Abstract
Aggressive angiomyxoma is a rare and locally aggressive mesenchymal tumour, predominantly occurring in women of reproductive age group. The term aggressive is attributed to the infiltrative nature and frequent local recurrences. They arise commonly from the vulvovaginal region, perineum or pelvis and are usually misdiagnosed as other common entities in these regions. Radiological investigations aid in the diagnosis and planning of surgery. However, the final diagnosis in most of the cases is established by histopathological examination. We herein report a case of a middle-aged woman presenting with recurrent large right vulvar mass highlighting the surgical challenges posed by its intrapelvic extension.
Keywords: obstetrics and gynaecology, radiology, vulvovaginal disorders, surgery
Background
Aggressive angiomyxoma (AA) is a rare mesenchymal tumour that occurs predominantly in women of reproductive age with a peak incidence in the 4–5th decades of life.1 They usually involve the deep soft-tissues of the vulvovaginal region, pelvis and perineum of women and analogous sites (inguinoscrotal region) in men.2 They are often misdiagnosed with other common pathological entities like Bartholin’s cyst, Gartner’s duct cyst, lipoma and hernia. Most AAs present either as a large painless mass or with local pressure effect and often measure larger than 10 cm in size at the time of diagnosis.3
Case presentation
A 37-year-old woman presented with swelling over the right side of the vulva for 2½ years, which was insidious in onset and gradually increased in size. There was no associated pain, fever, dysmenorrhoea, bleeding, ulceration, dyspareunia or any difficulty in defecation. She had two children and the younger one of the two was born 9 years ago. She did not conceive thereafter. She had a history of excision of similar mass 3 years ago elsewhere which was reported as a benign fibroma on histopathological examination. However, the patient received no further treatment or evaluation after the surgery as she never turned up for any follow-up.
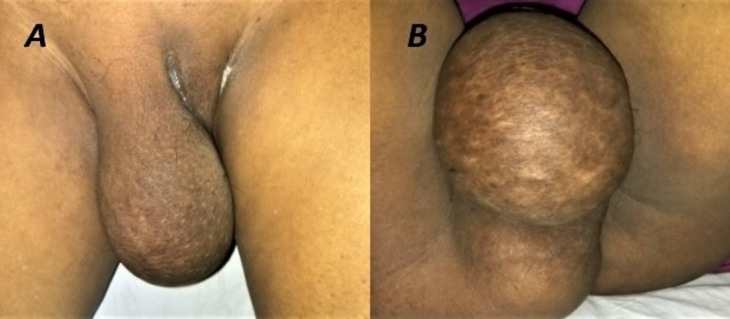
Clinical evaluation at presentation revealed a globular, lobulated firm to hard swelling of size 10×15 cm over the right vulvar region reaching up to the perineum (figure 1). It was felt going deep into the right ischiorectal fossa. The skin over the lump had dilated veins. Bilateral inguinal lymph nodes were not palpable and digital rectal examination suggested an extramural mass whose upper border could not be reached. The right lateral vaginal wall was pushed medially by the mass and was densely adhered to it.
Figure 1.
A globular mass of size 15×10 cm present over the (A) right vulvar region (B) extending into the perineum and right ischiorectal fossa.
Investigations
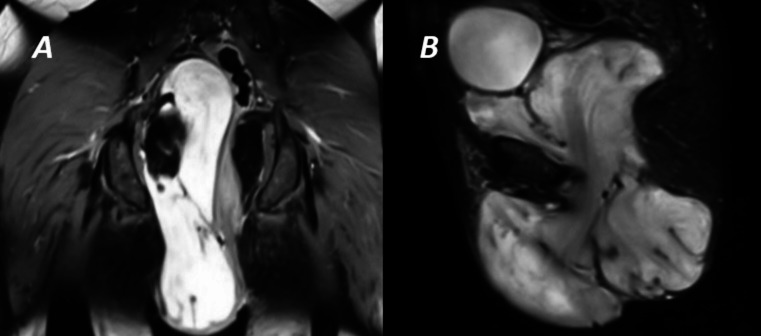
Ultrasound-guided fine-needle aspiration cytology showed a mixed cellularity aspirate in blood mixed myxoid background with minimal atypia. MRI of lower abdomen and pelvis revealed a heterogeneous encapsulated mass lesion in the pelvis extending up-to-the presacral region superiorly and abutting urinary bladder anteriorly without infiltration with characteristic swirled low-intensity signal on T2-weighted image. Inferiorly the mass occupied the entire right ischiorectal fossa abutting the anorectal canal and obturator internus muscle without obvious evidence of infiltration (figure 2A, B).
Figure 2.
T-2 weighted MRI showing (A) coronal and (B) sagittal view of the mass extending from presacral space down to the subcutaneous plane of right vulva and perineum with characteristic swirled low-intensity signal.
Differential diagnosis
AA needs to be differentiated from its various clinical and histopathological counterparts. They may clinically resemble a Bartholin’s cyst, Gartner’s duct cyst, vaginal polyp, lipoma, hernia and other rare causes of labial mass. We considered the histopathological differential diagnosis of vulvar angiomyofibroblastoma, fibroepithelial stromal polyps, superficial angiomyxoma, fibroma, myxofibrosarcoma, myxoid leiomyoma, myxoid lipoma/liposarcoma, myxoma and malignant fibrous histiocytoma (MFH).
Angiomyofibroblastoma is well-circumscribed tumour usually less than 5 cm. They are present in superficial soft-tissue of vulva and has much lower recurrence. In contrast, AAs are larger in size and have high recurrence. Both have low mitotic activity. Ultrasonography and MRI may be helpful in further differentiating the two.4
Fibroepithelial stromal polyps may be associated with pregnancy or hormone supplementation and are relatively common. They are polypoid with myxoid stroma and minimal or hyperplastic squamous epithelium and have an indolent course. They may occasionally exhibit a sarcomatous change.
Superficial angiomyxoma is mesenchymal tumours with recognisable multinodular or lobulated tissue architecture and are dermal or subcuticular in origin. They are generally negative for oestrogen and progesterone receptor and may show S100 positivity.4
The presence of adipose tissue may indicate lipomatous tumours like myxoid lipoma or liposarcoma. MRI and CT may also facilitate in their diagnosis.3 4 Myxoid leiomyoma and sarcoma are other differentials and has area of transition between classic smooth muscle and myxoid differentiation of the tumour and the diagnosis is mainly based on morphology as the immunophenotyping is similar to AA. MFH on the other hand are heterogeneous fibroblastic tumours with poorly differentiated fibroblasts, myofibroblasts, histiocyte-like cells with bizarre multinucleate giant cells and storiform pattern.4
However, in our case, the characteristic MRI findings pointed towards AA which was confirmed on histopathological evaluation. This was in fact a recurrence of the primary lesion with an incorrect initial diagnosis probably due to inadequate workup and histological evaluation while she received treatment for the first time.
Treatment
Excision of the mass through the perineal approach was planned with caution for the need of laparotomy. After an elliptical skin incision over the right vulvar region, the mass was carefully dissected from the lateral vaginal wall. On entering the pelvic cavity, the tumour was meticulously dissected off the uterus, adnexa and the urinary bladder intended to provide a wide margin. It occupied the entirety of right ischiorectal fossa and projected from the right labia towards the perineum and finally reaching the pelvis through the pelvic diaphragm. It abutted the right obturator internus without any infiltration. However, the entire mass could be resected out through the perineal approach.
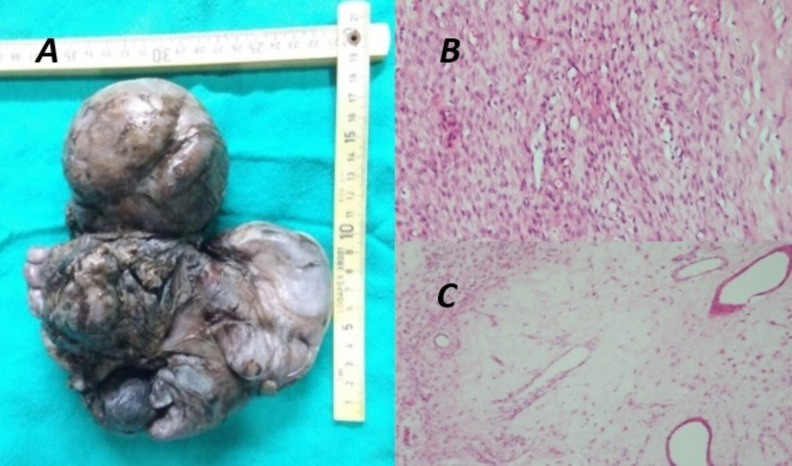
On gross examination, the outer surface was irregular with nodularity and multiple lobulations (figure 3A). Histopathology evaluation showed spindle to stellate cell tumour with variable cellularity, myxoid areas showing several scattered interspersed thin to medium calibre blood vessel without atypical mitosis suggestive of AA (figure 3B, C). The tumour tissue stained positively for oestrogen and progesterone receptors and the margins were negative for tumour cells.
Figure 3.
(A) A tan grey, vaguely circumscribed, bulky tumour specimen with histopathology evaluation showing (B) spindle-shaped tumour cells in the background of myxoid stroma with (C) variable sized haphazardly placed blood vessels.
Outcome and follow-up
The postoperative course was uneventful and the patient was discharged 3 days after the surgery. The patient has been doing well in the follow-up of 2 years wherein she is followed up every 3 monthly with clinical examination and ultrasonography of the lower abdomen and pelvis along with yearly MRI of the same. She was started on tamoxifen oral tablets after 1 month of surgery in a once daily dose of 20 mg as adjuvant hormonal therapy considering the oestrogen and progesterone receptor positivity of the tumour.
Discussion
AAs are rare mesenchymal tumours having a female predilection (female to male ratio 6.6:1).5 The term aggressive emphasises the infiltrative nature of the tumour and its frequent association with local recurrence. They grow in the perineal region and display atypical growth patterns with translevator extension. Tumour recurrence is a significant cause of patient morbidity and fatal cases of distant metastasis have been reported in the literature.6 However, the overall prognosis is good. The local recurrence rate varies from 9% to 72%, and the recurrence can occur between 2 months and 15 years following the initial diagnosis.7 In our case, it reappeared after 6 months of previous surgery. It was in fact a recurrence of the primary lesion with incorrect histological diagnosis. Hence, it cannot be overemphasised more that a correct establishment of initial diagnosis and adequate follow-up is a must to look for early recurrence in order to address the same. At the same time, long-term follow-up is required before the possibility of a recurrence can be dismissed. It is also worth mentioning the need for assessment of any distant metastasis at the time of initial evaluation or during the recurrence of the tumour.
On sonographic imaging, AAs typically appear as a hypoechoic or cystic mass.8 AAs are best diagnosed on MRI where these tumours are usually hyperintense on T2-weighted images related to their high water content and loose myxoid matrix. On T1-weighted images, the tumours are isointense to muscle. Characteristically, the mass shows internal areas of ‘swirled’ linear low-intensity signal on both T1-weighted and T2-weighted images, thought to be related to the fibrovascular stroma. MRI also helps in defining the extent and infiltration of the lesion in the surrounding tissue.9 In our case, MRI helped in establishing the diagnosis with its characteristic appearance which was later confirmed on histopathology examination.
The tumours are tan grey to pink and have a rubbery consistency with a gelatinous and glistening cut surface on gross evaluation.8 9 Histologically, AAs are sparsely cellular, composed of spindle and stellate-shaped cells on a soft myxoid background with scattered thick walled, medium-sized vessels in between.1 8 9 AAs do not have any specific immunohistochemistry markers but it does exhibit a characteristic pattern of reactivity. It generally shows diffuse immunopositivity for CD-34 and vimentin.8 9 Similarly, presence of smooth muscle actin highlights myoid bundles which may be present in individual tumour cells. S-100 reactivity maybe observed in entrapped nerves in the tumour. Perhaps the most characteristic feature is the characteristic positivity for oestrogen and/or progesterone receptors, suggesting a hormonal role in the development of the tumour.1 8 9 The high prevalence of these tumours in reproductive age group with reported cases of tumour growth during pregnancy further establishes the same.10
Oestrogen and progesterone receptor-positive AA tend to respond to therapy with gonadotropin-releasing hormone (GnRH) agonists, raloxifene and tamoxifen, both preoperatively and after recurrence.11 12 Several reports have published the use of GnRH agonists as a sole treatment or as an adjuvant hormonal therapy after surgery for the management of AA.11 12 In our case, tamoxifen was added as an adjuvant hormonal therapy after 1 month of surgery as recurrences are common between 2 months and 15 years. The cytogenetic analysis and fluorescent in situ hybridisation have shown chromosomal translocation involving 12q13-15 leading to HMGA2 gene rearrangement in these tumours.1 12
Treatment involves wide local surgical excision of the mass and hormonal therapy in the postoperative period, however, the duration of it is not clearly defined. Hormonal therapy can also be incorporated to shrink the tumour before surgery. These tumours have high propensity for recurrence if inadequately excised. Therefore, widest margin of excision is recommended as possible without undue morbidity.13 Other treatment modalities like angiographic embolisation can be used in adjuvant setting and in cases where surgery is a contraindication with a dismal possibility of obtaining a negative margin during resection. The role of chemotherapy and radiotherapy is not well defined and are of limited use. Radiotherapy has been reported to be of some use in multiple recurrent disease after poor results with surgical excision.14 There is no consensus over the adequate treatment modality. Hence, treatment must be individualised on a case to case basis after discussing the same in a multidisciplinary team. In our case, the intrapelvic extension of the tumour posed a surgical challenge to achieve a margin free resection. However, with meticulous dissection, the goal was achieved.
Patient’s perspective.
I had undergone surgery for a similar swelling 3 years back. However, it reappeared after 6 months. I got anxious when my doctors told me that I needed another surgery. After the scans, I underwent surgery and the mass was completely removed. Although I am well after 2 years of surgery, I fear it coming back again.
Learning points.
Aggressive angiomyxoma is a rare mesenchymal tumour occurring predominantly in vulvovaginal, pelvis and perineum of females of reproductive age group.
They are locally aggressive and has a high risk of recurrence. Metastasis has been reported in the literature. However, the overall prognosis is good.
Wide local excision is the treatment of choice along with hormonal therapy to prevent a recurrence.
Acknowledgments
The authors are grateful to Dr Mohit Mangla from Department of General Surgery and Dr Ashish Verma from the Department of Radiology for their expert advice and assistance.
Footnotes
Twitter: @pratikjha17
Contributors: PKJ, VS, AKV and MAA were involved in concept, design and draft of the manuscript. The patient received treatment and care under MAA, VS, PKJ. VS, PKJ and AKV were responsible for editing and proofreading.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Sutton BJ, Laudadio J. Aggressive angiomyxoma. Arch Pathol Lab Med 2012;136:217–21. 10.5858/arpa.2011-0056-RS [DOI] [PubMed] [Google Scholar]
- 2.Amezcua CA, Begley SJ, Mata N, et al. Aggressive angiomyxoma of the female genital tract: a clinicopathologic and immunohistochemical study of 12 cases. Int J Gynecol Cancer 2005;15:140–5. 10.1136/ijgc-00009577-200501000-00021 [DOI] [PubMed] [Google Scholar]
- 3.Fetsch JF, Laskin WB, Lefkowitz M, et al. Aggressive angiomyxoma: a clinicopathologic study of 29 female patients. Cancer 1996;78:79–90. [DOI] [PubMed] [Google Scholar]
- 4.McCluggage WG. A review and update of morphologically bland vulvovaginal mesenchymal lesions. Int J Gynecol Pathol 2005;24:26–38. [PubMed] [Google Scholar]
- 5.Chan YM, Hon E, Ngai SW, et al. Aggressive angiomyxoma in females: is radical resection the only option? Acta Obstet Gynecol Scand 2000;79:216e20. [PubMed] [Google Scholar]
- 6.Siassi RM, Papadopoulos T, Matzel KE. Metastasizing aggressive angiomyxoma. N Engl J Med 1999;341:2:1772. 10.1056/NEJM199912023412315 [DOI] [PubMed] [Google Scholar]
- 7.Huang C-C, Sheu C-Y, Chen T-Y, et al. Aggressive angiomyxoma: a small palpable vulvar lesion with a huge mass in the pelvis. J Low Genit Tract Dis 2013;17:75–8. 10.1097/LGT.0b013e3182507df8 [DOI] [PubMed] [Google Scholar]
- 8.Narang S, Kohli S, Kumar V, et al. Aggressive angiomyxoma with perineal herniation. J Clin Imaging Sci 2014;4:23. 10.4103/2156-7514.131740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayama C, Ikeda M, Yasaka M, et al. Aggressive angiomyxoma of the vulva with no recurrence on a 5-year follow up: a case report. Tokai J Exp Clin Med 2016;41:42e5. [PubMed] [Google Scholar]
- 10.Xu H, Sun P, Xu R, et al. Aggressive angiomyxoma in pregnancy: a case report and literature review. J Int Med Res 2020;48:030006052093641. 10.1177/0300060520936414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCluggage WG, Jamieson T, Dobbs SP, et al. Aggressive angiomyxoma of the vulva: dramatic response to gonadotropin-releasing hormone agonist therapy. Gynecol Oncol 2006;100:623–5. 10.1016/j.ygyno.2005.09.033 [DOI] [PubMed] [Google Scholar]
- 12.Shinohara N, Nonomura K, Ishikawa S, et al. Medical management of recurrent aggressive angiomyxoma with gonadotropin-releasing hormone agonist. Int J Urol 2004;11:432–5. 10.1111/j.1442-2042.2004.00816.x [DOI] [PubMed] [Google Scholar]
- 13.Raptin C, Lucot J-P, Bassil A, et al. Aggressive angiomyxoma of the perineal region. SAGE Open Med Case Rep 2019;7:2050313X19843391 10.1177/2050313X19843391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari RN, Dragun AE, Aguero EG, et al. External beam radiotherapy for perirectal angiomyxoma results in a dramatic clinical response and allows a patient to avoid abdominoperineal resection. Am J Clin Oncol 2006;29:318–9. 10.1097/01.coc.0000170581.28275.e9 [DOI] [PubMed] [Google Scholar]