Abstract
Background
Diabetic ketoacidosis (DKA) is a potentially life-threatening metabolic disorder that can occur with manifestation of type 1 diabetes mellitus (T1D). The aim of this study was to analyze the incidence of DKA at the time of the diagnosis of T1D in childhood and adolescence, the risk factors, and regional approaches to reduce the incidence of ketoacidosis.
Methods
We investigated the proportion of patients under 18 years of age with DKA (defined as pH <7.3, severe DKA pH <7.1) at the manifestation of T1D in Germany in the period 2000–2019, based on data from the German–Austrian registry of diabetes (Diabetes-Patienten-Verlaufsdokumentation, DPV). The influence of the following factors was evaluated: year of manifestation, age, sex, family history of migration (MiH), and distance from the hospital. Moreover, data from the region with and the region without a pilot screening project from 2015 onwards were compared.
Results
Of the 41 189 patients with manifestation of T1D, 19.8% presented with DKA (n = 8154, slight increase [p <0.001] over the study period) and 6.1% (n = 2513) had severe DKA. Children under 6 years of age had DKA more often than adolescents (12–17 years) (21.7% versus 18.6%, OR 1.22 95% CI: [1.14; 1.30]). Girls had a higher rate of DKA than boys (20.5% versus 19.2%, OR 1.10 [1.03; 1.14]), and patients with MiH were more likely to have DKA than those without MiH (21.4% versus 18.2%, OR 1.40 [1.32; 1.48]). In the region with a pilot screening project, the DKA rate stayed the same, at 20.6%, while in the control region the rate was 22.7% with a decreasing tendency.
Conclusion
The frequency of DKA at the time of diagnosis of T1D did not decrease between 2000 and 2019 and increased towards the end of the observation period. Children with MiH, children under 6, and girls were at a higher risk of DKA.
In Germany, an estimated 32 000 children and adolescents under 20 years and under have insulin-dependent type 1 diabetes mellitus (T1D) (1, 2), that is one child in 500. Each year, over 3000 children and adolescents aged under 15 years—about 23 in 100 000—are newly diagnosed with T1D (2, 3). This number of newly diagnosed T1D cases is increasing at a rate of 3% to 4% per year (2, 3). If the disease is not diagnosed at the time the first signs and symptoms of diabetes (polyuria, polydipsia, fatigue, weight loss) manifest, there is a risk that the young patient develops diabetic ketoacidosis (DKA) (4) which may lead to cerebral edema (5, 6) and, in rare cases, to death (7, 8). Mid- and long-term risks include an unfavorable course of the disease with a shortened period of remission and poor metabolic control (9, 10) as well as permanent intellectual and cognitive impairments (11). Consequently, early diagnosis of a manifestation of diabetes is crucial to prevent the development of DKA. Given the continuous rise in the incidence of T1D over the past years, increased awareness of the disease would be expected, leading to early diagnosis. This assumption is supported by the fact that DKA rates are lower in countries with a high incidence of diabetes compared to countries with a low incidence (12). Thus, awareness campaigns and screening programs (13) could help diagnose T1D at an early stage and thus prevent the development of DKA.
Research questions
What is the proportion of children and adolescents presenting with ketoacidosis at the manifestation of T1D? Has this proportion decreased over the last two decades? What are the risk factors, if any, predisposing to ketoacidosis at manifestation of T1D? Is there evidence that the incidence of DKA is reduced by a diabetes screening program? Our study aimed to address these questions.
Methods
Patient data
Since 1995, the German–Austrian registry of diabetes (Diabetes-Patienten-Verlaufsdokumentation, DPV) has continuously been collecting data of children with diabetes in Germany, Austria, Switzerland, and Luxembourg (14). Data of a large proportion of children with diabetes are now being collected in Germany: currently, more than 25 000 patients (with an estimated overall prevalence of 32 000).
This study analyzed DPV registry data documented by 202 healthcare providers in Germany that treated children newly diagnosed with T1D between 2000 and 2019. Of a total of 41,331 datasets, 41,189 were included in the analysis; 142 (0.34%) datasets were excluded because the reporting institutions did not provide information about the pH value at the time of manifestation.
For the diagnosis of DKA at the time of first diagnosis of diabetes mellitus, pH values (venous/capillary) below 7.3 or bicarbonate levels <15 mmol/L were required. Severe DKA was defined as pH values below 7.1 (2).
Potential risk factors
In order to identify potential risk factors for the development of DKA, the following data were collected:
Year of manifestation
Age at the time of first diagnosis
Sex
Family history of migration (MiH) (patient or at least one parent not born in Germany)
Distance from the hospital providing initial treatment (linear distance, calculated based on the postcodes of the patient’s place of residence and the hospital)
Regional approaches
Additional individual analyses were performed for the states of Bavaria and Baden-Wuerttemberg, because an antibody screening study focusing on small children aged 2 to 5 years (Fr1da study [15]) has been underway in Bavaria since 2015 and an awareness program was conducted in parts of Baden-Wuerttemberg between 2015 and 2017 [16]).
The Ethics Committee of the University of Ulm and the data protection supervisors of the participating institutions approved the analysis of anonymized data from the DPV registry.
Statistical analysis
All data analyses were performed using the statistical software package SAS, version 9.4 (TS1M5, SAS Institute Inc., Cary, NC, USA). Changes in the proportion of DKA over the years of treatment were depicted linearly in the figures to visualize the time trend. The effects of potential risk factors were assessed using logistic regression analysis with the following fixed effects: year of manifestation (linear), sex, age group (three categories), and family history of migration (estimator: maximum likelihood). The frequency of DKA is reported in percent and the results of group comparisons are quoted as odds ratios with 95% confidence intervals. Given the large number of cases, a p-value of <0.01 was considered statistically significant.
Results
Diabetes manifestation with ketoacidosis in Germany: incidence and trend over time
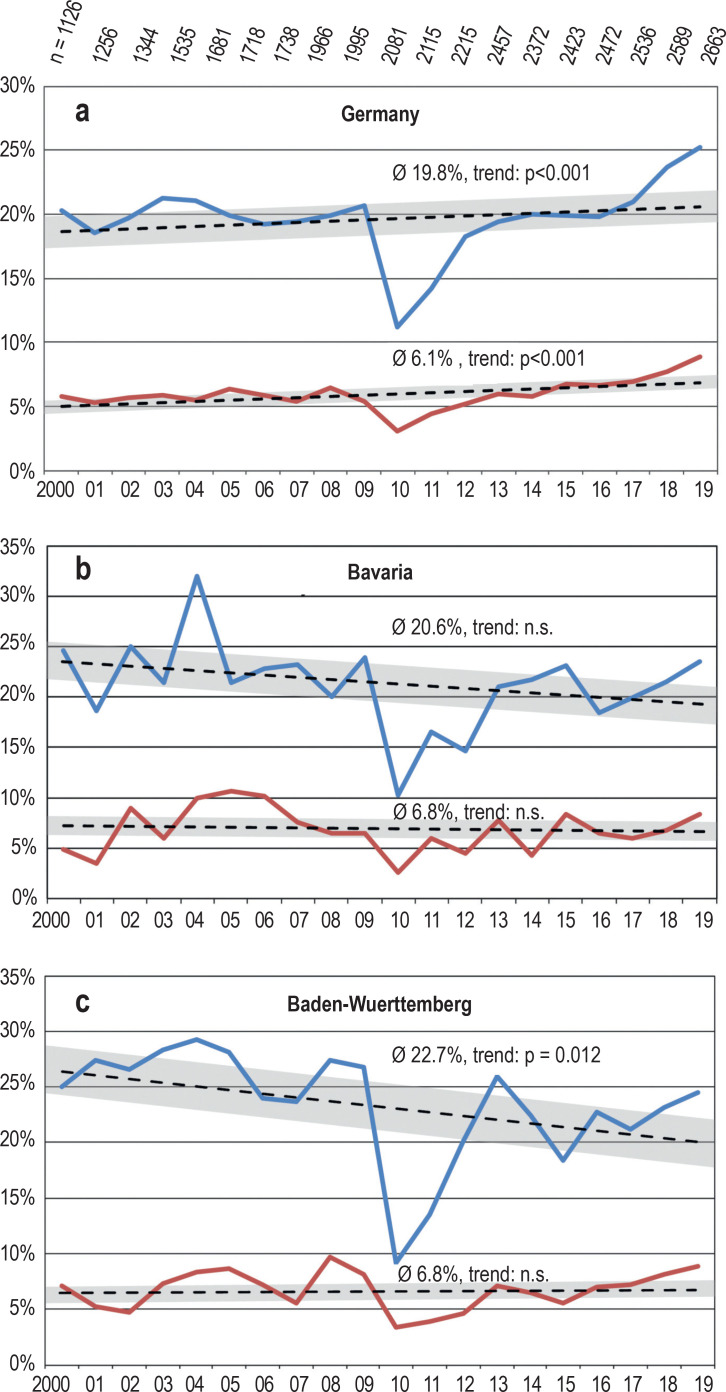
In the period from 2000 to 2019, the proportion of DKA at manifestation of T1D in childhood and adolescence was 19.8% in Germany. Up to 2016, this proportion remained essentially unchanged; from 2017 onwards, an increase was noted (Figure 1a). Across Germany, 6.1% of the newly diagnosed patients presented with severe DKA. This proportion has recently increased slightly, but statistically significantly (Figure 1a). Eight patients were documented as deceased.
Figure 1.
Proportion (%) of patients with diabetic ketoacidosis (pH<7.3, blue line) and severe ketoacidosis (pH<7.1, red line) at manifestation of type 1 diabetes mellitus in the period 2000–2019 (a) in Germany (small numbers: number of documented manifestations in each year), (b) in Bavaria and (c) Baden-Wuerttemberg. Linear trend with 95% confidence interval; n.s., non-significant
The year 2010 was an exception in that the incidence rates of ketoacidosis were lower than in the years before and after, both nationwide and in the separately analyzed German states.
Associations with potential risk factors
The patient’s age at the time of manifestation of diabetes had a significant effect on the rate of DKA: Children aged under six years were at a significantly increased risk of developing DKA compared to older children and adolescents (table). Girls had a slightly higher rate of DKA at the time of diabetes manifestation (table) compared to boys.
Table. Age, sex, family history of migration: Factors associated with an increased risk of ketoacidosis (pH<7.3) and severe ketoacidosis (pH<7.1) at manifestation of type 1 diabetes mellitus.
| Age | ||||
| < 6 yrsn = 9 277 | 6–11 yrsn = 16 688 | 12–17 yrsn = 11 835 | Odds ratio (95% CI)<6 yrs vs. 12–17 yrs6–11 yrs vs. 12–17 yrs | |
| pH<7.3 | 21.7 % | 17.9 % | 18.6 % | 1.22 [1.14;1.30] 0.96 [0.91; 1.02] |
| pH<7.1 | 6.9 % | 5.4 % | 5.2 % | 1.37 [1.23; 1.52] 1.03 [0.93; 1.14] |
| Sex | ||||
| Female n = 18 864 | Male n = 22 325 | Odds ratio (95% CI) | ||
| pH<7.3 | 20.5 % | 19.2 % | 0.91 [0.88; 0.97] | |
| Family history of migration | ||||
| no MiHn = 35 668 | with MiHn = 8 521 | Odds ratio (95% CI) | ||
| pH<7.3 | 18.2 % | 21.4 % | 1.40 [1.32; 1.48] | |
yrs., years of age; CI, confidence interval; MiH, family history of migration; pH, pH value
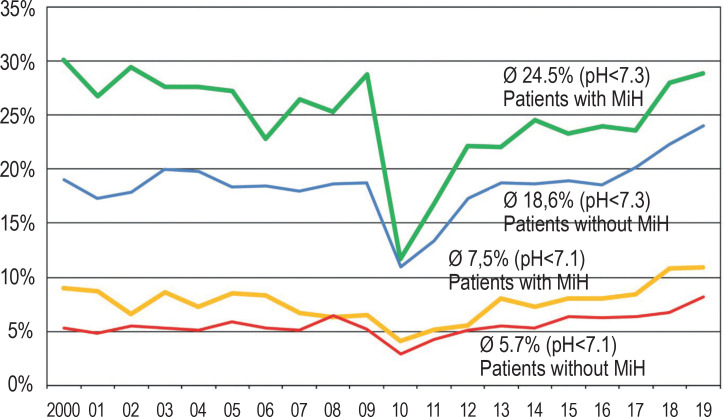
Patients with a family history of migration were significantly more frequently affected by DKA at manifestation compared to children without MiH (Table, Figure 2). During the period from 2000 to 2019, the proportion of patients with MiH increased from 11.9% to 26.2%; the overall rate was 20.7%. The increasing proportion of patients with MiH is not responsible for the slight increase in DKA incidence in Germany: Overall, the DKA rate was slightly lower in patients without MiH, but also showed a slight upward trend towards the end of the observation period (figure 2).
Figure 2.
Proportion (%) of patients with diabetic ketoacidosis with a family history of migration (bold lines, green: pH<7.3; yellow: pH<7.1) in the years 2000–2019. For comparison the proportion (%) of patients without family history of migration (thin lines, blue: pH<7.3; red: pH<7.1)
MiH, family history of migration; pH, pH value
The (linear) distance to the hospital providing the initial treatment was 19.6 km for patients with DKA and 20.2 km for patients without DKA (mean: 20.0 km). No association between the distance from the hospital and the risk of developing ketoacidosis was found.
Special assessment of two German federal states
In Bavaria (antibody-screening project, n = 4726), the overall frequency of DKA between 2000 and 2019 was 20.6%; no relevant change was found during the observation period (p = 0.32; Figure 1b). In Baden-Wuerttemberg (awareness program for a limited period; n = 4944), the overall frequency was 22.7%, with a slightly decreasing trend (p = 0.012; Figure 1c). The incidence of severe DKA was 6.8% in Bayern and in Baden-Wuerttemberg; no statistically relevant change was noted.
Discussion
The incidence of diabetic ketoacidosis in international comparison
In our analysis, the DKA rate at manifestation of T1D in children and adolescents under 18 years of age was with almost 20% similar to the rate of 21.1% in a survey covering the period 1995–2007 (17). Consequently, the rates in Germany are higher than the 16% to 19% found in Sweden (5, 18). This could be explained by the higher incidence and thus higher awareness of the disease in Scandinavia (19), but also by structured care as well as prevention and screening activities (20).
Data from a comparable patient population in Colorado (USA) show a proportion of patients with DKA of more than 50% (21). Recent Italian data indicate DKA rates as high as nearly 50% (22). The highest rate of almost 80% was found in Saudi-Arabia (23).
In respect to the few deaths documented for Germany in the DPV registry, we cannot completely rule out under-reporting.
Development over the years
Contrary to our expectations, the proportion of DKA at manifestation of diabetes has not decreased, but remained constant across Germany for years and has even increased recently.
Age and sex as risk factors
In our study, both young children and girls were found to be at an increased risk of developing DKA at manifestation of diabetes. It is conceivable that the autoimmune reaction underlying the development of T1D progresses more rapidly in affected young children compared to older children and adolescents (24).
Yound children are at a greater risk of developing DKA due to their smaller bodies and because interpreting their clinical signs and symptoms is more challenging.
In a large Finish study population, girls were found to present with greater weight loss, longer diagnosis latency and higher HbA1c levels (24). In Germany, the slight female preponderance was already noted in earlier studies (17, 18), but, despite being statistically significant, this finding should not be over-interpreted: A US study found no effect of gender on the risk of developing DKA (21). All in all, the available evidence on sex as a risk factor is inconclusive (18, 19).
Distance from the hospital
The distance from the hospital providing the initial treatment is not associated with the development of DKA. One should, however, note that the average linear distance is small anyway. Thus, the concern that long transfer times to far-away hospitals could increase the incidence of DKA can be dismissed. In Germany (and Austria), apparently, children with DKA are not presented and treated in a hospital with greater expertise in pediatric diabetology, which may be further away, but in the nearest (children’s) hospital (25). In Colorado (USA), a rural address is significantly associated with the occurrence of DKA, in contrast to urban residential addresses, regardless of the distance from the hospital providing the initial treatment (21).
Family history of migration as a risk factor
Our data analysis revealed an association between MiH and the occurrence of DKA at manifestation of diabetes. The hypothesis that an increasing proportion of patients with MiH concealed a decrease in the proportion of diabetes manifestations with DKA among German patients was rejected.
We can only speculate about the factors underlying the higher incidence of DKA in children and adolescents with MiH: initial symptoms may only be noticed with delay, the language barrier may interfere with taking a detailed medical history, patients may be referred later to medical examination, or patients may find it more difficult to access medical care (26).
As we know from other diseases (e.g. obesity), health risks of children are linked to the socioeconomic status of their families (27, 28). Families with MiH are frequently disadvantaged in this respect. Not surprisingly, children and adolescents with diabetes manifestation and MiH appear to be at a greater health risk.
Significance of health literacy for prevention
Early diagnosis of new cases of diabetes is crucial to prevent serious complications (29). Characteristic symptoms are frequently classed as “abnormal behavior“ and not perceived as being related to a disease (30). Knowledge of initial symptoms is likely to differ in the various groups of the population, similar to health literacy in general which is influenced by age, level of education, literacy, migration background, and socioeconomic status (31). Unfortunately, socioeconomic status data could not be used in this study as they were only incompletely recorded in the registry.
Regional differences, awareness campaigns, screening studies
The trends in the individual German states appear to differ. As examples, the data of Baden-Württemberg and Bavaria were analyzed. While the proportion of DKA slightly declined in Baden-Wuerttemberg, no change was noted in Bavaria during the observation period (figure 1). The proportion of severe DKA was stable at 6.8% in the two states. The background behind such regional differences cannot be elucidated on the basis of the observational data available to us. Here, activities of regional quality circles and patient support groups, continuing medical education events and awareness campaigns, such as the one in Stuttgart (16), may play a role.
In Italy, too, increased awareness resulted in a temporary success (32), but showed no lasting effect (22). In Australia (33), a two-year awareness campaign achieved a reduction in the rate of DKA from 38% to 14%. In Austria, an intensified awareness campaign did not result in a clear reduction in the rate of DKA (34).
Screening programs, such as those currently offered in Germany in the states of Bavaria, Lower Saxony and Saxony, are undertaken with the hope of lowering the rates of DKA. These programs include genetic testing at birth or during the first months postpartum (35, 37), as well as screening for B cell antibodies as part of the public health screening examinations of children (15, 36, 38).
The Fr1da study in Bavaria is an example of this (38). Since 2015, more than 90 000 young children have been included in this study. Of the participants identified as at-risk children, 62 were diagnosed with overt T1D between 2015 and 2019. Of these, only two (3%) showed signs and symptoms of DKA at manifestation of T1D (38). However, no impact on the incidence of DKA in Bavaria has (yet) been noticed. It is conceivable that the number of children included is still too small (38) and the observation period is too short to assess their effectiveness at the population level.
The validity of the data collected
The increase in absolute numbers (figure 1) of children and adolescents with diabetes manifestation in the period from 2000 to 2019 is reflective of both the increase in the incidence of T1D and the more comprehensive data collection by the DPV database. Since about 80% of the pediatric T1D patients (aged 0–20 years) are documented in the DPV registry, our analyses can be considered representative of this patient population.
The results of the DPV data analyses with respect to the rates of DKA at manifestation show variation from year to year. This variation is most pronounced in the year 2010. The anomaly may have been triggered by the 2009 flu season, which led to a change in infection pattern in 2010. (39). A possible explanation would be that T1D can be started by an autoimmune reaction and the clinical manifestation of the disease is frequently triggered by an infection, but the influenza wave may have left a state of heightened immunity. A similar association was observed in 1996 (17).
Conclusions
The rate of diabetes manifestation with ketoacidosis in children and adolescents in Germany which was mostly stable between 2000 and 2019 but showed an increase towards the end of the observation period, is not in line with what is expected of a modern health care system. The reasons for the lack of improvement remain unclear. It is of concern that an already achieved level of quality—that is a rather early diagnosis of diabetes in children and adolescents—cannot be further improved, and perhaps not even maintained.
Although patients with MiH are at significantly higher risk of developing ketoacidosis at the manifestation of diabetes, this does not explain the lack of a decline in the incidence rates of DKA. Thus, physicians/pediatricians as well as caregivers and teachers, should pay special attention to children with MiH feeling unwell to ensure serious illnesses are not missed.
Various approaches are available to achieve an earlier diagnosis of type 1 diabetes, i.e. prior to the development of DKA:
Open-ended awareness campaigns to improve the knowledge about initial symptoms of diabetes manifestation both among healthcare professionals and in the general population
Identification of at-risk individuals with a family history of T1D or an increased genetic risk. However, only some of the individuals who will become T1D patients later in life can be identified using this approach.
Population-based screening for T1D-associated autoantibodies
Special attention to early signs of T1D in patients at an increased risk for T1D (e.g. family history of T1D, autoimmune diseases such as celiac disease).
Conceivably, the manifestation of diabetes with DKA is not solely the consequence of a delayed response to initial symptoms, but, at least in some cases, indication of a special course of the disease with particularly rapid destruction of beta cells, as the higher incidence and often rapid development in young children suggests. As early as 2013, the existence of particularly aggressive disease courses among young children was suspected based on epidemiological evidence (34). Despite this, DKA does not appear to be an inevitable, fateful event, as evidenced by the low rates in Sweden.
In any case, knowledge of the initial signs and symptoms patients present with at manifestation of diabetes is essential in both the general population and the medical community at large as it may help to reduce the incidence of severe ketoacidosis in children and adolescents.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
Prof. Neu received lecture fees and consultancy fees from Novo Nordisk, Roche, Lilly, and Abbott.
Dr. Sindichakis received lecture fees from Nova Biomedical GmbH.
Dr. Dost received lecture fees from RG Gesellschaft für Information und Organisation mbH.
The remaining authors declare no conflict of interest.
References
- 1.Rosenbauer J, Bächle C, Stahl A, for German Pediatric Surveillance Unit DPV Initiative, German Competence Network Diabetes mellitus: Prevalence of type 1 diabetes mellitus in children and adolescents in Germany. Diabetologia. 2012;55 [Google Scholar]
- 2.Ziegler R, Neu A. Diabetes in childhood and adolescence—a guideline-based approach to diagnosis, treatment, and follow-up. Dtsch Arztebl Int. 2018;115:146–156. doi: 10.3238/arztebl.2018.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendas A, Rothe U, Kiess W, et al. Trends in incidence rates during 1999-2008 and prevalence in 2008 of childhood type 1 diabetes mellitus in Germany - model-based national estimates. Obukhov AG (Hrsg.): PLoS One. 2015;10 doi: 10.1371/journal.pone.0132716. e0132716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mencher SR, Frank G, Fishbein J. Diabetic ketoacidosis at onset of type 1 diabetes: rates and risk factors today to 15 years ago. Glob Pediatr Health. 2019;6 doi: 10.1177/2333794X19870394. 2333794X1987039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanas R, Lindgren F, Lindblad B. Diabetic ketoacidosis and cerebral oedema in Sweden - a 2-year paediatric population study. Diabet Med. 2007;24:1080–1085. doi: 10.1111/j.1464-5491.2007.02200.x. [DOI] [PubMed] [Google Scholar]
- 6.Glaser NS, Wootton-Gorges SL, Buonocore MH, et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diabetes. 2006;7:75–80. doi: 10.1111/j.1399-543X.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 7.Black AB, Malins JM. Diabetic ketosis; a comparison of results of orthodox and intensive methods of treatment based on 170 consecutive cases. Lancet Lond Engl. 1949;1:56–59. doi: 10.1016/s0140-6736(49)90385-2. [DOI] [PubMed] [Google Scholar]
- 8.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999;81:318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagl K, Hermann JM, Plamper M, et al. Factors contributing to partial remission in type 1 diabetes: analysis based on the insulin dose-adjusted HbA1c in 3657 children and adolescents from Germany and Austria. Pediatr Diabetes. 2017;18:428–434. doi: 10.1111/pedi.12413. [DOI] [PubMed] [Google Scholar]
- 10.Piccini B, Schwandt A, Jefferies C, et al. Association of diabetic ketoacidosis and HbA1c at onset with year-three HbA1c in children and adolescents with type 1 diabetes: Data from the International SWEET Registry. Pediatr Diabetes. 2020;21:339–348. doi: 10.1111/pedi.12946. [DOI] [PubMed] [Google Scholar]
- 11.Ghetti S, Kuppermann N, Rewers A, et al. Cognitive function following diabetic ketoacidosis in children with new-onset or previously diagnosed type 1 diabetes. Diabetes Care. 2020;43:2768–2775. doi: 10.2337/dc20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55:2878–2894. doi: 10.1007/s00125-012-2690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler C, Schober E, Ziegler A-G, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13:308–313. doi: 10.1111/j.1399-5448.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 14.Hofer SE, Schwandt A, Holl RW, Austrian/German DPV Initiative Standardized documentation in pediatric diabetology: experience from Austria and Germany. J Diabetes Sci Technol. 2016;10:1042–1049. doi: 10.1177/1932296816658057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raab J, Haupt F, Scholz M, et al. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011144. e011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holder M, Ehehalt S. Significant reduction of ketoacidosis at diabetes onset in children and adolescents with type 1 diabetes—The Stuttgart Diabetes Awareness Campaign, Germany. Pediatr Diabetes. 2020;21:1227–1231. doi: 10.1111/pedi.13064. [DOI] [PubMed] [Google Scholar]
- 17.Neu A, Hofer SE, Karges B, et al. Ketoacidosis at diabetes onset is still frequent in children and adolescents: a multicenter analysis of 14,664 patients from 106 institutions. Diabetes Care. 2009;32:1647–1648. doi: 10.2337/dc09-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherubini V, Grimsmann JM, Åkesson K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia. 2020;63:1530–1541. doi: 10.1007/s00125-020-05152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343 doi: 10.1136/bmj.d4092. d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elding Larsson H. A Swedish approach to the prevention of type 1 diabetes: a Swedish approach to the prevention of type 1 diabetes. Pediatr Diabetes. 2016;17:73–77. doi: 10.1111/pedi.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso GT, Coakley A, Pyle L, Manseau K, Thomas S, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado children, 2010-2017. Diabetes Care. 2020;43:117–121. doi: 10.2337/dc19-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabbone I, Maltoni G, Tinti D, et al. Diabetic ketoacidosis at the onset of disease during a national awareness campaign: a 2-year observational study in children aged 0-18 years. Arch Dis Child. 2020;105:363–366. doi: 10.1136/archdischild-2019-316903. [DOI] [PubMed] [Google Scholar]
- 23.Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with Human Development Index (HDI): an updated systematic review, meta-analysis, and meta-regression. Horm Metab Res. 2018;50:209–222. doi: 10.1055/s-0044-102090. [DOI] [PubMed] [Google Scholar]
- 24.Turtinen M, Härkönen T, Parkkola A, Ilonen J, Knip M. the Finnish Pediatric Diabetes Register: Sex as a determinant of type 1 diabetes at diagnosis. Pediatr Diabetes. 2018;19:1221–1228. doi: 10.1111/pedi.12697. [DOI] [PubMed] [Google Scholar]
- 25.Nagl K, Rosenbauer J, Neu A, et al. Children with onset-ketoacidosis are admitted to the nearest hospital available, regardless of center size. J Pediatr Endocrinol Metab. 2020;33:751–759. doi: 10.1515/jpem-2020-0038. [DOI] [PubMed] [Google Scholar]
- 26.Schienkiewitz A, Brettschneider AK, Damerow S, Schaffrath Rosario A. Übergewicht und Adipositas im Kindes- und Jugendalter in Deutschland - Querschnittergebnisse aus KiGGS Welle 2 und Trends. J Health Monitoring. 2018;3:16–23. [Google Scholar]
- 27.Razum O, Geiger I, Zeeb H, Ronellenfitsch U. Gesundheitsversorgung von Migranten. Dtsch Arztebl. 2004;10 12882. [Google Scholar]
- 28.Hölling H, Schlack R, Petermann F, Ravens-Sieberer U, Mauz E. Psychische Auffälligkeiten und psychosoziale Beeinträchtigungen bei Kindern und Jugendlichen im Alter von 3 bis 17 Jahren in Deutschland - Prävalenz und zeitliche Trends zu 2 Erhebungszeitpunkten (2003-2006 und 2009-2012) Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2014;57:807–819. doi: 10.1007/s00103-014-1979-3. [DOI] [PubMed] [Google Scholar]
- 29.Muñoz C, Floreen A, Garey C, et al. Misdiagnosis and diabetic ketoacidosis at diagnosis of type 1 diabetes: patient and caregiver perspectives. Clin Diabetes Publ Am Diabetes Assoc. 2019;37:276–281. doi: 10.2337/cd18-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankin D, Harden J, Waugh N, et al. Pathways to diagnosis: a qualitative study of the experiences and emotional reactions of parents of children diagnosed with type 1 diabetes. Pediatr Diabetes. 2014;15:591–598. doi: 10.1111/pedi.12124. [DOI] [PubMed] [Google Scholar]
- 31.Schaeffer D, Berens EM, Vogt D. Health literacy in the German population— results of a representative survey. Dtsch Arztebl Int. 2017;114:53–60. doi: 10.3238/arztebl.2017.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children: An 8-year study in schools and private practices. Diabetes Care. 1999;22:7–9. doi: 10.2337/diacare.22.1.7. [DOI] [PubMed] [Google Scholar]
- 33.King BR, Howard NJ, Verge CF, et al. A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes. 2012;13:647–651. doi: 10.1111/j.1399-5448.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 34.Fritsch M, Schober E, Rami-Merhar B, Hofer S, Fröhlich-Reiterer E, Waldhoer T. Diabetic ketoacidosis at diagnosis in Austrian children: a population-based analysis, 1989-2011. J Pediatr. 2013;163:1484–1488. doi: 10.1016/j.jpeds.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Hommel A, Haupt F, Delivani P, et al. Screening for type 1 diabetes risk in newborns: The Freder1k Pilot Study in Saxony. Horm Metab Res. 2018;50:44–49. doi: 10.1055/s-0043-120921. [DOI] [PubMed] [Google Scholar]
- 36.Kordonouri O, Lange K, Boettcher I, et al. New approach for detection of LDL-hypercholesterolemia in the pediatric population: The Fr1dolin-trial in Lower Saxony, Germany. Atherosclerosis. 2019;280:85–91. doi: 10.1016/j.atherosclerosis.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Ferrat LA, Vehik K, Sharp SA, et al. A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med. 2020;26:1247–1255. doi: 10.1038/s41591-020-0930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler AG, Kick K, Bonifacio E, et al. Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA. 2020;323:339–351. doi: 10.1001/jama.2019.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gröndahl B, Ankermann T, von Bismarck P, et al. The 2009 pandemic influenza A(H1N1) coincides with changes in the epidemiology of other viral pathogens causing acute respiratory tract infections in children. Infection. 2014;42:303–308. doi: 10.1007/s15010-013-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]