Abstract
A 58-year-old man with a history of end-stage degenerative joint disease developed a postsurgical infection at the right hip 4 weeks after hip replacement surgery. He underwent surgical washout of the right hip without opening the joint capsule. Arthrocentesis returned positive for Mycobacterium fortuitum. He was started on antibiotics with the recommendation to remove the prosthesis. The prosthesis was retained. Based on antimicrobial susceptibilities, he was treated with 4 weeks of intravenous therapy using cefoxitin and amikacin and later switched to oral ciprofloxacin and doxycycline for 5 additional months. Eighteen months from his initial hip replacement surgery, he continues to do well. Joint aspiration culture is important to make a diagnosis of prosthetic joint infection (PJI) when periprosthetic culture is not available. In the absence of serious systemic or comorbid joint conditions, PJI due to M. fortuitum can be managed medically without having to remove the prosthesis or debride the joint.
Keywords: hip prosthesis implantation, infections
Background
Total joint replacements are important therapeutic interventions in alleviating the morbidity associated with severe degenerative joint disease. It is estimated that by the year 2030, approximately 2 million total hip or knee replacements will be done yearly in the USA.1 With the increase in the number of joint replacements, however, comes associated infection complications. Prosthetic joint infections (PJIs) account for 1%–2% of primary total joint replacement surgeries including 7% of revision surgeries.2–4 Rapidly growing mycobacterium (RGM) PJI is rare.5 6 From a recent extensive literature review, there are 45 cases of RGM causing PJI of either hip or knee, out of which only 7 cases were attributable to Mycobacterium fortuitum of the hip.2–4 This suggests that M. fortuitum PJI of the hip is very rare.
Historically, the cure for PJI due to RGM is generally achieved by a combination of antibiotics and surgical intervention which in many cases require prosthesis explantation, joint space debridement or revision surgery.2–6 However, there is no consensus on the optimal treatment when it comes to rare organisms such as M. fortuitum causing PJI.7 8 To our knowledge, medical management alone, without any surgical intervention targeted at the infected prosthesis, has not been successfully employed to treat a hip PJI due to RGM. Here, we present a case of a right hip PJI caused by M. fortuitum in a 58-year-old man who was successfully treated with medical management without debridement/polyethylene removal or prosthesis explantation.
Case presentation
This is a 58-year-old man with a history of end-stage degenerative joint disease who successfully underwent a right hip replacement. Four weeks after his surgery, he presented with an area of redness, which was initially considered to be a postsurgical haematoma (figure 1). He subsequently underwent limited superficial irrigation and debridement of his right hip wound and arthrocentesis of the right hip joint under anaesthesia and fluoroscopic guidance. The joint aspirate was sent for bacterial, fungal and mycobacterial cultures. Cloudy-appearing fluid was expressed from the distalmost aspect of the wound with two separate cultures also sent for bacterial, fungal and mycobacterial cultures. At surgery, inspection as well as digital palpation of the deeper fascia revealed there was no communicating sinus between the superficial wound and the deeper structures. The decision was made at that time to not dissect through the fascia and enter the right hip joint as this could contaminate the prosthesis. Two of three separate aspirates of the joint cavity, however, returned positive for M. fortuitum. Other superficial and deep wound cultures at this time were negative for any other pathogens. There was no loosening of hardware per X-ray of the hip obtained during this short admission. He was treated empirically with 10 days of Bactrim.
Figure 1.
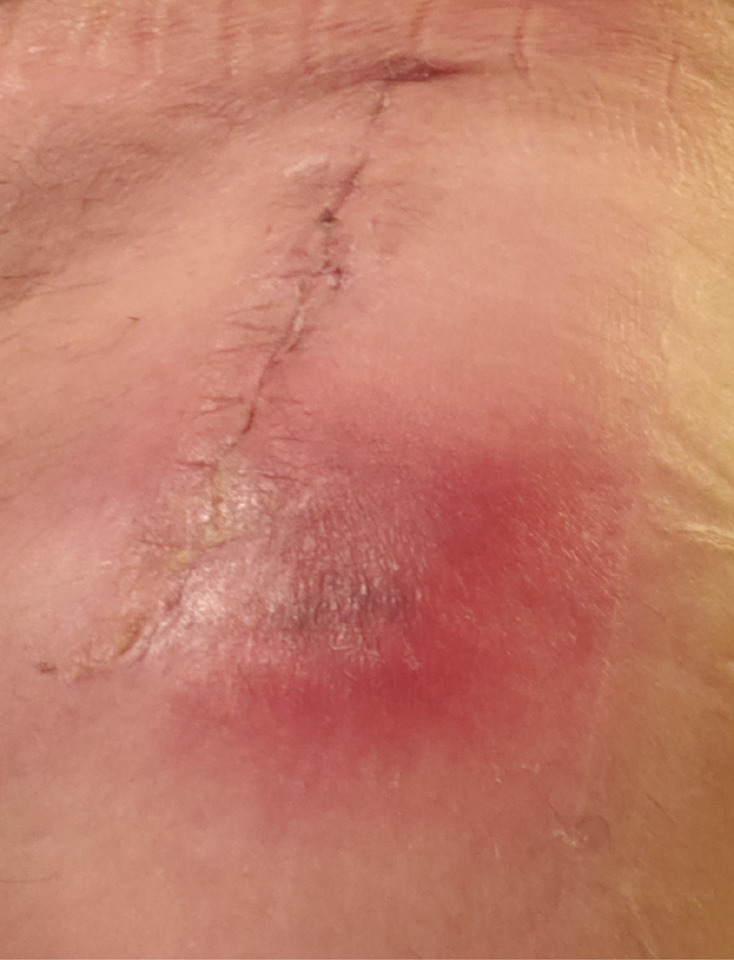
Postsurgical haematoma 4 weeks after right total hip replacement.
The patient, however, presented again 5 weeks after his last wound washout due to an open wound with persistent drainage at the right hip (figure 2). He did not have any fevers, chills or trauma to the right hip. Vitals were within normal limits. Physical examination showed a 2 cm open wound with persistent purulent drainage. There was no leucocytosis present on peripheral blood test. Serum C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were normal at <0.1 mg/dL and 7 mm/hour, respectively. Based on his history, clinical examination and report of two separate aspiration joint specimens growing M. fortuitum, the infectious diseases consult team recommended to remove the prosthesis. He was started empirically on cefoxitin 2 g intravenously every 6 hours and amikacin 15 mg/kg intravenously every 24 hours. Antimicrobial sensitivities for the M. fortuitum were requested which showed susceptibilities to amikacin, cefoxitin, ciprofloxacin, doxycycline, imipenem, linezolid, moxifloxacin and trimethoprim/sulfamethoxazole. He had a repeat washout at this new hospital admission with findings consistent with 1.5×1.5 cm subcutaneous abscess underneath the area of wound dehiscence again without extension to deeper structures. Intraoperative joint aspiration was again obtained and samples were sent for bacterial, fungal and mycobacterial studies from superficial, deep and intra-articular joint aspirations. The results of microbiological studies show M. fortuitum from the superficial wound cultures; however, deep cultures and joint aspiration cultures were negative for any growth. The prosthesis was not removed as a result of reluctance from the patient and the orthopaedic surgeon, negative inflammatory markers and concern that the infection was superficial. He was treated with 4 weeks of intravenous therapy using cefoxitin and amikacin (same doses as indicated above) and later transitioned to oral ciprofloxacin 750 mg two times per day and oral doxycycline 100 mg two times per day for 5 additional months. At his follow-up visit 6 months later, he was doing well with good range of motion and no evidence of new dehiscence. Twelve months after his initial hip replacement surgery, he presented with pain again at the right hip area. His hip X-ray showed no loosening of prosthesis but hip ultrasound suggested a small hip joint effusion. Joint aspiration was done and joint fluid was analysed for synovial fluid cell count (white blood count (WBC) 170, neutrophils 36%, Gram stain negative), cultures (negative for bacterial, fungal or mycobacterial growth) and synovial fluid (alpha defensin—negative; CRP <0.4 mg/dL (cut-off >3).
Figure 2.

Open wound with persistent drainage 9 weeks after right total hip replacement.
His new hip pain was felt to be due to iliopsoas tendonitis. His hip PJI has responded satisfactorily to antibiotic therapy without intra-articular prosthesis debridement or prosthesis removal.
Outcome and follow-up
At the time of this report, which approximates 18 months from his initial hip replacement surgery, he continues to do well.
Discussion
PJIs are potentially damaging infections if not diagnosed and treated early. There are several unique points that merit further discussion in this case in comparison to previously reported cases.
Foremost, the authors are convinced that this case represents PJI of the hip. Generally speaking, PJI diagnosis due to RGM organisms is often difficult and challenging. The pathogen may take several weeks to grow in culture and several notable elements that support a PJI diagnosis may be absent, like in our patient. Features that support PJI in this case are clinical symptoms related to the hip joint such as wound drainage, wound dehiscence, tenderness, as well as two positive separate joint aspiration cultures. Features against PJI are normal ESR and CRP, negative deep intraoperative cultures and no evidence of hardware loosening. Per 2012 Infectious Disease Society of America (IDSA) guidelinesand 2018 Musculoskeletal Infection Society (MSIS) guidelines,9 10 several criteria can be used to diagnose PJI. Of these, the patient appears to satisfy the criteria of having two positive periprosthetic cultures with phenotypically identical organisms. Of note, however, there were no periprosthetic cultures obtained in this case and the best culture material we have in close proximity to the prosthesis is the synovial fluid. There is fairly good evidence to support that synovial fluid culture is a good reflection of intraoperative (periprosthetic culture).11–13 The 2019 IDSA/American Academy of Orthopaedic surgeons guideline also noted the important contribution of synovial fluid culture to PJI diagnosis. This most recent PJI guideline suggests moderate strength evidence of the use of synovial fluid aerobic and anaerobic bacterial cultures to aid in the diagnosis of PJI.14
Second, the standard of care for PJI is antibiotic therapy, prosthesis removal with or without prosthesis reimplantation, or debridement and retention, which often involves removal of the polyethylene liner.
Of the seven cases of M. fortuitum noted earlier, only one case did not require prosthesis removal15 but even this case required joint debridement and retention. Our case is the first case of hip M. fortuitum PJI treated with antibiotics alone with no prosthesis removal or joint space debridement. This case is therefore unique in that it shows that a patient can have a good clinical response with only medical management for a M. fortuitum PJI especially when surgery is refused or accompanied with excessive risk–benefit ratio. Even though no hip PJI due to M. fortuitum has been previously reported to be successfully treated medically, there are very few cases that support this approach as a viable option for other PJIs. One such case was that of a M. fortuitum prosthetic knee infection successfully treated with debridement and retention plus antibiotic therapy.8 Other similar cases have been reported but all require at least debridement of the joint space.7 16 17 In a review by Napaumpaiporn et al comprising 1529 subjects with non-tuberculous mycobacterial infections 14% of which are PJI, only 18% of the identified cohort were treated with medical management only.18
Often times, patients with PJI are discharged on empiric antibiotics targeting Gram-positive organisms when nothing initially grows in culture. When Mycobacterium culture is eventually confirmed, combination therapy with at least two in-vitro active antimicrobial agents (amikacin plus either cefoxitin or imipenem or quinolone) for at least 6 months is often recommended.9 19–21 Other antibiotic options if susceptible include linezolid, sulfamethoxazole/trimethoprim and tetracyclines including doxycycline and minocycline. Our patient was initially discharged home on 10 days of Bactrim and was subsequently appropriately treated with 4 weeks of intravenous cefoxitin and amikacin followed by 5 months of ciprofloxacin and doxycycline.7
There are a few limitations to our case. First, the joint was never opened therefore we did not have a periprosthetic culture, which could have strengthened or further confirmed our impression of M. fortuitum PJI. Second, synovial fluid studies at the time the PJI was diagnosed did not include a synovial fluid cell count. Finally, antibiotics were started before cultures were obtained at the time of his repeat washout procedure. This could have accounted for negative deep wound cultures.
In conclusion, in the absence of serious systemic or comorbid joint conditions, PJI due to M. fortuitum can be managed medically without having to remove or debride the joint. However, each case has to be judged on its own merits and further case reports, case series or retrospective analyses of mycobacterium PJI are needed to make any definitive recommendations.
Patient’s perspective.
“I was initially frustrated when it was recommended that I had to have the prosthesis removed as I did not want to limit my mobility.”
“I was very happy I was able to do so well with medical management while retaining my prosthesis.”
“I would like to include this case in the growing body of literature on prosthetic joint infections to help other physicians make the decision of medically managing an infection while retaining a prosthesis.”
Learning points.
Prosthetic joint infections (PJIs) due to Mycobacterium fortuitum can be medically managed without prosthesis removal or joint debridement.
Joint aspiration culture is an important tool that can assist in making a diagnosis of PJI when the joint is not surgically opened since synovial fluid culture is a good reflection of intraoperative (periprosthetic culture).
Starting antibiotics before cultures are obtained is a frequent reason for negative intraoperative cultures as far as PJI is concerned and could have accounted for negative deep wound cultures at the time of repeat washout.
Footnotes
Twitter: @foluayoade
Contributors: Both authors were involved in direct patient care. I, MR, was involved in writing the manuscript. FA was involved in editing and proofreading the manuscript. All authors have critically discussed, read and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100:1455–60. 10.2106/JBJS.17.01617 [DOI] [PubMed] [Google Scholar]
- 2.Ong KL, Kurtz SM, Lau E, et al. Prosthetic joint infection risk after total hip arthroplasty in the medicare population. J Arthroplasty 2009;24:105–9. 10.1016/j.arth.2009.04.027 [DOI] [PubMed] [Google Scholar]
- 3.Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;466:1710–5. 10.1007/s11999-008-0209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz SM, Lau E, Schmier J, et al. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 2008;23:984–91. 10.1016/j.arth.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 5.Herold RC, Lotke PA, MacGregor RR. Prosthetic joint infections secondary to rapidly growing Mycobacterium fortuitum. Clin Orthop Relat Res 1987;216:183???186–6. 10.1097/00003086-198703000-00029 [DOI] [PubMed] [Google Scholar]
- 6.Henry MW, Miller AO, Kahn B, et al. Prosthetic joint infections secondary to rapidly growing mycobacteria: two case reports and a review of the literature. Infect Dis 2016;48:453–60. 10.3109/23744235.2016.1142673 [DOI] [PubMed] [Google Scholar]
- 7.Cheung I, Wilson A. Mycobacterium fortuitum infection following total knee arthroplasty: a case report and literature review. Knee 2008;15:61–3. 10.1016/j.knee.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 8.Taweekitikul P, Ngarmukos S, Tanavalee A. Early onset prosthetic knee joint infection by mycobacterium fortuitum: a case report and literature review. JRCOST 2019;43:33–43. [Google Scholar]
- 9.Osmon DR, Berbari EF, et al. Infectious diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the IDSA. Clin Infect Dis 2013;56:e1–25. 10.1093/cid/cis803 [DOI] [PubMed] [Google Scholar]
- 10.Second international consensus on periprosthetic joint infection July 25-27, 2018. Available: https://icmphilly.com/wp-content/uploads/2018/11/ICM-update-111218.pdf [Accessed 09 Apr 2021].
- 11.Ayoade F, Todd J. Clinical outcomes of prosthetic knee joint infection in a United States tertiary healthcare center. Open Forum Infect Dis 2017;4:S99. 10.1093/ofid/ofx163.080 [DOI] [Google Scholar]
- 12.Jennings JM, Dennis DA, Kim RH, et al. False-positive cultures after native knee aspiration: true or false. Clin Orthop Relat Res 2017;475:1840–3. 10.1007/s11999-016-5194-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Merchan EC. Preoperative aspiration culture (PAC) for the diagnosis of infection in a prosthetic knee joint. Arch Bone Jt Surg 2018;6:342–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Tubb CC, Polkowksi GG, Krause B. Diagnosis and prevention of periprosthetic joint infections. J Am Acad Orthop Surg 2020;28:e340–8. 10.5435/JAAOS-D-19-00405 [DOI] [PubMed] [Google Scholar]
- 15.Fix WC, Sheth NP, Braffman MN. Mycobacterium fortuitum prosthetic joint infection after total hip arthroplasty: a case report. JBJS Case Connect 2020;10:e0343. 10.2106/JBJS.CC.18.00343 [DOI] [PubMed] [Google Scholar]
- 16.Eid AJ, Berbari EF, Sia IG, et al. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis 2007;45:687–94. 10.1086/520982 [DOI] [PubMed] [Google Scholar]
- 17.Buser GL, Laidler MR, Cassidy PM, et al. Outbreak of nontuberculous mycobacteria joint prosthesis infections, Oregon, USA, 2010-2016. Emerg Infect Dis 2019;25:849–55. 10.3201/eid2505.181687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napaumpaiporn C, Katchamart W. Clinical manifestations and outcomes of musculoskeletal nontuberculous mycobacterial infections. Rheumatol Int 2019;39:1783–7. 10.1007/s00296-019-04392-8 [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, et al. Species distribution and macrolide susceptibility of M. fortuitum. Antimicrob Agents Chemother 2019;63:e02331–18. doi:10.1128%2FAAC.02331-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelius L, Reddix R, Burchett C, et al. Cluster of mycobacterium fortuitum prosthetic joint infections. J Surg Orthop Adv 2007;16:196–8. [PubMed] [Google Scholar]
- 21.Venter RG, Solomon C, Baartman M. Mycobacterium fortuitum as infectious agent in a septic total knee replacement: case study and literature review. SA orthop 2015;14:52–6. 10.17159/2309-8309 [DOI] [Google Scholar]


