Abstract
Survival in cases involving childhood malignancy is reaching nearly 80% in high-income countries, yet cancer remains one of the leading disease-related causes of death in children. In adult oncology the role of targeted therapies is established, but information regarding the use of these therapies in children is limited, largely because targeted therapies were developed in the context of adult pathologies. The few pediatric reports regarding crizotinib, an anaplastic lymphoma kinase (ALK) inhibitor, seem promising. This case of an 8-year-old male with an ALK-positive anaplastic large cell lymphoma highlights the challenges of treating children with crizotinib. Our experience with crizotinib was more challenging than described in the limited pediatric reports. Not only was the tumor response poorer than described in the reports, but a substantial amount of side-effects and practical difficulties, such as the method of administration and dosing, made management challenging. Many challenges for the use of targeted therapy in pediatric care currently persist. The limited research in pediatric populations leaves uncertainty regarding efficacy and short- and long-term side effects as well as practical difficulties. Despite a clear underlying biological rationale for certain targeted therapies, their contribution toward improving the outcome of childhood cancer remains largely unclear.
Keywords: anaplastic large cell lymphoma, crizotinib, molecular targeted therapy, off-label use, oncology, pediatrics
Background
In the last decade, the development of targeted therapy has risen, and there has been an increased focus on research in pediatric populations.1 Targeted cancer therapies comprise anti-neoplastic drugs that target specific genes, proteins, or other molecules, interfering with tumor growth and survival. Monoclonal antibodies have been extensively studied in multiple diseases in children.2,3 In this article we focus on the role of small-molecule inhibitors, in particular crizotinib, in pediatric oncology.
In pediatric oncology, there has been a remarkable improvement in survival since the 1970s because of better risk stratification, improved understanding of pathophysiology, increased intensity of treatment protocols, and better supportive care.1,4 Small-molecule inhibitors have been applied to possibly improve outcomes even further, but their role remains unclear.1,5 These targeted therapies are usually designed for adult diseases, and the pediatric application is often derived from the adult indications, although this generally requires specific studies in children to establish safety and efficacy in the relevant age groups.1,5 Because of the difference in etiology and biology between childhood and adult diseases,5 the degree of extrapolation of efficacy of these new drugs has indeed been limited.1,5 Historically, children were seen as a vulnerable group that should be protected from research.6 Today it is generally agreed that clinical trials in children are necessary to ensure that prescribers can base their decisions on a level of evidence comparable to that of adult patients—children can best be protected through research, not by shielding them from research.6 However, in the context of the relatively good prognosis of childhood cancer,1 compared with that seen in adults, and given the substantially lower patient numbers available for studies, the design of attractive and informative clinical trials involving new anti-cancer medicines for the pediatric population remains challenging.5,6
The aim of this article is to illustrate how small-molecule inhibitors, with crizotinib as an example, could possibly address an unmet need in childhood cancer. Furthermore, this case report of a child with anaplastic large cell lymphoma (ALCL) and the challenges of treating him with crizotinib highlights the current difficulties in the studies, the development, and daily administration of small-molecule inhibitors in children.
Case Report
An 8-year-old male was diagnosed with an intra-abdominal anaplastic lymphoma kinase (ALK)–mutation–positive, metastatic ALCL. The primary tumor was localized posterior to the colon ascendens, with mediastinal metastases. An initial good response to frontline chemotherapy, according to the guidelines of the ALCL99 protocol (Table 1), was seen. Three months after diagnosis the tumor progressed, and second-line chemotherapy (Table 1) with ifosfamide, carboplatin, and mitoxantrone was administered without effect. Crizotinib, an ALK and ros oncogene 1 (ROS-1) inhibitor, was subsequently administered as third-line treatment.
Table 1.
Overview of Chemotherapy
|
ALCL99—Protocol high-risk group, November 22, 2018–January 13, 2019 Result: Incomplete response | ||
| Pre-phase | ||
| Dexamethasone (oral) | 5 mg/m2/day | Days 1–2 |
| Dexamethasone (oral) | 5 mg/m2 twice/day | Days 3–5 |
| Intrathecal injection | MTX 12 mg, ARA-C 30 mg, HSHC 10 mg | Day 1 |
| Cyclophosphamide (IV) | 200 mg/m2 over 1 hr | Days 1–2 |
|
| ||
| Continuation | ||
| AM 1 + BM 1 + AM 2 - stopped because of progression | ||
|
| ||
| AM course | ||
| Dexamethasone (oral) | 5 mg/m2 twice/day | Days 1–5 |
| Methotrexate (IV) | 3 g/m2 over 3 hr | Day 1 |
| Ifosfamide (IV) | 800 mg/m2 over 1 hr | Days 1–5 |
| Cytarabine (IV) | 150 mg/m2 over 1 hr every 12 hr | Days 4 and 5 |
| Etoposide (IV) | 100 mg/m2 over 2 hr | Days 4 and 5 |
|
| ||
| BM course | ||
| Dexamethasone (oral) | 5 mg/m2 twice/day | Days 1–5 |
| Methotrexate (IV) | 3 g/m2 over 3 hr | Day 1 |
| Cyclophosphamide (IV) | 200 mg/m2 over 1 hr | Days 1–5 |
| Doxorubicin (IV) | 25 mg/m2 over 1 hr | Days 4 and 5 |
|
| ||
|
ALCL—Relapse, February 8, 2019 Result: Incomplete response | ||
| ICM course | ||
| Intrathecal injection | MTX 12 mg, ARA-C 30 mg, PRED 10 mg | Day 1 |
| Mitoxantrone (IV) | 8 mg/m2/day over 30 min | Days 1 and 2 |
| Carboplatin (IV) | 200 mg/m2/day continuous infusion over 4 days | Days 2–5 |
| Ifosfamide (IV) | 2 g/m2/day continuous infusion over 5 days | Days 2–6 |
|
ALCL—Third-line therapy, February 15, 2019 Result: Response limited (administration problems)* | ||
| Crizotinib (oral) | 280 mg/m2 2 times/day | 2 wk |
| ALCL—Third-line therapy + weekly vinblastine, February 28, 2019 | ||
| Crizotinib (nasogastric) | 280 mg/m2 2 times/day | 3 wk |
| Vinblastine (IV) | 6 mg/m2 1 time/wk | |
| Stopped because of toxicity | ||
ALCL, anaplastic large cell lymphoma; ARA-C, cytarabine; HSHC, hydrocortisone; MTX, methotrexate; PRED, prednisolone.
* Based on Mossé.11
In the absence of a European Union (EU) marketing authorization for pediatric ALCL, the drug was procured via a single patient medical need program. Because of swallowing difficulties attributed to the size of the capsules, which were designed for adult use, small-sized capsules were prepared by the hospital pharmacy.
Imaging studies showed stable disease after 7 days of crizotinib administration (Figure 1A and B). On the CT scan, a pronounced thickening of the esophageal wall was demonstrated, correlating with clinical complaints of swallowing difficulties. On gastroscopy, a distinct esophagitis, with marked debris adhering to the esophageal wall, was seen (Figure 2). A biopsy confirmed the presence of crystals, presumably crizotinib residues. A nasogastric tube was placed to ensure an effective administration of crizotinib and to reduce the risk of further esophageal injury. To allow administration by nasogastric tube, an oral liquid formulation was prepared extemporaneously according to the manufacturers' instructions (Table 2).7 Weekly intravenous vinblastine was added in an attempt to achieve an anti-tumor effect. Two weeks after administering crizotinib through the nasogastric tube in combination with vinblastine, a decrease in tumor mass was demonstrated (Figure 1C). The response was unfortunately time limited, and rapid tumor progression was subsequently evident.
Figure 1.
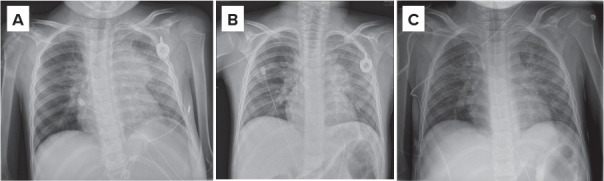
Intermittent treatment response of the mediastinal tumor mass on crizotinib, as seen on chest x-ray. A) Apical (AP) chest x-ray before administration of crizotinib; B) Stable disease after 7 days of daily crizotinib (oral capsules) on AP chest x-ray; C) Improved anti-tumor effect on day 28 after 14 days of crizotinib via nasogastric tube and intravenous vinblastine.
Figure 2.
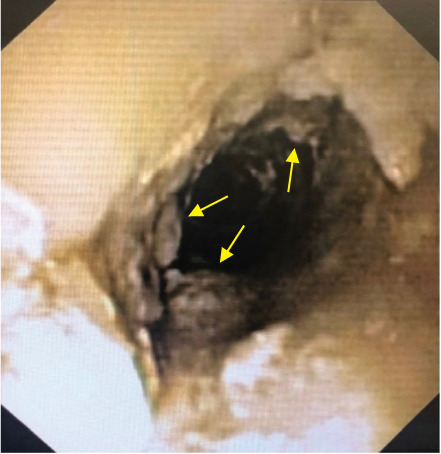
Esophageal crizotinib crystal residue on gastroscopy and reported after a tissue biopsy.
Table 2.
Two Methods for Preparing Crizotinib in a Suspension *
| Method 1: Preparation of the suspension in our center * | |
| Prepare in a clean glass. Protect yourself from contact with the suspension by wearing gloves. The suspension must be prepared directly before administration. | |
| Step | Action |
| 1 | Boil 50 mL of bottled water. |
| 2 | Transfer 15 mL of water into the clean glass. |
| 3 | Add crizotinib capsule (Xalkori)† without opening the capsule. |
| 4 | Stir with a spoon for 2 min until the capsule disintegrates completely. |
| 5 | Add another 10 mL of warm water and stir for another 2 min. |
| 6 | Allow the suspension to cool to a drinkable temperature. |
| 7 | Stir once again and give to the patient to drink or administer the suspension through a nasogastric tube. |
| 8 | Rinse the spoon and glass with water (or Coca-Cola‡) and administer to the patient. |
| 9 | Repeat step 8 two more times. |
| Method 2: Preparation and Administration of Oral Liquid Suspension Formulation as Used in Clinical Studies.7 | |
| Step | Action |
| 1 | Prepare 1 cup of boiling water (tap or bottled water). |
| 2 | Measure 15 mL of the above boiling water and pour into the drinking glass. |
| 3 | Add the prescribed crizotinib dosage to the drinking glass. Note: Do not open the capsule. |
| 4 | Using the spoon, stir the solution in step 3 continuously for at least 2 min. |
| 5 | Administer a mint candy to the patient (optional), allowing it to dissolve in the mouth to aid in masking the undesirable taste. |
| 6 | Measure another 15 mL of boiling water and pour into the drinking glass containing the crizotinib dosage. |
| 7 | Stir this solution with a spoon continuously for another 2 min. |
| 8 | Pour an additional 15 mL of room-temperature or cold water into the solution. Rinse the stirring spoon in the process so that any solids on the spoon are removed and rinsed into the glass. |
| 9 | Remove any undissolved candy from the patient’s mouth. |
| 10 | Swirl the drinking glass contents vigorously for 10 sec immediately before dosing. |
| 11 | Administer the suspension to the patient. |
| 12 | To the same drinking glass, add 15 mL of room-temperature water, rinsing the inside walls of the glass in the process. Swirl the contents vigorously for 10 sec and then administer to the patient. |
| 13 | Repeat step 12 two more times. |
| 14 | Administer 1 mint candy to the patient (optional). |
Note: Specific pharmacokinetic studies have not been performed to evaluate the method of capsule disintegration for ease of oral administration, as described in this letter using the clinical supply crizotinib formulation. Therefore, Pfizer cannot guarantee that the use of this method will result in comparable safety or efficacy outcomes to that observed with oral administration of the intact, commercially available crizotinib capsule formulation.
* In our center preparation of the suspension (Method 1) was based on the Pfizer medical information.7
† Commercially available crizotinib capsule, 250 mg.
‡ Coca-Cola may be used to wash down the suspension to prevent residue.
The combined toxicity from co-administration of crizotinib and vinblastine on top of the recent ifosfamide–carboplatin–mitoxantrone course, resulted in a state of extended bone marrow depression with a deep neutropenia, lasting for several weeks. The patient became both platelet and red blood cell transfusion dependent and suffered from long-lasting grade 3 to 4 mucositis and multiple infections. During the course of treatment, there was evidence of bacteremia with multiple isolated germs, including Staphylococcus epidermidis, Streptococcus viridans, Pseudomonas aeruginosa, and Enterococcus faecium. In addition, lung infiltrates were seen on CT, suspect for pulmonary aspergillosis. Five months after diagnosis, cerebral lesions were seen on MRI, likely because of brain metastases, although a cerebral fungal infection could not be ruled out (Figure 3). The patient suffered from pronounced anorexia, requiring parenteral nutrition, especially in the combination with frequent vomiting and diarrhea because of the combined toxicity of crizotinib and conventional chemotherapy. The patient also developed renal failure, presumably of multifactorial origin, with possible causes including lymphoma invasion, nephrotoxic medication (possibly including crizotinib-related renal hemorrhage), pre-renal intravascular volume depletion because of gastrointestinal fluid loss, and post-renal obstruction because of hemorrhagic cystitis (likely caused by ifosfamide).
Figure 3.
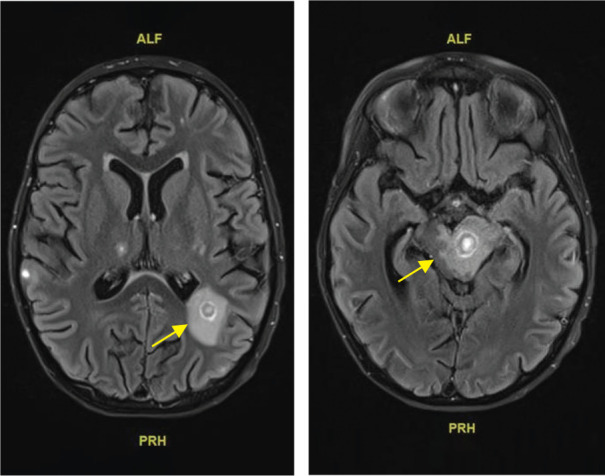
Magnetic resonance imaging demonstrating cerebral lesions suspected to represent brain metastases.
The patient developed extensive skin lesions with blistering (Figure 4). After crizotinib-associated toxic epidermal necrosis was considered, culture-negative staphylococcal scalded skin syndrome was diagnosed, which was confirmed by microscopy. He was transferred to the pediatric intensive care unit, where he developed massive melena secondary to severe neutropenic colitis. With these severe complications, crizotinib was discontinued.
Figure 4.
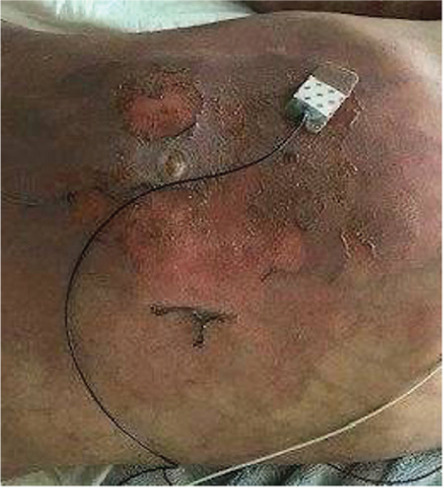
Staphylococcal scalded skin syndrome.
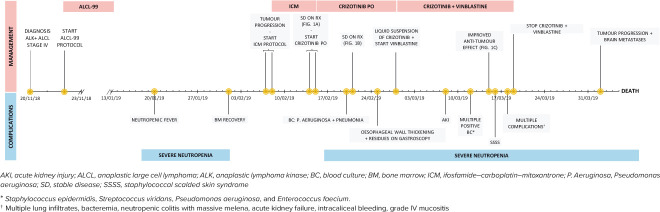
Five months after diagnosis, all treatment with curative intent was discontinued because of worsening condition with dismal oncological and neurological prognosis. The patient ultimately died because of multiple organ failure (Figure 5).
Figure 5.
Timeline of the disease process.
Discussion
The use of targeted therapies in pediatrics, especially pediatric oncology, is increasing in an effort to improve patient outcomes.1,5 A successful example is the introduction of the tyrosine kinase inhibitor imatinib, directed against BCR-ABL1 fusions, and its standard inclusion in frontline therapy for children with chronic myeloid leukemia and Philadelphia-positive acute lymphoblastic leukemia, with significant improvement in the outcome.1,5
Moreover, targeted therapy may be used for temporary disease control after failure of standard therapies: vemurafenib, a selective BRAF inhibitor, is used as a treatment option in refractory multisystem, BRAF-positive Langerhans cell histiocytosis.8 Although it may not yield a cure and while there is a high incidence of relapse after discontinuation, it may contribute to disease control.8 However, in the context of vastly improved prognosis through traditional combination chemotherapy, the role of small-molecule inhibitors in the therapeutic armamentarium against childhood cancer remains unclear, even where a sound pharmacological rationale is present. Our case illustrates this duality for crizotinib.
Crizotinib is an ALK and ROS-1 inhibitor that was developed for the treatment of lung carcinoma with an EML4-ALK translocation in adults, and its use was authorized in 2011.9 It has long been established that ALK mutations are highly prevalent in pediatric ALCL and in a few cases of neuroblastoma,9 making it potentially very relevant in pediatric oncology as well. Why, then, is it that almost 10 years later, expectations have not been met in the field of pediatric oncology? The reasons are manifold.
Initiation of the necessary clinical studies in children has traditionally been delayed for various reasons: Because children are a vulnerable population, additional safety considerations are made at the time of clinical trial authorization.6 In addition, pediatric clinical trials are more challenging to perform because of lower patient numbers and age-related considerations.5,6 Biomarkers may require separate validation in the pediatric population, hampering patient selection.4,5
Crizotinib has been developed for the treatment of some ALK-positive non–small cell lung carcinomas in adults, but the ALK variant in childhood cancers, including ALCL and neuroblastoma, is different from the variant in adult lung carcinoma.4 Therefore, the exposure-response curve may differ. Mossé et al10 demonstrated that crizotinib was well tolerated up to the dose of 280 mg/m2 twice daily, which is nearly twice that of the adult standard dose. In our case report, the patient was treated with a dose of 280 mg/m2 twice daily based on these recommendations.
For crizotinib, a few small academic studies have demonstrated its safety and effi cacy in pediatric ALCL. In the study by Mossé et al,11 26 patients with relapsed or refractory ALK-positive ALCL received crizotinib orally twice daily. These results were promising, with overall response rates of 83% and 90%, respectively, for those patients treated with doses of 165 mg/m2 and 280 mg/m2 with acceptable toxicity, highlighting the importance of the ALK pathway in pediatric ALCL. In this trial,11 at least one Grade 3 or 4 adverse event occurred in 83% of patients receiving the dose of 165 mg/m2 and in all the patients receiving the higher dose of 280 mg/m2. The most common adverse event was a decrease in neutrophil count.11 These results are, however, based on small populations, and no follow-up data are available on the patients who had discontinued ALK inhibitor therapy, which needs to be investigated prospectively.11 In our case, the patient encountered a substantial number of side effects, possibly in part as a result of crizotinib. This illustrates the difficulty of using off-label drugs with limited knowledge of possible (side) effects.
Vinblastine was added as part of third-line treatment in our case, based on literature12 that states that vinblastine reduces the risk of treatment failure in relapsed ALCL, although the benefit seems to disappear on long-term follow-up. After adding vinblastine, in combination with the administration of crizotinib by nasogastric tube, a decrease in tumor mass was seen in our case. This can be explained by better absorption of crizotinib administered through the nasogastric tube, compared with the oral administration of capsules; by the addition of vinblastine; or by a combination of both. The study by Hudson et al13 describes a possible synergistic effect of crizotinib in combination with vinblastine. However, the combination of crizotinib with conventional chemotherapy, like vinblastine, and its safety profile need further research.
Uncertainties about dosing and a lack of age-appropriate formulations constitute further barriers to safe and effective use of small-molecule inhibitors in children. While oral administration may be an advantage for adults, it can be a big challenge for children, especially in neonates and toddlers.1 A clear example of this challenge in daily practice is presented in this case. Some of the small-molecule inhibitors are highly insoluble, making it difficult to develop an oral liquid formulation for children.1 Bio-availability and stability may be affected by changes in the formulation.1 In this case, the liquid formulation was given at the same dose as the capsules, based on the results of bioequivalence studies in adults, as stated in the article by Mossé et al.10 As is often the case with children, food was used as a vehicle to administer medication. The food-drug interaction is often unknown in children. In the EU, the development of an age-appropriate formulation is now included in the pediatric investigation plans for new medicines as part of the regulatory obligations of the marketing authorization holders.14
Pharmacokinetic clinical trials in children are challenging for many reasons, among which are limitations in blood sample volumes.15 As a result, knowledge about dosing is sometimes limited, raising doubts about possible clinical (side) effects.15 The concentration of a medicine in blood or tissue is determined by processes such as absorption, distribution, metabolism, and excretion.15 These processes can differ greatly within the pediatric population compared with adults because of differences in anatomy and physiology.15 There are also many physiological changes during childhood that can have an impact on the pharmacokinetic and dynamic profile of a drug, such as intestinal transit and body composition changes that come with age.15 In our case, although crizotinib plasma concentrations were not determined, it was assumed that absorption of the initial capsule formulation was inadequate, in view of the findings on endoscopy. In addition, the effect of crizotinib on the possible brain metastases was doubtful owing to uncertainties regarding blood-brain barrier penetration.16
As a result of the limited pediatric research of the recent past, the use of off-label drugs is common practice.17,18 However, this practice can create an ethical and legal paradox,17 because caregivers must rely on less conclusive information when no other authorized options are available.10,18 Currently there is a general agreement that children deserve timely access to medicines that are appropriately studied in their desired subgroup with well-documented effects and side effects.6 With this goal in mind, the European Paediatric Medicines Regulation was launched in 2007.9 The regulation requires a pharmaceutical company to propose a pediatric investigation plan (PIP) at an early stage of development.6,9 Since then, more new medicines have been authorized for use in children.6 It is expected that this will eventually result in a decrease in off-label use of adult medicines in pediatric care. In pediatric oncology, several PIPs have been completed, resulting in novel treatment options for cases involving high-grade glioma, rhabdomyosarcoma, and acute lymphoblastic leukemia.6 However, these positive results mainly seem to be concentrated in therapeutic fields in which the pediatric patients can take advantage of the adult development.6,9,19 In child-specific diseases, major advances have been limited.6,9,19 This is partly because the scope of a PIP is usually derived from the adult indication.9 As a result, more PIPs have been approved for extremely rare malignancies in children than for the more common diseases.9
Furthermore, the EU Paediatric Regulation allows for applicants to request a waiver to avoid obligations for development in children, on specific grounds. Class waivers were initially agreed upon to avoid burdensome regulatory procedures for cases in which the regulation could not impose obligations, such as for developments in adult indications that do not exist in children.6,9,19,20 One such example is the ALK-inhibitor crizotinib presented in this case. Because it has been developed in the context of lung carcinoma in adults, the marketing authorization holder of crizotinib could take advantage of a class waiver to avoid obligations in pediatrics in 2010, because this condition does not exist in children.9,20 However, it has long been known that ALK mutations occur frequently in pediatric tumors such as ALCL, making it potentially very relevant in pediatric oncology.9 This illustrates that class waivers represent a loophole for medicine development for childhood malignancies. Pearson et al20 reported that 147 waivers were granted for 89 cancer drugs between 2012 and 2015. Forty-eight of these 89 drugs had a mechanism of action (MOA) that is potentially relevant for pediatric development. The class waiver list has subsequently been revised by the Paediatric Committee of the European Medicines Agency to address this problem, but a pharmaceutical company can still request a product-specific waiver on the grounds that the condition does not exist in children. Histology-independent indications may render waivers on this ground more difficult to justify.
However, in recent years, steps forward have been taken to overcome the challenges mentioned above. The success of targeted medicines in adults is rapidly fragmenting the traditional morphologic disease classification into molecularly defined entities across age boundaries. This now opens up the possibility that one could justify an MOA-based PIP condition, such as in the case of histology-independent indications.21 In addition, some developers have chosen to agree to a PIP that is not linked to adult development, which potentially allows us to benefit from the reward system, as foreseen in the EU Paediatric Regulation as well. Globally, it is anticipated that the “Research to Accelerate Cures and Equity for Children Act” passed by the United States Congress in 2017 will further support an MOA-based model.19 When this law takes effect in 2020, it may leverage additional PIP submissions in the EU, because cancer medicine development, especially in children, is a global process. A collaborative international approach will be most beneficial in giving children with cancer and other diseases increased access to novel treatment options.
Conclusion
Cancer remains an important cause of death in childhood, and the impact of side effects of the current standard treatment, both during therapy and among survivors, should not be discounted. Novel strategies are urgently needed to complement current treatment where outcomes are suboptimal. Small-molecule inhibitors carry the promise of improving outcomes of certain pediatric malignancies. However, very promising small-molecule inhibitors, like crizotinib, have not yet proven to be of actual benefit in terms of overall survival in cases of childhood cancer, despite a clear underlying biological rationale and a proven rapid and robust tumor reduction in studies. To this day, the precise role and positioning of small-molecule inhibitors in the pediatric cancer treatment armamentarium remain largely unclear, with substantial barriers to daily clinical practice. Additional international collaborative clinical trials, with research on the combination of small-molecule inhibitors with traditional chemotherapy, are needed to address remaining knowledge gaps.
ABBREVIATIONS
- ALCL
anaplastic large cell lymphoma
- ALK
anaplastic lymphoma kinase
- CT
computed tomography
- EU
European Union
- MOA
mechanism of action
- PIP
pediatric investigation plan
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
Ethical Approval and Informed Consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and have been approved by the appropriate committees at our institution. Informed consent for publication has been received. Approval received from Pfizer to reproduce tables from XALKORI (crizotinib). Disintegration of Capsules for Patients Unable to Swallow Capsules. Pfizer Medical Information. Created on September 11, 2018; p 4.
References
- 1.Adamson PC. Improving the outcome for children with cancer: development of targeted new agents. CA Cancer J Clin. 2015;65(3):212–220. doi: 10.3322/caac.21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillaume-Czitrom S. Biologic targeted therapies in pediatric rheumatology. Joint Bone Spine. 2014;81(suppl 1):2–48. doi: 10.1016/S1297-319X(14)70020-X. [DOI] [PubMed] [Google Scholar]
- 3.Guariso G, Gasparetto M. Treating children with inflammatory bowel disease: current and new perspectives. World J Gastroenterol. 2017;23(30):5469–5485. doi: 10.3748/wjg.v23.i30.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forrest SJ, Geoerger B, Janeway KA. Precision medicine in pediatric oncology. Current Opin Pediatr. 2018;30(1):17–24. doi: 10.1097/MOP.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MA, Reaman GH. Remaining challenges in childhood cancer and newer targeted therapeutics. Pediatr Clin North Am. 2015;62(1):301–312. doi: 10.1016/j.pcl.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Commission State of paediatric medicines in the EU: 10 years of the EU Paediatric Regulation. Accessed January 8, 2018. https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrens-medicines_report_en.pdf (26 October 2017)
- 7.Pfizer Inc XALKORI® (crizotinib) Disintegration of Capsules for Patients Unable to Swallow Capsules. Pfizer Medical Information. September 11, 2018:1–4.
- 8.Heisig A, Sorensen J, Zimmermann SY et al. Vemurafenib in Langerhans cell histiocytosis: report of a pediatric patient and review of the literature. Oncotarget. 2018;9(31):22236–22240. doi: 10.18632/oncotarget.25277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassal G, Geoerger B, Morland B. Is the European Pediatric Medicine Regulation working for children and adolescents with cancer? Clin Cancer Res. 2013;19(6):1315–1325. doi: 10.1158/1078-0432.CCR-12-2551. [DOI] [PubMed] [Google Scholar]
- 10.Mossé YP, Lim MS, Voss SD et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472–480. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mossé YP, Voss SD, Lim MS et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children's Oncology Group study. J Clin Oncol. 2017;35(28):3215–3221. doi: 10.1200/JCO.2017.73.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prokoph N, Larose H, Lim MS et al. Treatment options for paediatric anaplastic large cell lymphoma (ALCL): current standard and beyond. Cancers. 2018;10(4):99. doi: 10.3390/cancers10040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson S, Wang D, Middleton F. Crizotinib induces apoptosis and gene expression changes in ALK+ anaplastic large cell lymphoma cell lines; brentuximab synergizes and doxorubicin antagonizes. Pediatr Blood Cancer. 2018;65(8):e27094. doi: 10.1002/pbc.27094. [DOI] [PubMed] [Google Scholar]
- 14.Guideline on pharmaceutical development of medicines for paediatric use. doi: 10.1016/j.ijpharm.2012.05.053. EMA/CHMP/QWP/805880/2012 Rev. 2. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf. [DOI] [PubMed]
- 15.Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395–404. doi: 10.1111/bcp.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruf S, Hebart H, Hjalgrim LL et al. CNS progression during vinblastine or targeted therapies for high-risk relapsed ALK-positive anaplastic large cell lymphoma: a case series. Pediatr Blood Cancer. 2018;65(6):e27003. doi: 10.1002/pbc.27003. [DOI] [PubMed] [Google Scholar]
- 17.Lenk C, Duttge G. Ethical and legal framework and regulation for off-label use: European perspective. Ther Clin Risk Manag. 2014;10:537–546. doi: 10.2147/TCRM.S40232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frattarelli DA, Galinkin JL, Green TP et al. Off-label use of drugs in children. Pediatrics. 2014;133(3):563–567. doi: 10.1542/peds.2013-4060. [DOI] [PubMed] [Google Scholar]
- 19.Pearson AD, Heenen D, Kearns PR et al. 10-year report on the European Paediatric Regulation and its impact on new drugs for children's cancers. Lancet Oncol. 2018;19(3):285–287. doi: 10.1016/S1470-2045(18)30105-0. [DOI] [PubMed] [Google Scholar]
- 20.Pearson ADJ, Pfister SM, Baruchel A et al. From class waivers to precision medicine in paediatric oncology. Lancet Oncol. 2017;18(7):e394–e404. doi: 10.1016/S1470-2045(17)30442-4. [DOI] [PubMed] [Google Scholar]
- 21.Pearson AD, Herold R, Rousseau R et al. Implementation of mechanism of action biology-driven early drug development for children with cancer. Eur J Cancer. 2016;62:124–131. doi: 10.1016/j.ejca.2016.04.001. [DOI] [PubMed] [Google Scholar]