Abstract
A 37-year-old nulliparous woman with abnormal uterine bleeding and a uterine mass suggestive of a leiomyoma not responding to medical therapy was submitted to two hysteroscopies with histological analysis. The first one showed a ‘leiomyoma’ and the second a ‘uterine smooth muscle tumour of uncertain malignant potential/epithelioid leiomyosarcoma, with positivity for hormonal receptors’. The patient was submitted to a total hysterectomy with bilateral salpingo-oophorectomy, and the microscopic examination of the tumour revealed a ‘uterine tumour resembling ovarian sex-cord tumours’. According to the literature, most cases are diagnosed in postmenopausal women and have a favourable prognosis. These rare tumours have uncertain malignant potential and have no established treatment protocol, but it appears that a fertility-sparing approach is possible once they are well diagnosed.
Keywords: cancer - see oncology, gynecological cancer
Background
Uterine tumour resembling ovarian sex-cord tumours (UTROSCT) is the definition used to characterise a rare uterine neoplasm, composed predominantly or exclusively of cells that resemble those seen in sex-cord tumours of the ovary. Since its first description in 1976, by Clement and Scully,1 less than 200 cases have been reported. It is included in the category of mesenchymal tumours specific to the uterus, in the 2020 WHO Classification of Female Genital Tumours.2 The immunohistochemical algorithm for the diagnosis of these tumours has been proposed in 2008.3
Originally, these tumours were divided into two histopathological groups: group 1, known as endometrial stromal tumours with sex-cord like elements (ESTSCLE), has less than 50% focal ovarian sex-cord patterns and expresses only one sex-cord marker, mostly calretinin; group 2, comprising classical UTROSCT, presents positivity for calretinin and at least for one of the other markers, including inhibin, CD99, Melan A, vimentin, oestrogen and progesterone receptors, keratin, actin and desmin.2 More recently, groups 1 and 2 have been considered as distinct entities as they present different cytogenic findings (JAZF1-JJAZ1 gene fusion is present in ESTSCLE and absent in UTROSCT)4 and clinical outcomes (less aggressive disease and better overall survival for UTROSCT than ESTSCLE).5
UTROSCT often affects middle-aged women, with mean age of 52.2, and only 30% of cases in women with less than 40 years old.5 It usually has a benign clinical course, even though it may cause metastasis and present recurrence. Most patients present with abnormal uterine bleeding (AUB) as postmenopausal vaginal bleeding or abnormal menstruation.5 This tumour is often polypoid and submucosal; however, it may be intramural, subserosal or cervical. It is typically well circumscribed and solid but an haemorrhagic cyst may be found in rather large tumours. The average diameter is 6.2 cm.5 The most common diagnostic imaging used is ultrasonography.5 A magnetic resonance pattern of intratumoural cystic spaces lined by granulosa cells and filled with fluid or clotted blood has been described.6
Regarding treatment options, most cases have been treated surgically, hysterectomy with or without bilateral salpingo-oophorectomy being the procedure of choice. In a small number of cases, conservative treatment has been applied via hysteroscopy7–9 and laparoscopy.10
Case presentation
The patient was a 37-year-old nulliparous woman, non-smoker, without relevant pathological history. She was medicated with ethinylestradiol 0.02 mg/gestodene 0.075 mg11 and non-steroidal anti-inflammatory drugs12 due to dysmenorrhoea. She had a family history of several types of neoplasms in every ancestral branch (breast cancer and classic Kaposi’s sarcoma).
On one occasion, the patient had massive uterine bleeding and attended the emergency room. She had pelvic pain, AUB, fatigue and urinary urgency symptoms in the previous months. She presented with moderate to severe uterine bleeding, but was haemodynamically stable. After administration of intravenous tranexamic acid, the bleeding diminished. Blood analysis showed haemoglobin of 64 g/L and a blood transfusion was performed. Transvaginal ultrasound at that time showed the presence of a nodular formation suggestive of a leiomyoma with 30 mm. The patient referred she knew about the ‘leiomyoma’. Two years before, it had 18 mm; the following year, it measured 35 mm, with an apparent submucosal component; 6 months later, she performed pelvic MRI, which confirmed the presence of the nodule, suggestive of leiomyoma, with stable dimensions (35×25 mm).
We assumed the acute uterine bleeding was due to the submucous leiomyoma. After being clinically stable, the patient was discharged to perform a routine hysteroscopy.
Investigations
The patient underwent hysteroscopy with partial myomectomy, the histological exam revealing a ‘fibroleiomyoma’.
The pelvic pain persisted and a new ultrasound showed the leiomyoma with approximately the same dimensions changes. This way, we decided to perform another hysteroscopy. The histological exam revealed a ‘uterine smooth muscle tumour of uncertain malignant potential/epithelioid leiomyosarcoma, favouring the latter because of its infiltrative growth’. Immunohistochemistry confirmed the presence of positive hormone receptors (oestrogens and progesterone) and was negative for CD10, Melan A, C-Kit, cytokeratin 7 and inhibin.
A thoracoabdominopelvic CT performed for staging showed no signs of disease spread. A new pelvic MRI was not performed.
Differential diagnosis
The differential diagnosis of UTROSCT is particularly difficult when the surgical specimen is not from a hysterectomy, such as a myomectomy, curettage or endometrial biopsy. It includes epithelioid leiomyoma, ESTSCLE, metastatic ovarian sex-cord stromal tumour, low-grade endometrial stromal sarcoma, adenosarcoma, carcinosarcoma, sertoliform endometrioid adenocarcinoma and plexiform tumourlet.13 As they have a different prognosis, it is important to differentiate UTROSCT from low-grade endometrial stromal sarcoma and endometroid carcinoma with sex-cord differentiation.14 Microscopic features of UTROSCT can include cells displayed in cords, trabeculae, sertoliform or retiform tubular structures.15 The most reliable immunohistochemistry markers are calretinin, inhibin, CD99 and Melan-A, with immunoreactivity for calretinin and at least one or the other being very suggestive of UTROSCT.16 Dickson et al identified the presence of NCOA1-3 gene fusion in 81.8% of a series, which included 26 cases of UTROSCT, favouring the hypothesis that this is a distinct entity and leading to a promising way to accurately diagnose it.17
Treatment
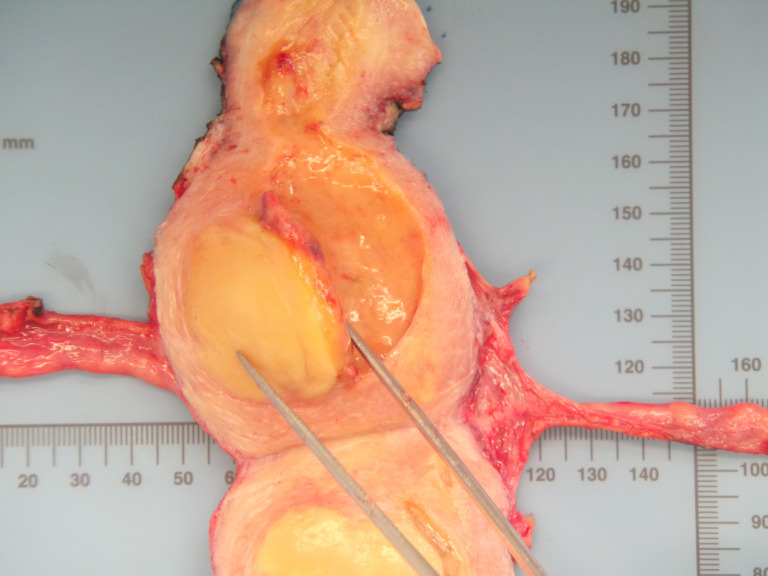
Total hysterectomy with bilateral salpingo-oophorectomy was performed with cryopreservation of ovarian tissue as a fertility conserving procedure (figure 1).
Figure 1.
Cross-section of the uterus showing the localisation of the tumour on the uterine corpus.
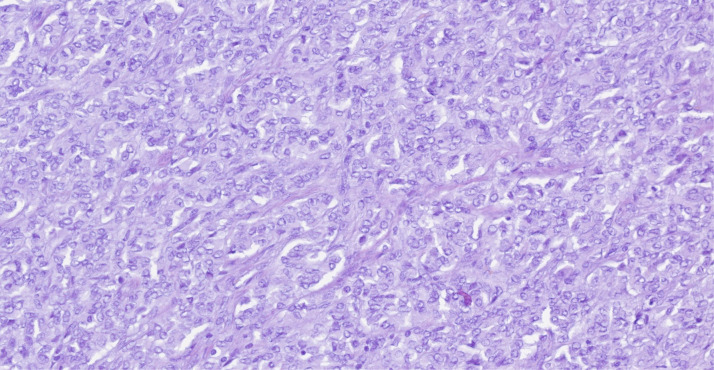
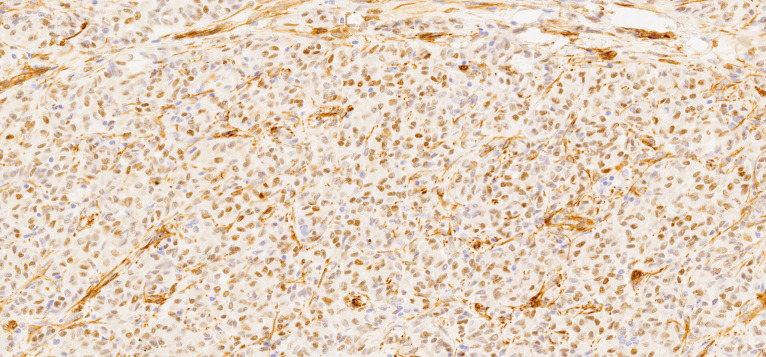
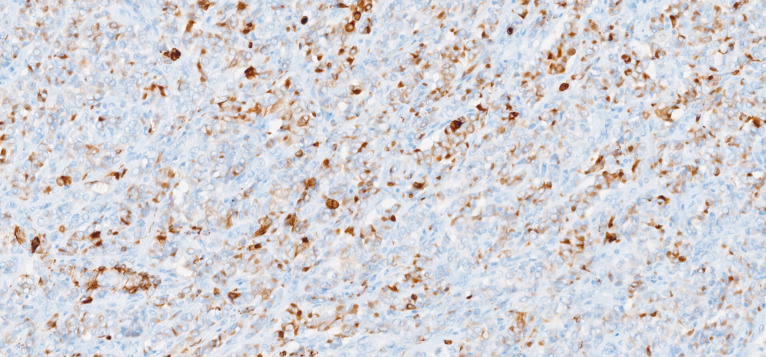
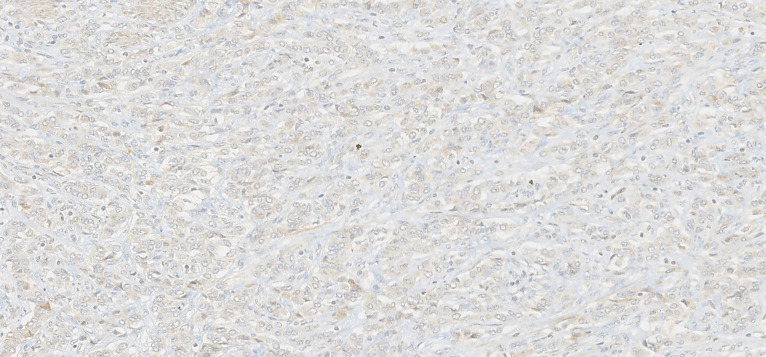
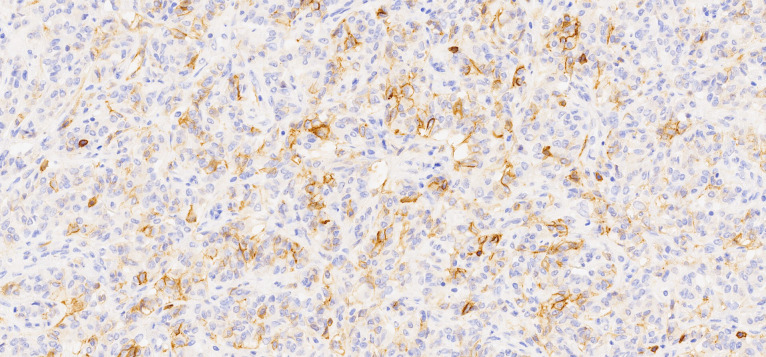
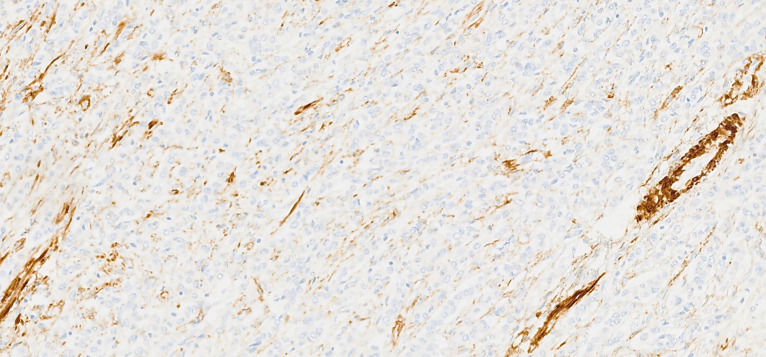
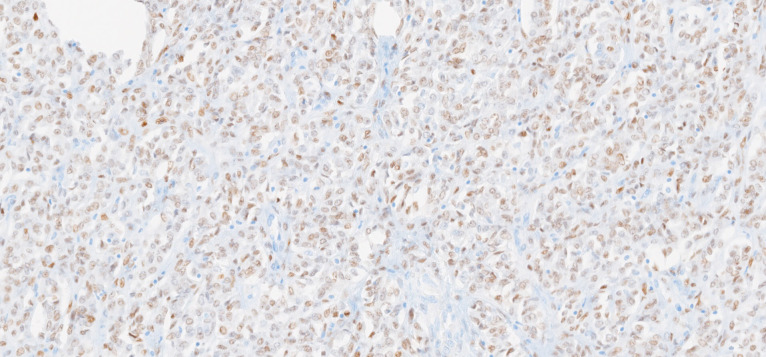
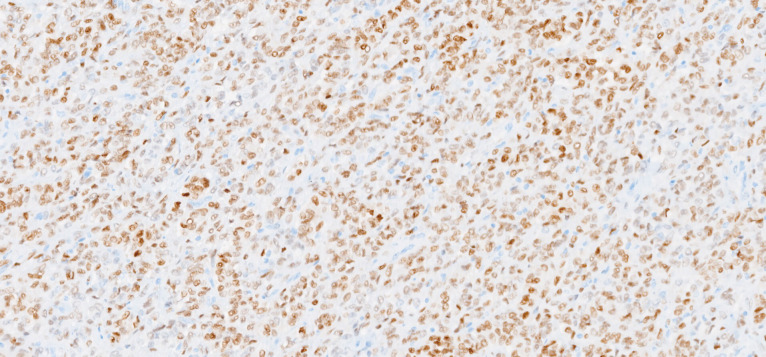
Macroscopically, the uterus had a regular appearance showing a 42 mm tumour, neoplasm with expansive, well-defined borders and yellow cut surface. The microscopic examination showed a neoplasm of solid, trabecular and cordonal patterns. Neoplastic cells had predominantly ovoid and some irregular nuclei, often with slits, and with eosinophilic cytoplasm (figure 2). The mitotic index was less than 1 mitosis/10 HPF. No images of lymphovascular invasion were identified. On the surface of the neoplasm, ischaemic necrosis foci were observed. The neoplasm infiltrated the myometrium but no endocervix invasion was identified. The immunohistochemical study showed a diffusely positive stain to calretinin (figure 3) and CD99 (figure 4), as well as multifocal positivity for Wilms tumor protein 1 (WT1) (figure 5) and desmin (figure 6); there was only focal or weak positivity for inhibin (figure 7), CD56 (figure 8) and caldesmon (figure 9). The cells expressed oestrogen (90%–100%) (figure 10) and progesterone (80%–90%) receptors (figure 11) and the proliferative index (Ki-67) was 5%–10% (figure 12). This histological and immunohistochemical study favoured the diagnosis of UTROSCT.
Figure 2.
H&E staining: high power view (×400) of the tumour.
Figure 3.
Immunohistochemical stain for calretinin (×40).
Figure 4.
Immunohistochemical stain for CD99 (×200).
Figure 5.
Immunohistochemical stain for Wilms tumor protein 1 (WT1) (×200).
Figure 6.
Immunohistochemical stain for desmin (×200).
Figure 7.
Immunohistochemical stain for inhibin (×200).
Figure 8.
Immunohistochemical stain for CD56 (×200).
Figure 9.
Immunohistochemical stain for caldesmon (×200).
Figure 10.
Immunohistochemical stain for oestrogen receptors (×200).
Figure 11.
Immunohistochemical stain for progesterone receptors (×200).
Figure 12.
Immunohistochemical stain for Ki-67 (×40).
Outcome and follow-up
The clinical case was discussed in a multidisciplinary meeting, having been decided to maintain clinical control in gynaecology consultation for at least 10 years: every 4 months in the first 2 years, every 6 months in the next 3 years and then annually. After 20 months of follow-up, the patient remains clinically well, without complications. Due to the unknown behaviour of the tumour, the patient had a pelvic MRI performed 12 months later, and it was negative for recurrence or metastasis. A genetic study was performed due to family history and was negative for germline mutations. The patient awaits results for decision-making options regarding oncofertility follow-up.
Discussion
UTROSCT is a rare uterine tumour, whose nature and natural history are not well established. There is no determined treatment protocol neither reliable prognostic factors. Surgery with negative margins is considered the standard in the treatment of this entity. Most cases are treated surgically, through hysterectomy, with or without bilateral salpingo-oophorectomy, but there are reports of a few cases treated conservatively, with a follow-up period of up to 28 months, without recurrence.7
Although these tumours seem to behave like a benign tumour, there are cases of recurrence18 and metastasis.19 20 In a 2019 case series, it was described the occurrence of extrauterine metastasis in 23.5%.20 Some histological characteristics such as an infiltrative border, vascular invasion, great mitotic activity, serosal rupture, tumour necrosis, stromal predominance and cytologic atypia seem to be associated with cases of recurrence or metastatic behaviour of UTROSCT tumours,12 14 19 but none of these were present in this case.
In light of the probably less aggressive tumour biology and concerning the patient’s autonomy, the uterus-preserving strategy can be considered in selected individuals, especially premenopausal women, willing to preserve fertility. Some authors suggest a hysteroscopic follow-up,8 with periodic endometrial biopsies. However, further case reports with long follow-ups are needed to prove the safety of the organ-preserving strategy in UTROSCT.
This case is uncommon because it occurred in a premenopausal woman. Diagnosis of UTROSCT is very difficult, as it is very similar to leiomyoma, clinically and radiologically. At first, the patient responded to the estroprogestative therapy and the growth of the tumour was not significant, which favoured the diagnosis. The decision to perform the bilateral salpingo-oophorectomy was due to the suspicion of uterine sarcoma with positivity for hormone receptors after the second hysteroscopy. The patient was referred to a fertility-preservation centre before surgery and opted for cryopreservation of the ovarian tissue. Having no experience with these tumours, the follow-up schedule decided to apply to the patient was based on the fact that these neoplasms are potentially malignant.
Learning points.
Uterine tumour resembling ovarian sex-cord tumours (UTROSCT) is a rare uterine tumour, usually seen is postmenopausal women with abnormal uterine bleeding. Diagnosis is very difficult in small surgical specimens.
In premenopausal women, UTROSCT is frequently misdiagnosed with leiomyomas, as its ultrasound appearance and symptoms (abnormal uterine bleeding) are the same.
UTROSCT seems to have a low malignant potential and a uterus-preserving strategy can be considered in selected individuals.
Footnotes
Contributors: SFP has contributed to the planning, bibliographic research and reporting. RS has contributed to the conception and reporting. LC has contributed to reporting. CA has contributed to reporting and with the figures. They were all involved as physicians with this case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Clement PB, Scully RE. Uterine tumors resembling ovarian Sex-cord tumors. American Journal of Clinical Pathology 1976;66:512–25. [DOI] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board . Female genital tumours. 4. 5th ed. Lyon (France: International Agency for Research on Cancer. WHO classification of tumours series, 2020. [Google Scholar]
- 3.Czernobilsky B. Uterine tumors resembling ovarian sex cord tumors: an update. Int J Gynecol Pathol 2008;27:229–35. 10.1097/PGP.0b013e3181569a21 [DOI] [PubMed] [Google Scholar]
- 4.Staats PN, Garcia JJ, Dias-Santagata DC, et al. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) lack the JAZF1-JJAZ1 translocation frequently seen in endometrial stromal tumors. Am J Surg Pathol 2009;33:1206–12. 10.1097/PAS.0b013e3181a7b9cf [DOI] [PubMed] [Google Scholar]
- 5.Blake EA, Sheridan TB, Wang KL, et al. Clinical characteristics and outcomes of uterine tumors resembling ovarian sex-cord tumors (UTROSCT): a systematic review of literature. Eur J Obstet Gynecol Reprod Biol 2014;181:163–70. 10.1016/j.ejogrb.2014.07.050 [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi M, Matsuzaki K, Bando Y, et al. A case of uterine tumor resembling ovarian Sex-cord tumor (UTROSCT) exhibiting similar imaging characteristics to those of ovarian Sex-cord tumor. Magn Reson Med Sci 2019;18:113–4. 10.2463/mrms.ci.2017-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watrowski R, Jäger C, Möckel J, et al. Hysteroscopic treatment of uterine tumor resembling ovarian sex Cord-like tumor (UTROSCT). Gynecol Endocrinol 2015;31:856–9. 10.3109/09513590.2015.1080682 [DOI] [PubMed] [Google Scholar]
- 8.Garuti G, Gonfiantini C, Mirra M, et al. Uterine tumor resembling ovarian sex cord tumors treated by resectoscopic surgery. J Minim Invasive Gynecol 2009;16:236–40. 10.1016/j.jmig.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Vilos AG, Zhu C, Abu-Rafea B, et al. Uterine tumors resembling ovarian sex cord tumors identified at Resectoscopic endometrial ablation: report of 2 cases. J Minim Invasive Gynecol 2019;26:105–9. 10.1016/j.jmig.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 10.Hillard JB, Malpica A, Ramirez PT. Conservative management of a uterine tumor resembling an ovarian sex cord-stromal tumor. Gynecol Oncol 2004;92:347–52. 10.1016/j.ygyno.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 11.Wong CL, Farquhar C, Roberts H. Oral contraceptive pill as treatment for primary dysmenorrhoea. Cochrane Database Syst Rev 2009:CD002120. 10.1002/14651858.CD002120.pub3 [DOI] [PubMed] [Google Scholar]
- 12.Marjoribanks J, Ayeleke R, Farquhar C. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea (review). Cochrane Database Syst Rev 2015:1–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauptmann S, Nadjari B, Kraus J, et al. Uterine tumor resembling ovarian sex-cord tumor--a case report and review of the literature. Virchows Arch 2001;439:97–101. 10.1007/s004280100455 [DOI] [PubMed] [Google Scholar]
- 14.Hashmi AA, Faridi N, Edhi MM, et al. Uterine tumor resembling ovarian sex cord tumor (UTROSCT), case report with literature review. Int Arch Med 2014;7:47. 10.1186/1755-7682-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan D, Mohanty SK, Dinesh Pradhan MD. Uterine tumors resembling ovarian sex cord tumors. Arch Pathol Lab Med 2013;137:1832–6. 10.5858/arpa.2012-0634-RS [DOI] [PubMed] [Google Scholar]
- 16.Irving JA, Carinelli S, Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Mod Pathol 2006;19:17–24. 10.1038/modpathol.3800475 [DOI] [PubMed] [Google Scholar]
- 17.Dickson BC, Childs TJ, Colgan TJ, et al. Uterine tumor resembling ovarian sex cord tumor: a distinct entity characterized by recurrent NCOA2/3 gene fusions. Am J Surg Pathol 2019;43:178–86. 10.1097/PAS.0000000000001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cömert GK, Kiliç Çiğdem, Çavuşoğlu D, et al. Recurrence in uterine tumors with ovarian sex-cord tumor resemblance: a case report and systematic review. Turk Patoloji Derg 2018;34:225–33. 10.5146/tjpath.2018.01429 [DOI] [PubMed] [Google Scholar]
- 19.Umeda S, Tateno M, Miyagi E, et al. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) with metastasis: clinicopathological study of two cases. Int J Clin Exp Pathol 2014;7:1051–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Moore M, McCluggage WG. Uterine tumour resembling ovarian sex cord tumour: first report of a large series with follow-up. Histopathology 2017;71:751–9. 10.1111/his.13296 [DOI] [PubMed] [Google Scholar]