Abstract
OBJECTIVE
Procalcitonin (PCT) is a biomarker used as an indicator for inflammation and bacterial infections. In October 2018, our PICU implemented a PCT monitoring protocol incorporating cutoffs established in previous studies to help guide antibiotic decision-making in patients undergoing sepsis evaluation. The study objective was to evaluate adherence to the protocol with regard to PCT monitoring and antibiotic use.
METHODS
This retrospective review included PICU patients with systemic inflammatory response syndrome ages > 1 month to 18 years with at least 1 PCT level and blood culture obtained during the 9 months following protocol implementation. Patients were excluded if they received < 48 hours of antibiotic therapy, were neutropenic, or had antibiotics initiated at another hospital. Patients were evaluated for protocol adherence, defined as antibiotic continuation or discontinuation per protocol guidance without excess PCT monitoring. Descriptive statistics were employed.
RESULTS
Out of 100 patients evaluated, 50 patients were included. Full adherence was observed in 17 patients (34%). Reasons for non-adherence were excess PCT monitoring (54.5%), antibiotic continuation (30.3%), or both (15.2%). Of patients who were non-adherent due to antibiotic continuation, 61.5% had a positive respiratory viral panel (RVP). A total of 49 excess PCT levels were drawn, resulting in an additional $2,000 in health care costs and $15,000 in patient charges.
CONCLUSIONS
Overall, the impact of our PCT monitoring protocol was difficult to evaluate due to non-adherence, but it highlights potential areas of focus for improving PCT monitoring and antimicrobial stewardship, such as inclusion of RVP results.
Keywords: antimicrobial stewardship, biomarkers, pediatrics, procalcitonin
Introduction
In patients being evaluated for sepsis, judicious use of antibiotics is essential for optimizing patient outcomes and promoting antimicrobial stewardship.1 Procalcitonin (PCT) is a biomarker produced in the body in response to microbial toxins and inflammatory mediators. It has been used as a surrogate marker for inflammation and bacterial infections to aid in antibiotic decision-making, often in combination with additional biomarkers such as C-reactive protein (CRP).2 Although PCT has been shown to be useful in evaluating the presence of bacterial infections, clinically relevant cutoffs are still being evaluated in pediatric patients.3,4
One study that evaluated levels of clinically relevant biomarkers in bacterial infections in pediatric patients was the Optimizing Antibiotic Strategies in Sepsis (OASIS) I study.5 OASIS I proposed cutoffs for the combined monitoring of PCT and CRP at < 1.75 ng/mL and < 4.0 mg/dL, respectively.5 The authors concluded that using the established cutoffs provided a reliable negative predictive value of 90% for identifying the presence of bacterial infections.5 In October 2018, our PICU implemented a PCT monitoring protocol based on the cutoffs determined in OASIS I to help guide antibiotic decision-making in patients with systemic inflammatory response syndrome (SIRS) and limit unnecessary PCT monitoring. The primary objective of this study was to evaluate practitioner adherence to the established protocol with regard to PCT monitoring and antibiotic use.
Materials and Methods
This study was a retrospective chart review conducted at a 114-bed academic pediatric hospital with an 18-bed PICU. Patients were included if they were between the ages of 1 month and 18 years, were admitted to the PICU, and had at least 1 PCT level and blood culture drawn during a 9-month period following protocol initiation (December 1, 2018, to August 31, 2019). Patients were excluded if they received antibiotics for < 48 hours, were immunocompromised with an absolute neutrophil count < 500 cells per microliter, or if antibiotics were initiated at an outside hospital.
For patients who met inclusion criteria, the following information was collected: patient demographics, microbiology results, PCT and CRP levels, reason for antibiotic initiation, and antibiotic continuation or discontinuation at protocol decision points. Protocol adherence was evaluated based on where patients fell in the decision tree (see Figure 1). The primary outcome of this study was complete adherence to the established protocol, defined as antibiotic continuation or discontinuation without excess PCT monitoring. The secondary outcomes evaluated common reasons for protocol non-adherence, which included excess PCT levels and antibiotic continuation despite protocol-recommended discontinuation.
Figure 1.

Guideline for the use of procalcitonin (PCT) for children with SIRS
Obtain initial PCT and CRP levels (baseline levels) and initiate antibiotics if clinical suspicion for bacterial infection
Study data were collected and managed using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, TN) tools hosted at Penn State Health Milton S. Hershey Medical Center and Penn State College of Medicine. REDCap is a secure, web-based application designed to support data capture for research studies.6 Data analysis was completed using descriptive statistics.
Results
Patients were assessed chronologically in order of presentation until a sample size of 100 patients was reached. A total of 50 patients were included in data analysis. The primary reason for exclusion was an antibiotic duration less than 48 hours (n=23). Other causes for exclusion included: missing lab data (n=9), outside of PICU during infectious work-up (n=8), < 1 month of age (n=5), antibiotic initiation at outside hospital (n=4), and ANC <500 cells/microliter (n=1).
Table 1 shows patient characteristics and disposition. Of the 8 patients who died, none were attributed to sepsis. The majority of patients required mechanical ventilation during the study period. The most common indication for initiation of antibiotics was respiratory failure/distress (see Figure 2). Neurologic concerns, including meningitis and encephalitis, were the least common. Of the 50 patients, 15 patients (30%) had more than 1 concern listed leading to the initiation of antibiotics.
Table 1.
Patient Characteristics
| Parameter | Value |
|---|---|
| Age, median (IQR), yr | 2.3 (0.3–11.1) |
| Age categories, n (%) | |
| <1 yr | 20 (40) |
| 1–10 yr | 17 (34) |
| >10 yr | 13 (26) |
| Sex, n (%), male | 35 (70) |
| Mechanical ventilation, n (%) | 40 (80) |
| Invasive, n (%) | 32 (64) |
| Medical device* | 46 (92) |
| Surgical intervention | 12 (24) |
| Disposition | |
| Discharge | 34 (68) |
| Transfer | 8 (16) |
| Death | 8 (16) |
* Medical devices included foley catheters, central line devices, and chest tubes.
Figure 2.
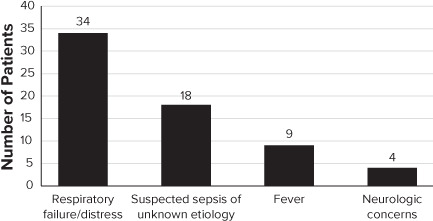
Indication for antibiotic initiation.
Microbiology testing results are displayed in Table 2. All but 1 patient had a respiratory viral panel (RVP) obtained, 51% of which were positive. In regards to bacterial cultures, 20 patients had at least 1 positive culture. Of these patients, 9 also had a positive RVP. Of the 30 culture-negative patients, 16 had a positive RVP.
Table 2.
Positive Microbiology Culture Results
| Culture | Value, n (%) |
|---|---|
| Blood* (n = 50) | 4 (8) |
| Urine (n = 31) | 6 (19.4) |
| Respiratory (n = 36) | 15 (41.7) |
| Wound (n = 1) | 1 (100) |
| Tissue (n = 1) | 0 (0) |
| Cerebrospinal fluid (n = 6) | 0 (0) |
| Pleural fluid (n = 1) | 1 (100) |
| Respiratory viral panel (n = 49) | 25 (51) |
* Two positive blood cultures were deemed contaminants.
All 50 patients were included in the analysis for the primary outcome. Overall, complete protocol adherence was observed in 17 patients (34%). Reasons for non-adherence were excess PCT monitoring (54.5%), continuation of antibiotics (30.3%), and both PCT monitoring and continued antibiotics (15.2%). For evaluation of secondary outcomes, patients were categorized based on bacterial culture results. In the 20 culture-positive patients, 11 patients (55%) had additional PCT levels drawn beyond protocol guidance and were classified as non-adherent. In the 30 culture-negative patients, 12 patients (40%) had additional PCT levels drawn beyond protocol guidance and were classified as non-adherent. Five of the 30 patients in the culture-negative group had a repeat PCT level ≥ 1.75 ng/mL and therefore were not recommended to have antibiotics discontinued per protocol. Thirteen of the 25 culture-negative patients (52%) with repeat PCT levels below the 1.75 ng/mL cutoff were classified as non-adherent due to continuation of antibiotics despite protocol guidance. Notably, 8 of these 13 patients had a positive RVP.
Throughout the study period, a total of 49 excess PCT levels were drawn on the included patients. These additional levels led to an excess of approximately $2,000 in health care costs and $15,000 in patient charges for the study population. Multiple CRP levels were also drawn on the majority of patients but were not included in the analysis because the protocol does not provide guidance on obtaining additional CRP levels.
Discussion
Surrogate markers for inflammation and bacterial infections have been used as prognostic factors to help stratify risk in pediatric patients with sepsis; however, few studies have been published evaluating the use of these biomarkers in an algorithm to help guide clinical and antibiotic decision-making.3 In OASIS II, a before and after study evaluating a biomarker-based algorithm on broad-spectrum antibiotic prescribing in PICU patients with SIRS, Downes et al.8 found that the combined use of CRP and PCT reduced antibiotic durations when both values were low at SIRS onset. However, because the majority of SIRS cases in this study did not fall into the low-risk category based on the biomarker cutoffs, antibiotic prescribing did not change overall.8
In our study, high rates of protocol non-adherence limited the potential impact of PCT and CRP monitoring on antibiotic use in PICU patients with SIRS. Similar to the OASIS II study, we also found that antibiotic decision-making varied among SIRS cases, despite being culture-negative, especially when either PCT or CRP fell above their respective low-risk cutoffs of 1.75 ng/mL and 4 mg/dL, respectively. However, our study was able to provide insight into real-world application of a PCT monitoring algorithm and identify potential areas of improvement. By improving provider education and adherence to our protocol, we could prevent unnecessary cost expenditures from excess laboratory monitoring and reduce antibiotic exposure in patients with low suspicion for infection.
Of particular interest was the potential impact of our protocol for patients presenting with respiratory distress. The majority of our patients who had antibiotics continued despite negative cultures and low PCT and CRP levels also had a positive RVP. In these patients, a viral infection serves as a potential alternative cause for the symptoms resulting in the infectious work-up. With respiratory viruses being a common cause of respiratory distress in pediatric patients, improving adherence to our protocol and including RVP results in the algorithm has the potential to significantly impact antibiotic use within this population.4,8 Using clinical judgement to correlate biomarker results with the overall clinical picture, sources of infection, and potential imaging when available is essential for optimizing protocol utility.
It is important that we continue to develop and refine biomarker-monitoring algorithms in pediatric patients with SIRS in order to optimize antibiotic use, reduce antibiotic resistance, and prevent unnecessary health care costs, adverse events, and hospital length-of-stays. Although there are few studies that have evaluated the implementation and effectiveness of these algorithms, the data on sepsis biomarkers suggest there is a place in therapy for biomarker-based protocols.7,8
Our study was limited by the single-center, retrospective study design, as we were unable to account for additional factors in clinical decision-making through our chart review, such as clinical picture and provider intentions. The results of our study could also be due to the small sample size and selection bias as we captured more patients who were direct admits to the ICU rather than transfers, which may highlight a more severe presentation. In relation to our protocol, the initial evaluation occurs after a standard 48-hour sepsis rule out timeframe. Therefore, we were unable to capture if antibiotics were stopped sooner than 48 hours, which may have excluded patients with lower concern for infection. Finally, we were unable to assess the effect of our protocol on clinical outcomes due to non-adherence. In the future, improving protocol adherence will allow us to evaluate antibiotic use pre- and post-protocol initiation, as well as gauge the effectiveness of our chosen PCT and CRP cutoffs.
Conclusion
Overall, we found high rates of non-adherence to our PCT monitoring protocol. Evaluating the use of the protocol highlights potential areas of focus for improving provider education in regards to PCT monitoring and antimicrobial stewardship. Future studies are needed to evaluate the use and outcomes of protocols using clinically relevant biomarkers in patients with SIRS. Our study suggests that there is potential for cost-savings and reduction of antibiotic use with an established PCT-monitoring protocol, which may be enhanced by consideration of RVP results in patients presenting with respiratory symptoms.
ABBREVIATIONS
- ANC
absolute neutrophil count
- CRP
C-reactive protein
- ICU
intensive care unit
- PCT
procalcitonin
- PICU
pediatric intensive care unit
- REDCap
Research Electronic Data Capture
- RVP
respiratory viral panel
- SIRS
systemic inflammatory response syndrome
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all data and take responsibility for the integrity and accuracy of the data analysis. REDCap grant disclaimer: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR002014 and Grant UL1 TR00045. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Ethical Approval and Informed Consent. Given the nature of this study, the institutional review board/ethics committee review approved this study under “exempt” status, and informed consent was not required.
References
- 1.Weiss SL, Peters MJ, Alhazzani W et al. Surviving sepsis campaign international guidelines for management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 2.Vijayan AL. Vanimaya, Ravindran S et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5(2017):51. doi: 10.1186/s40560-017-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanziotti VS, Povoa P, Soares M et al. Use of biomarkers in pediatric sepsis: literature review. Rev Bras Ter Intesiva. 2016;28(4):472–482. doi: 10.5935/0103-507X.20160080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotula JJ, 3rd, Moore WS, 2nd, Chopra A, Cies JJ. Association of procalcitonin value and bacterial coinfections in pediatric patients with viral lower respiratory tract infections admitted to the pediatric intensive care unit. J Pediatr Pharmacol Ther. 2018;23(6):466–472. doi: 10.5863/1551-6776-23.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downes KJ, Weiss SL, Gerber JS et al. Pragmatic biomarker-driven algorithm to guide antibiotic use in the pediatric intensive care unit: the Optimizing Antibiotic Strategies in Sepsis (OASIS) study. J Pediatr Infect Dis Soc. 2017;6(2):134–141. doi: 10.1093/jpids/piw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downes KJ, Fitzgerald JC, Schriver E et al. Implementation of a pragmatic biomarker-driven algorithm to guide antibiotic use in the pediatric intensive care unit: the Optimizing Antibiotic Strategies in sepsis (OASIS) II study. J Pediatr Infect Dis Soc. 2020;9(1):36–43. doi: 10.1093/jpids/piy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito S, Tagliabue C, Picciolli I et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med. 2011;105(12):1939–1945. doi: 10.1016/j.rmed.2011.09.003. [DOI] [PubMed] [Google Scholar]


