 pg/mL [37]. Intra-assay CV was 6.4% and inter-assay CV was 8.8%. The antiserum displayed significant cross-reactivity with LVP but <1% cross-reactivity with OT, AVP and desmopressin. Serum IGF-1 was measured in serum using liquid chromatography–mass spectrometry (Quest Diagnostics, San Juan Capistrano, CA, USA). Serum prolactin was measured using a competitive ELISA kit (ALPCO, Salem, NH, USA) with a minimum detection limit of 2 ng/mL, an inter-assay CV of 5.3%, and an intra-assay CV of 3.7%. Basic metabolic panel, lipids, glucose, free T4, sex hormone binding globulin (SHBG), and total testosterone were obtained using standard techniques by LabCorp (Burlington, NC, USA). Free testosterone was calculated from total testosterone and SHBG by the laws of mass action [38].
Statistical analysis
Statistical analyses were performed using STATA software V.14.2, StataCorp LLC (College Station, TX). Quantitative data are expressed as mean and standard deviation (SD) (Gaussian distribution) or as median and interquartile range (IQR) (non-Gaussian distribution), and categorical data are expressed as percentages. Data distribution was analyzed using the Shapiro Wilk test, and if non-normally distributed, variables were log-transformed or non-parametric testing was performed. Means across CDI and APD were compared using Student’s t-test or Wilcoxon rank-sum test accordingly. Fisher’s exact test was performed to compared categorical variables across the groups. For primary outcome measures, associations between bone characteristics (BMD and HSA variables) and dichotomized posterior pituitary hormone levels (below or above the median levels of fasting OT, 1h-pool of OT and fasting VP) among patients with hypopituitarism were assessed using Student’s t-test or Wilcoxon rank-sum test accordingly. For secondary outcome measures, the correlations between bone characteristics and posterior pituitary hormones levels among CDI and APD participants were assessed using linear regression analyses with Pearson correlation coefficients. t. P values were adjusted for multiple comparisons using Holm-Bonferroni correction. Stepwise regression analyses were performed to further investigate determinants of bone variables. Two-tailed P values of <0.05 were considered statistically significant.
Results
Clinical characteristics of the study participants
Mean age was 45.8±11.8 years, BMI was 30.8±5.1 kg/m2 and WHR was 0.95±0.08 with no differences between the groups (p≥0.484) (Table 1). No participants endorsed current tobacco smoking or heavy alcohol consumption [39]. Twelve CDI (71%) and thirteen (65%) APD reported regular physical activity with similar number of hours of exercise a week (5.4±3.7 and 4.3±5.2 hours/week, respectively, p=0.482). Nine (24%) participants were taking vitamin D supplementation and two (5%) were receiving a combination of calcium plus vitamin D supplements without significant differences across groups (p=0.725). None of the participants had any previous history of osteoporotic fracture, and by design, none of the participants were on any medication for osteoporosis. Two participants were on antiepileptic drugs (one CDI was on clonazepam and one APD was on levetiracetam).
Table 1.
Participant clinical characteristics, hormone levels, bone mineral density and hip geometry in CDI vs. APD (mean±SD)
| CDI participants (N=17) |
APD participants (N=20) |
P | |
|---|---|---|---|
| Clinical characteristics and hormone data | |||
| Age (years) | 44.4±12.6 | 47.0±11.3 | 0.507 |
| Body mass index (kg/m2) | 30.1±5.1 | 31.3±5.2 | 0.484 |
| Pituitary disease | 0.413 | ||
| Non-functioning pituitary adenoma | 4 (24%) | 5 (25%) | |
| Craniopharyngioma | 5 (29%) | 4 (20%) | |
| Cushing’s disease | 1 (6%) | 3 (15%) | |
| Acromegaly | 1 (6%) | 2 (10%) | |
| Neurosarcoidosis | 2 (12%) | 0 | |
| Germinoma | 2 (12%) | 0 | |
| Chondrosarcoma | 0 | 2 (10%) | |
| Prolactinoma | 0 | 2 (10%) | |
| Pituitary hypoplasia | 1 (6%) | 1 (5%) | |
| Pituitary stalk enlargement | 1 (6%) | 0 | |
| Medulloblastoma | 0 | 1 (5%) | |
| Number of APD | 3.3±0.9 | 3.2±0.9 | 0.885 |
| Sodium (mmol/L) | 139.2±4.3 | 139.1±2.6 | 0.395 |
| Free-T4 (ng/dL) | 1.38±0.06 | 1.25±0.27 | 0.228 |
| Prolactin (ng/mL) | 8.4±5.8 | 12.0±6.8 | 0.101 |
| Testosterone (ng/dL) | 555.6±447.6 | 603.6±331.7 | 0.711 |
| IGF-1 (ng/mL) | 166.0±75.8 | 143.9±78.6 | 0.393 |
| Fasting oxytocin (pg/mL) | 1.00±0.38 | 0.99±0.31 | 0.972 |
| 1-h pool of oxytocin (pg/mL) | 1.08±0.29 | 1.32±0.34 | 0.024 |
| Fasting vasopressin (pg/mL) | 0.24±0.10 | 0.45±0.23 | 0.001 |
| Bone mineral density | |||
| PA lumbar spine BMD Z-score | 0.03±1.42 | 0.17±0.28 | 0.760 |
| Total hip BMD Z-score | 0.81±.122 | 0.38±0.86 | 0.213 |
| Femoral neck BMD Z-score | 0.33±1.03 | −0.08±0.81 | 0.187 |
| Distal 1/3 radius BMD Z-score | −0.01±1.13 | 0.18±1.20 | 0.632 |
| Subtotal body BMD Z-score | −0.38±1.04 | −0.69±0.74 | 0.306 |
| Hip structural analysis | |||
| NN cortical thickness (cm) | 0.21±0.04 | 0.19±0.03 | 0.139 |
| NN section modulus (cm3) | 2.12±0.47 | 1.97±0.31 | 0.239 |
| NN buckling ratio | 9.92±2.17 | 11.17±2.98 | 0.175 |
| IT cortical thickness (cm) | 0.49±0.10 | 0.45±0.07 | 0.223 |
| IT section modulus (cm3) | 6.03±1.76 | 5.93±1.08 | 0.837 |
| IT buckling ratio | 7.11±1.69 | 7.55±1.49 | 0.405 |
| FS cortical thickness (cm) | 0.76±0.19 | 0.73±0.13 | 0.891 |
| FS section modulus (cm3) | 3.10±0.72 | 3.20±0.59 | 0.651 |
| FS buckling ratio | 2.28±0.67 | 2.36±0.52 | 0.716 |
Abbreviations: APD, anterior pituitary deficiencies; CDI, central diabetes insipidus; PA, posterioanterior; BMD, bone mineral density; NN, narrow neck; IT, intertrochanteric region; FS, femoral shaft.
Mean age at diagnosis of pituitary disease was 31.4±14.1 years and number of anterior pituitary deficiencies was 3.3±0.9 without differences between groups (p≥0.772). Childhood onset of the pituitary disease was observed in six (16%) of the participants [three (18%) CDI and three (15%) APD, p=0.828]. The most frequent pituitary diseases were non-functioning pituitary adenomas in nine (24%) and craniopharyngioma in nine (24%) participants, followed by Cushing’s disease in four (11%), acromegaly in three (8%), prolactinoma in two (5%), neurosarcoidosis in two (5%), chondrosarcoma in two (5%), pituitary hypoplasia in two (5%), and other pituitary diseases in three (8%) participants. Twelve (71%) CDI and seventeen (85%) APD participants underwent pituitary surgery (p=0.254), and five (35%) CDI and fifteen (75%) APD participants had received radiotherapy (p=0.022). All patients with hyperfunctioning tumors were either in long-term biochemical remission (N=35) or were biochemically controlled with cabergoline (one with acromegaly for 54 months and the other with a macroprolactinoma for 78 months). Median (interquartile range) time of biochemical control of Cushing’s disease was 5.4 (8.1) years and of acromegaly was 2.2 (0.8) years. All patients were on stable hormone replacement therapy. A free testosterone level in the normal range was an inclusion criterion; 15 (88%) of CDI and 19 (95%) APD subjects were receiving testosterone replacement therapy (p=0.438). Mean daily hydrocortisone equivalent dose in patients with central adrenal insufficiency was 16.2±2.7 mg without differences between groups (p=0.617). Eleven (65%) CDI and thirteen (65%) APD patients had confirmed growth hormone deficiency (p=0.879); of those, 90% and 77% were on recombinant human growth hormone replacement respectively (p=0.743). All CDI patients were on desmopressin replacement therapy: nine (53%) on oral desmopressin, six (35%) on intranasal desmopressin, and two (12%) on both intranasal and oral desmopressin dose from one to three times a day. Mean fasting hormone levels (free T4, prolactin, testosterone, IGF-1 and fasting OT) did not differ between CDI and APD (p≥0.101), except for lower 1-h pool of OT and fasting VP levels in CDI compared to APD (1-h pool of OT: p=0.024 and VP: p=0.001) (Table 1).
Results of DXA showed that mean total fat (CDI: 31±9 kg vs. APD: 33±10 kg, p=0.434), lean body mass (CDI: 65±11 kg vs. ADP: 65±12 kg, p=0.971) and visceral adipose tissue (CDI: 811±318 cm3 vs. APD: 953±370 cm3, p=0.226) did not differ between groups. Mean BMD Z-scores and hip structural characteristics (section modulus, cortical thickness and BR) did not differ between CDI and APD participants at any site (all p values ≥ 0.175) (Table 1).
Relationship between hormones and BMD
Table 2 shows mean BMD Z-scores and body composition parameters according to fasting OT levels below or above the median (0.983 pg/mL) for all participants. Mean hormone levels (free T4, prolactin and IGF-1) (p≥0.175), number and type of anterior pituitary deficiencies (p≥0.254), etiology of pituitary disease (p=0.417), pituitary surgery (p=0.621), type of surgery (p=0.700) and radiotherapy exposure (p=0.743) did not differ between groups (OT below or above the median). While mean age, BMI, fat mass, and lean body mass did not differ in those with lower (≤0.983 pg/mL) vs. higher fasting OT levels (p≥0.348), mean BMD Z-scores at all sites were lower in those with lower (≤0.983 pg/mL) vs. higher fasting OT levels (p≥0.018). These differences remained significant after Holm-Bonferroni correction. Mean age, BMI, body composition variables and BMD Z-scores did not differ between those with lower (≤1.167 pg/mL) vs. higher (>1.167 pg/mL) 1-h pool of OT levels (p≥0.247) or those with lower (≤0.322 pg/mL) vs. higher (>0.322 pg/mL) fasting baseline VP levels (p≥0.583) based on median cutoffs.
Table 2:
Mean BMD Z-scores and HSA characteristics above compared to below the median fasting baseline oxytocin and vasopressin levels across all study participants
| Lower OT (N=19) (OT≤0.983 pg/mL) |
Higher OT (N=18) (OT>0.983 pg/mL) |
P | Lower VP (N=19) (VP≤0.322 pg/mL) |
Higher VP (N=18) (VP>0.322 pg/mL) |
P | |
|---|---|---|---|---|---|---|
| Bone mineral density | ||||||
| PA lumbar spine BMD Z-score | −0.28±1.44 | 0.52±1.06 | 0.018* | 0.06±1.38 | 0.14±1.29 | 0.855 |
| Total hip BMD Z-score | 0.17±1.03 | 0.99±0.91 | 0.016* | 0.56±1.23 | 0.59±0.86 | 0.942 |
| Femoral neck BMD Z-score | −0.28±0.80 | 0.51±0.89 | 0.008* | 0.2211.11 | −0.01±0.71 | 0.456 |
| Distal 1/3 radius BMD Z-score | −0.38±1.06 | 0.59±1.06 | 0.009* | 0.12±1.28 | 0.06±1.04 | 0.884 |
| Subtotal body BMD Z-score | −0.91±0.78 | −0.17±0.87 | 0.010* | −0.45±1.06 | −0.66±0.71 | 0.489 |
| Hip structural analysis variables | ||||||
| NN cortical thickness (cm) | 0.19±0.03 | 0.21±0.03 | 0.058 | 0.20±0.04 | 0.19±0.02 | 0.299 |
| NN section modulus (cm3) | 1.90±0.29 | 2.19±0.43 | 0.024 | 2.15±0.45 | 1.92±0.29 | 0.232 |
| NN buckling ratio | 11.3±3.09 | 9.84±1.98 | 0.121 | 10.46±3.15 | 10.72±2.15 | 0.778 |
| IT cortical thickness (cm) | 0.43±0.06 | 0.51±0.09 | 0.003* | 0.48±0.11 | 0.46±0.05 | 0.505 |
| IT section modulus (cm3) | 5.47±1.29 | 6.52±1.37 | 0.022* | 6.00±1.67 | 5.95±1.13 | 0.926 |
| IT buckling ratio | 8.01±1.48 | 6.66±1.41 | 0.008* | 7.37±1.92 | 7.32±1.17 | 0.913 |
| FS cortical thickness (cm) | 0.68±0.13 | 0.82±0.17 | 0.009* | 0.76±0.20 | 0.73±0.12 | 0.680 |
| FS section modulus (cm3) | 3.02±0.63 | 3.29±0.66 | 0.078 | 3.14±0.68 | 3.17±0.63 | 0.877 |
| FS buckling ratio | 2.54±0.57 | 2.11±0.53 | 0.032 | 2.34±0.68 | 2.3110.48 | 0.919 |
significant after Holm-Bonferroni correction.
Abbreviations: BMD, bone mineral density; HSA, hip structural analysis; OT, oxytocin; VP, vasopressin; PA, posteroanterior; NN, narrow neck; IT, intertrochanteric region; FS, femoral shaft.
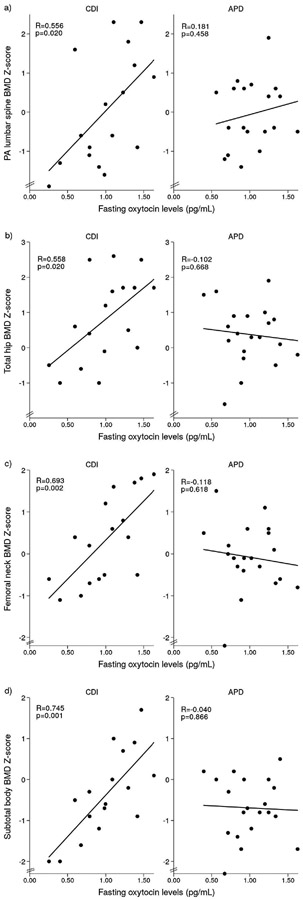
Table 3 shows the relationships between fasting OT levels and BMD Z-scores in participants with CDI. There were robust positive associations between fasting OT and BMD Z-scores at the PA lumbar spine, femoral neck, distal 1/3 radius and subtotal body in CDI participants (p≤0.025) (Figure 1). All associations, except at the distal radius, remained significant after Holm-Bonferroni correction. In contrast to participants with CDI, in those with APD, there were no significant relationships between fasting OT levels and BMD Z-scores at any site (p≥0.487) (Figure 1). There were no significant associations between 1-h pool of OT, VP or serum sodium levels and BMD Z-scores at any site in either CDI or APD participants (p≥0.226).
Table 3.
Associations between fasting oxytocin and vasopressin levels and BMD and HSA characteristics in CDI and APD participants.
| Oxytocin | Vasopressin | |||||||
|---|---|---|---|---|---|---|---|---|
| CDI participants (N=17) |
APD participants (N=20) |
CDI participants (N=17) |
APD participants (N=20) |
|||||
| R | P | R | P | R | P | R | P | |
| Bone mineral density | ||||||||
| PA lumbar spine BMD Z-score | 0.556 | 0.020* | 0.181 | 0.458 | −0.153 | 0.555 | 0.168 | 0.478 |
| Total hip BMD Z-score | 0.558 | 0.020* | −0.102 | 0.668 | 0.193 | 0.457 | 0.037 | 0.874 |
| Femoral neck BMD Z-score | 0.693 | 0.002* | −0.118 | 0.618 | −0.233 | 0.368 | −0.074 | 0.758 |
| Distal 1/3 radius BMD Z-score | 0.540 | 0.025 | 0.112 | 0.638 | −0.031 | 0.905 | −0.377 | 0.101 |
| Subtotal body BMD Z-score | 0.745 | 0.001* | −0.040 | 0.866 | −0.189 | 0.468 | 0.113 | 0.634 |
| Hip structural analysis variables | ||||||||
| NN cortical thickness (cm) | 0.547 | 0.023 | −0.177 | 0.455 | −0.174 | 0.503 | −0.169 | 0.474 |
| NN section modulus (cm3) | 0.523 | 0.031 | 0.088 | 0.712 | −0.458 | 0.068 | −0.199 | 0.400 |
| NN buckling ratio | −0.448 | 0.071 | 0.078 | 0.744 | −0.159 | 0.540 | 0.197 | 0.405 |
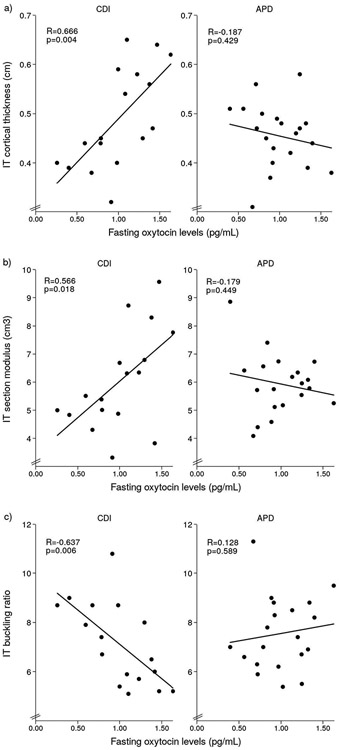
| IT cortical thickness (cm) | 0.666 | 0.004* | −0.187 | 0.429 | −0.017 | 0.947 | 0.117 | 0.623 |
| IT section modulus (cm3) | 0.566 | 0.018* | −0.179 | 0.449 | −0.155 | 0.551 | 0.167 | 0.480 |
| IT buckling ratio | −0.637 | 0.006* | 0.128 | 0.589 | −0.196 | 0.451 | −0.051 | 0.830 |
| FS cortical thickness (cm) | 0.510 | 0.036 | −0.015 | 0.951 | 0.131 | 0.615 | 0.220 | 0.351 |
| FS section modulus (cm3) | 0.373 | 0.140 | −0.139 | 0.560 | −0.242 | 0.286 | 0.145 | 0.541 |
| FS buckling ratio | −0.400 | 0.111 | 0.029 | 0.904 | −0.339 | 0.182 | −0.228 | 0.333 |
R, Pearson coefficient; P, P value
significant after Holm-Bonferroni correction.
Abbreviations: BMD, bone mineral density; CDI, central diabetes insipidus; PA, posteroanterior; NN, narrow neck; IT, intertrochanteric region; FS, femoral shaft.
Figure 1.
Significant linear relationships between fasting OT levels and BMD Z-scores at the a) PA lumbar spine, b) total hip, c) femoral neck and d) subtotal body in CDI participants. No significant relationships were found in APD participants.
To evaluate the impact of fasting OT on variability in BMD Z-scores at different sites in participants with CDI, we performed stepwise regression models. When fasting OT was entered into the model with lean body mass and IGF-1 levels, key determinants of BMD [40, 41], fasting OT accounted for 20-45% and lean body mass for 18-40% of the variance in BMD Z-scores at all sites (p<0.05), while IGF-1 was not a significant contributor to variability at any site.
Relationship between hormones and HSA variables
Table 2 shows mean HSA variables in all participants according to fasting OT levels below or above the median. Mean IT and FS cortical thickness (cm) and NN and IT section modulus (cm3) were lower in those participants with lower (≤0.983 pg/mL) vs. higher (> 0.983 pg/mL) fasting OT levels (p≤0.024); while, mean IT and FS BR were higher in those with fasting OT below vs. above the median (p≤0.032). After Holm-Bonferroni correction, the differences between participants with OT levels below vs. above the median in all IT characteristics and FS cortical thickness remained significant. Mean HSA variables did not differ in those with lower (≤1.167 pg/mL) vs. higher (>1.167 pg/mL) 1-h pool of OT levels or lower (≤0.322 pg/mL) vs. higher (>0.322 pg/mL) fasting VP levels (p≥0.232) based on median cutoffs.
Table 3 shows the relationships between fasting OT levels and HSA variables in CDI and APD. There were positive associations between fasting OT and cortical thickness at all sites and both NN and IT section modulus (p≤0.036) and a negative relationship between fasting OT and IT BR in CDI participants (p=0.006). After Holm-Bonferroni correction the relationships between fasting OT and IT sites remained significant. In contrast to participants with CDI, in those with APD there were no significant relationships between fasting OT and HSA variables (p≥0.145) (Figure 2). There were no significant associations between 1-h pool of OT, VP or serum sodium levels and HSA variables in either CDI or APD participants (p≥0.173).
Figure 2.
Significant linear relationships between fasting OT levels and IT HSA characteristics, including a) cortical thickness, b) section modulus and c) buckling ratio in CDI participants. No significant relationships were found in APD participants.
When fasting OT was entered into stepwise regression models with lean body mass and IGF-1 in participants with CDI, fasting OT and lean body mass, respectively, accounted for 34% and 24% of the variability in IT cortical thickness (p=0.003 and p=0.001, respectively); 22% and 47% of the variability in IT section modulus (p=0.004 and p<0.001, respectively); and 34% and 8% of the variability in IT BR (p=0.004 and p=0.103, respectively), while IGF-1 was not a significant contributor to variability at any site.
All analyses were repeated after excluding the four participants with a history of Cushing’s disease (one CDI and three APD participants), all of whom were in remission at the time of the study. After excluding the four participants with Cushing’s from analyses, all between group differences in bone variables and relationships between OT levels and bone variables remained significant other than the difference between IT section modulus in participants with OT levels below vs. above the median (p=0.08).
Discussion/Conclusion
To the best of our knowledge, this is the first study to evaluate the relationship between OT and VP levels and bone characteristics in men with hypopituitarism. We demonstrate that individuals with hypopituitarism and lower fasting OT levels have lower BMD and less favorable hip geometry than those with higher OT levels. Further, in patients with CDI, lower fasting OT levels are associated with lower BMD Z-scores at multiple sites and inferior hip geometry and strength. In multivariate regression models, OT levels accounted for at least as much of the variance in DXA and HSA variables as lean body mass, a key predictor of BMD [41, 42]. Together these data suggest that low OT in patients with hypopituitarism and CDI may have negative effects on bone. In addition, these findings give further support to the clinical relevance of an OT deficient-state in patients with hypopituitarism and posterior pituitary dysfunction.
Specifically, our data showed lower BMD Z-scores at all sites, and lower IT and FS cortical thickness and NN and IT section modulus in patients with lower fasting OT levels (below median) compared to those with higher OT levels. In contrast, mean BR, an estimate of femoral strength that has been associated with fracture risk [33], was higher at IT and FS sites in patients with lower fasting OT levels (below median) compared to those with higher OT levels. Furthermore, we showed that fasting OT levels were positively correlated with BMD Z-scores at the PA lumbar spine, femoral neck, total hip and subtotal body, as well as all hip structure IT variables in men with CDI but not with APD only.
Preclinical studies have shown that OT is anabolic to bone. The positive associations between OT levels and BMD and favorable HSA characteristics reported in this study, although novel in hypopituitarism, are consistent with data in other populations. Fasting serum OT levels have been found to be lower in postmenopausal women compared to those without osteoporosis, independent of other factors that may impair bone status (age, BMI, history of fractures, smoking, steroid use, hormone replacement therapy, leptin or estradiol) [17]. Further, the OPUS study of 1097 postmenopausal women showed a significant positive correlation between serum OT levels and total hip BMD in individuals with undetectable serum estradiol levels, even after adjustment for confounding factors [20]. In another study, young female athletes with amenorrhea and estrogen deficiency had lower serum levels of nocturnal OT than controls, associated with lower BMD at the lumbar spine and impaired bone microarchitecture at the distal tibia and radius [18, 43]. Across the weight spectrum in women, nocturnal OT levels have been shown to be positively associated with BMD Z-scores at the spine and hip and favorable hip geometry [21]. While low OT levels are consistently associated with less favorable bone characteristics in females, there are few data on OT levels and bone in males. The MINOS study, which included 552 men, showed a weak negative association between fasting serum OT levels and fracture risk [44]. To our knowledge, our study is the first to demonstrate a relationship between peripheral OT levels and BMD or HSA in men.
Multiple studies have assessed bone homeostasis in patients with hypopituitarism, and skeletal fragility is a common complication despite adequate hormone replacement therapy [7]. Only one prior study examined BMD in patients with CDI vs. APD [3]. Consistent with their findings of no between-group differences in BMD at the lumbar spine [3], we found no significant differences in BMD at any site or in hip geometry and strength in CDI vs. APD. It is possible that OT has protective effects on bone homeostasis and may explain similar BMD between APD and CDI despite low levels of catabolic VP. Importantly, all participants were on stable and appropriate hormone replacement. Lower doses of hydrocortisone (≤ 20 mg) are associated with better long-term bone health than higher doses [45], and the mean hydrocortisone daily-equivalent dose of our participants was relatively low at 16 mg/d. Further, most of the patients with GH deficiency were receiving GH replacement. Despite this complex endocrine milieu, we found strong and robust associations between OT and bone variables at most sites, suggesting that, similar to preclinical studies [16], OT might have a key role in bone homeostasis in patients with hypopituitarism and CDI.
In the present study, we report significant correlations between a single fasting timepoint but not 1h-pool of plasma OT levels and bone characteristics in men with CDI, suggesting that basal OT may be more relevant to bone metabolism than pulsatile secretion captured in the 1h-pool. We demonstrated similar baseline (single timepoint) plasma OT levels but lower 1 h pooled plasma OT levels in patients with CDI compared to APD indicative of an overall relative OT deficient state in CDI [30]. These two measures of OT were not correlated (data not shown), and therefore may be different indicators of OT release (e.g., basal vs. pulsatile). Further research using deconvolution analyses to determine OT secretory patterns will be important in further evaluating the relationship between OT and bone. Despite the small number of participants and similar DXA and HSA characteristics between CDI and APD participants in this study, correlations between OT and bone were only significant in CDI patients. These data suggest that lower OT levels may have negative effects on bone in the context of VP deficiency and it is consistent with animal data showing interaction between OT and VP signaling in bone homeostasis [22]. In addition, a relative OT deficiency in CDI patients may impact bone despite appropriate hormone replacement therapy.
Preclinical data indicate that VP is catabolic to bone [23, 24, 15, 28], but the effects of VP on bone metabolism in humans are still unclear. We did not observe significant relationships between VP and bone characteristics in men with hypopituitarism across the sample or by subgroup (CDI or APD). In contrast, we found significant and strong relationships between OT and bone characteristics in patients with CDI. One possible explanation for these divergent results is that OT plays a more important role in bone homeostasis than VP. Alternatively, the suppressed levels and lack of variability in VP, particularly in patients with CDI, may have masked a relationship between VP and bone in these patients. If VP is catabolic to bone in humans as reported in animal models, we might expect that low VP secretion would prevent bone loss in patients with CDI. This could explain why BMD and HSA-derived variables did not differ in men with CDI vs. APD, despite evidence for an OT deficiency in the former group. While patients with CDI were replaced with desmopressin, this drug acts primarily at AVPR2 on the kidney and has been shown to have no effects on bone [46]. Along these lines, desmopressin doses were not associated with bone characteristics in our study. Since chronic hyponatremia may also contribute to bone loss via mobilization of sodium from bone [23-25, 27], we assessed for correlations between sodium levels and bone variables and found no significant relationships. This might be expected as sodium levels were predominantly within the normal range. Larger studies are needed to define the interaction between OT and VP with bone characteristics in human physiology and specifically in patients with hypothalamic-posterior pituitary hormone deficiencies.
Although this is the first report showing associations between OT and bone in hypopituitarism, our study is not without limitations. This was a cross-sectional study and therefore causality cannot be determined. OT circulates at low levels in humans close to the limit of detection. We used extracted RIA, with negligible cross-reactivity with VP and desmopressin, for the measurement of OT in our study, allowing for the lowest limit of detection currently available. Given the rarity of CDI, our sample size was relatively small, and the types of pituitary diseases included were heterogeneous. Nevertheless, we observed differences in BMD according to OT levels and strong relationships between OT and bone characteristics. We ensured that participants with Cushing’s disease or acromegaly were characterized by long-term biochemical control in order to minimize effects of pituitary hyperfunction on BMD. Recovery of BMD has been reported after remission of hypercortisolism, and BMD assessed by DXA is not typically altered in acromegaly [7]. Importantly, all study participants were on appropriate and stable testosterone replacement. Since estradiol impacts OT levels and receptor distribution, we studied men only in this first investigation [47-50]. OT has sex specific effects, and future studies in women with hypopituitarism will be important.
We have demonstrated that low OT levels are associated with lower BMD and less favorable hip geometry, in particular in patients with CDI, who are at higher risk for OT deficiency. These data support the presence of a clinically relevant OT-deficient state in patients with hypopituitarism and CDI. Whether exogenous OT could be used therapeutically to prevent or improve bone loss in patients with hypopituitarism is an important area for future investigation.
Acknowledgement
We thank the Massachusetts General Hospital Neuroendocrine and Pituitary Tumor Clinical Center staff, the Translational and Clinical Research Center staff, and study participants for their assistance in successfully completing this study
Funding Sources
The project described was supported by Grant Numbers 1UL1TR002541-01, 1UL1TR001102-01, 8 UL1 TR000170-05, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science and a Clinical Research Grant from Catalan Society of Endocrinology. AA is supported by a grant from Fundación Alfonso Martín Escudero. Funding sources had no role in the design of the study, data analysis, or writing of the manuscript.
Footnotes
Statement of Ethics
The study was approved by the Institutional Review Board of Partners Healthcare and informed consent was obtained from all participants
Disclosure Statement
EAL has a financial interest in OXT Therapeutics, a company developing an intranasal oxytocin and long-acting analogs of oxytocin to treat obesity and metabolic disease. EAL’s interests were reviewed and are managed by Massachusetts General Hospital and Partners Healthcare in accordance with their conflict of interest policies. Other authors have no financial interests to declare.
References
- 1.Degerblad M, Bengtsson BA, Bramnert M, Johnell O, Manhem P, Rosen T, et al. Reduced bone mineral density in adults with growth hormone (GH) deficiency: increased bone turnover during 12 months of GH substitution therapy. Eur J Endocrinol. 1995August;133(2):180–8. [DOI] [PubMed] [Google Scholar]
- 2.Rosen T, Wilhelmsen L, Landin-Wilhelmsen K, Lappas G, Bengtsson BA. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol. 1997September;137(3):240–5. [DOI] [PubMed] [Google Scholar]
- 3.Wuster C, Abs R, Bengtsson BA, Bennmarker H, Feldt-Rasmussen U, Hernberg-Stahl E, et al. The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J Bone Miner Res. 2001February;16(2):398–405. [DOI] [PubMed] [Google Scholar]
- 4.Johannsson G, Sunnerhagen KS, Svensson J. Baseline characteristics and the effects of two years of growth hormone replacement therapy in adults with growth hormone deficiency previously treated for Cushing's disease. Clin Endocrinol (Oxf). 2004May;60(5):550–9. [DOI] [PubMed] [Google Scholar]
- 5.Hoybye C, Ragnarsson O, Jonsson PJ, Koltowska-Haggstrom M, Trainer P, Feldt-Rasmussen U, et al. Clinical features of GH deficiency and effects of 3 years of GH replacement in adults with controlled Cushing's disease. Eur J Endocrinol. 2010April;162(4):677–84. [DOI] [PubMed] [Google Scholar]
- 6.van Varsseveld NC, van Bunderen CC, Franken AA, Koppeschaar HP, van der Lely AJ, Drent ML. Fractures in pituitary adenoma patients from the Dutch National Registry of Growth Hormone Treatment in Adults. Pituitary. 2016August;19(4):381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazziotti G, Frara S, Giustina A. Pituitary Diseases and Bone. Endocr Rev. 2018August1;39(4):440–88. [DOI] [PubMed] [Google Scholar]
- 8.Colao A, Di Somma C, Pivonello R, Loche S, Aimaretti G, Cerbone G, et al. Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab. 1999June;84(6):1919–24. [DOI] [PubMed] [Google Scholar]
- 9.Tritos NA. Focus on growth hormone deficiency and bone in adults. Best practice & research Clinical endocrinology & metabolism. 2017February;31(1):49–57. [DOI] [PubMed] [Google Scholar]
- 10.Drake WM, Rodriguez-Arnao J, Weaver JU, James IT, Coyte D, Spector TD, et al. The influence of gender on the short and long-term effects of growth hormone replacement on bone metabolism and bone mineral density in hypopituitary adults: a 5-year study. Clin Endocrinol (Oxf). 2001April;54(4):525–32. [DOI] [PubMed] [Google Scholar]
- 11.Elbornsson M, Gotherstrom G, Bosaeus I, Bengtsson BA, Johannsson G, Svensson J. Fifteen years of GH replacement improves body composition and cardiovascular risk factors. Eur J Endocrinol. 2013May;168(5):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barake M, Klibanski A, Tritos NA. Effects of recombinant human growth hormone therapy on bone mineral density in adults with growth hormone deficiency: a meta-analysis. J Clin Endocrinol Metab. 2014March;99(3):852–60. [DOI] [PubMed] [Google Scholar]
- 13.Copland JA, Ives KL, Simmons DJ, Soloff MS. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology. 1999September;140(9):4371–4. [DOI] [PubMed] [Google Scholar]
- 14.Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochem Biophys Res Commun. 2002September27;297(3):442–5. [DOI] [PubMed] [Google Scholar]
- 15.Tamma R, Sun L, Cuscito C, Lu P, Corcelli M, Li J, et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc Natl Acad Sci U S A. 2013November12;110(46):18644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci U S A. 2009April28;106(17):7149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breuil V, Amri EZ, Panaia-Ferrari P, Testa J, Elabd C, Albert-Sabonnadiere C, et al. Oxytocin and bone remodelling: relationships with neuropituitary hormones, bone status and body composition. Joint Bone Spine. 2011December;78(6):611–5. [DOI] [PubMed] [Google Scholar]
- 18.Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, et al. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry. 2011November;72(11):1546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson EA, Ackerman KE, Estella NM, Guereca G, Pierce L, Sluss PM, et al. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur J Endocrinol. 2013March;168(3):457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breuil V, Panaia-Ferrari P, Fontas E, Roux C, Kolta S, Eastell R, et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: analysis of the OPUS cohort. J Clin Endocrinol Metab. 2014April;99(4):E634–41. [DOI] [PubMed] [Google Scholar]
- 21.Schorr M, Marengi DA, Pulumo RL, Yu E, Eddy KT, Klibanski A, et al. Oxytocin and Its Relationship to Body Composition, Bone Mineral Density, and Hip Geometry Across the Weight Spectrum. J Clin Endocrinol Metab. 2017August01;102(8):2814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Tamma R, Yuen T, Colaianni G, Ji Y, Cuscito C, et al. Functions of vasopressin and oxytocin in bone mass regulation. Proc Natl Acad Sci U S A. 2016January5;113(1):164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010March;25(3):554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2011March25;286(12):10864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sejling AS, Pedersen-Bjergaard U, Eiken P. Syndrome of inappropriate ADH secretion and severe osteoporosis. J Clin Endocrinol Metab. 2012December;97(12):4306–10. [DOI] [PubMed] [Google Scholar]
- 26.Sejling AS, Thorsteinsson AL, Pedersen-Bjergaard U, Eiken P. Recovery from SIADH-associated osteoporosis: a case report. J Clin Endocrinol Metab. 2014October;99(10):3527–30. [DOI] [PubMed] [Google Scholar]
- 27.Usala RL, Fernandez SJ, Mete M, Cowen L, Shara NM, Barsony J, et al. Hyponatremia Is Associated With Increased Osteoporosis and Bone Fractures in a Large US Health System Population. J Clin Endocrinol Metab. 2015August;100(8):3021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negri AL, Ayus JC. Hyponatremia and bone disease. Rev Endocr Metab Disord. 2017March;18(1):67–78. [DOI] [PubMed] [Google Scholar]
- 29.Murthy K, Ondrey GJ, Malkani N, Raman G, Hodge MB, Marcantonio AJ, et al. The Effects of Hyponatremia on Bone Density and Fractures: A Systematic Review and Meta-Analysis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2019April;25(4):366–78. [DOI] [PubMed] [Google Scholar]
- 30.Aulinas A, Plessow F, Asanza E, Silva L, Marengi DA, Fan W, et al. Low Plasma Oxytocin Levels and Increased Psychopathology in Hypopituitary Men with Diabetes Insipidus. J Clin Endocrinol Metab. 2019March18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990June;51(6):1106–12. [DOI] [PubMed] [Google Scholar]
- 32.Ramamurthi K, Ahmad O, Engelke K, Taylor RH, Zhu K, Gustafsson S, et al. An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT. Osteoporos Int. 2012February;23(2):543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melton LJ 3rd, Beck TJ, Amin S, Khosla S, Achenbach SJ, Oberg AL, et al. Contributions of bone density and structure to fracture risk assessment in men and women. Osteoporos Int. 2005May;16(5):460–7. [DOI] [PubMed] [Google Scholar]
- 34.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008December;23(12):1892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amico JA, Ervin MG, Leake RD, Fisher DA, Finn FM, Robinson AG. A novel oxytocin-like and vasotocin-like peptide in human plasma after administration of estrogen. J Clin Endocrinol Metab. 1985January;60(1):5–12. [DOI] [PubMed] [Google Scholar]
- 36.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. Am J Physiol. 1986February;250(2 Pt 2):R267–75. [DOI] [PubMed] [Google Scholar]
- 37.Hew-Butler T, Hoffman MD, Stuempfle KJ, Rogers IR, Morgenthaler NG, Verbalis JG. Changes in copeptin and bioactive vasopressin in runners with and without hyponatremia. Clin J Sport Med. 2011May;21(3):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999October;84(10):3666–72. [DOI] [PubMed] [Google Scholar]
- 39.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. The American journal of gastroenterology. 2018February;113(2):175–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachmann KN, Fazeli PK, Lawson EA, Russell BM, Riccio AD, Meenaghan E, et al. Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. J Clin Endocrinol Metab. 2014December;99(12):4664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho-Pham LT, Nguyen UD, Nguyen TV. Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab. 2014January;99(1):30–8. [DOI] [PubMed] [Google Scholar]
- 42.Yakar S, Werner H, Rosen CJ. Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol. 2018July;61(1):T115–T37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawson EA, Holsen LM, Santin M, DeSanti R, Meenaghan E, Eddy KT, et al. Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. J Clin Psychiatry. 2013May;74(5):e451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breuil V, Fontas E, Chapurlat R, Panaia-Ferrari P, Yahia HB, Faure S, et al. Oxytocin and bone status in men: analysis of the MINOS cohort. Osteoporos Int. 2015December;26(12):2877–82. [DOI] [PubMed] [Google Scholar]
- 45.Behan LA, Kelleher G, Hannon MJ, Brady JJ, Rogers B, Tormey W, et al. Low-dose hydrocortisone replacement therapy is associated with improved bone remodelling balance in hypopituitary male patients. Eur J Endocrinol. 2014January;170(1):141–50. [DOI] [PubMed] [Google Scholar]
- 46.Pivonello R, Colao A, Di Somma C, Facciolli G, Klain M, Faggiano A, et al. Impairment of bone status in patients with central diabetes insipidus. J Clin Endocrinol Metab. 1998July;83(7):2275–80. [DOI] [PubMed] [Google Scholar]
- 47.Amico JA, Seif SM, Robinson AG. Oxytocin in human plasma: correlation with neurophysin and stimulation with estrogen. J Clin Endocrinol Metab. 1981May;52(5):988–93. [DOI] [PubMed] [Google Scholar]
- 48.Stock S, Bremme K, Uvnas-Moberg K. Plasma levels of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Hum Reprod. 1991September;6(8):1056–62. [DOI] [PubMed] [Google Scholar]
- 49.Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998March30;9(5):933–6. [DOI] [PubMed] [Google Scholar]
- 50.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001April;81(2):629–83. [DOI] [PubMed] [Google Scholar]