Abstract
Introduction:
Percutaneous endoscopic gastrostomy (PEG) is the main accepted method for long-term tube feeding. The aim of this study is to investigate the risk factors associated with early mortality after PEG.
Methods:
It is a retrospective survival analysis in a tertiary-level hospital. We reviewed the medical records of 277 patients with PEG placement. The data were analyzed by the Kaplan-Meier method. Multivariable Cox proportional regression models were also built to test the effects of PEG on mortality.
Results:
A total of 277 patients who submitted to PEG were studied. One-hundred and sixty (58%) were female, mean age of 73.3 ± 15.7 years. Ninety-three patients (33.6%) had diabetes mellitus and 165 (59.6%) had blood hypertension. The indications for PEG placement were chronic neurologic dysphagia in 247 (89.5%) patients and tumors and other diseases in 29 (10.5%). The 30 days proportional mortality probability rate was 13%. In a multivariate Cox proportional regression model, preoperative ICU hospitalization (HR 1.79, 95% CI 1.36–2.36, P = 0.000) and hemoglobin (HR 0.91, 95% CI 0.85–0.98, P = 0.015) were predictors of early mortality.
Conclusion:
In patients who had underwent PEG tube insertion for long-term nutrition, anemia and previous ICU admission were predictors of mortality at four weeks. These factors may guide physicians to discourage the indication for PEG.
Keywords: Deglutition disorders, Malnutrition, Enteral nutrition, Critical illness, PEG tube
INTRODUCTION
Percutaneous endoscopic gastrostomy (PEG) has become the mode of choice for long-term enteral feeding over the last decades.1 Gauderer et al2 first reported a PEG tube insertion in pediatric patients with dysphagia but its use has now spread to adults as well. The objective of the procedure is to stabilize and improve the patient’s nutritional status and, consequently, their weight.3 Dysphagia due to cerebrovascular disease, oropharyngeal, and esophageal tumors are the most common indications for PEG tube placement.4
The PEG tube placement should be performed when the need for enteral nutrition is permanent or longer than six weeks. Life expectancy lower than two months and end-stage chronic diseases are contraindications to the procedure.5 Although this procedure is fast and safe, it is not free of complications and is associated with early mortality rates,3,6,7 demonstrating the need for a better and objective patient selection criterion to avoid futile indications and wasting resources. Different authors have identified risk factors associated with early mortality, and, thus, it is important to elaborate a clinical protocol to protect patients who would not benefit from this endoscopic procedure.1,3–22
The aim of the present study is to identify risk factors associated with early mortality in patients availing the PEG insertion to establish a protocol for clinical practices that could help in making decisions regarding undergoing the procedure.
METHODS
Study Design
This is a retrospective study with patients who underwent PEG placement between Jan 2012 and Apr 2019 in a tertiary-care hospital. This hospital is highly certified by the Canadian Council for Health Service Accreditation due to its quality of patient safety, security, and clinical protocols. Furthermore, it has an electronic medical records system certified by the Healthcare Information and Management System Society in its highest level.
Data Collection
Data was collected from adult patients requiring long-term enteral nutrition and submitted to PEG placement for the first time. The exclusion criteria included patients submitted to the PEG replacement, outpatients, patients with incomplete charts, and patients with unsuccessful procedures. PEG placement was indicated by the primary doctor responsible for the patient after a complete assessment of the patient’s ability to no longer feed themselves or with help of another person and were dependent of tube feeding. The doctors discussed its risks, complications, and advantages with the patient or with their family, when they could not decide for themselves.
The following variables were collected from the patient’s charts: age, gender, main diagnosis (chronic neurologic diseases, tumors, dementia), comorbidities (hypertension, diabetes mellitus, renal disease), laboratory exams (albumin, C-reactive protein, hemoglobin, sodium, potassium, urea), surgical complications, time of survival, and endoscopic report. We considered bleeding, dermatitis, peritonitis, abscess, buried bumper syndrome, and avulsion as surgical complications.
Pre- and postoperative data were also collected: dialysis, orotracheal tube procedure, hospitalization in intensive care unit (ICU). We considered a period of two months before and after the PEG placement.
Surgical Procedure
All procedures were performed by the same surgical team with the support of the anesthesia team. All patients received antibiotic prophylaxis with 2 g of cefazolin unless they were taking antibiotics for other reasons. Sedation was performed with the anesthesiologist’s assistance when necessary. The technique described by Gauderer et al2 was used to place the PEG tubes using a standard forward-view endoscope (GIFH-180; Olympus America, Center Valley, PA). The procedure was performed in an endoscopy operative room that meets the requirements of the Brazilian legislation. Either a PEG 24 Pull Method Kit (Cook Endoscopy, Indiana, USA) or EndoViveTM enteral access initial placement system (24 Fr Peg-Kit, Boston Scientific, Marlborough, MA, USA) was employed at the team’s judgement. Feeding through the tube was partially initiated six hours after the procedure and, after 24 hours, the patients were fed according to the nutrition team. The surgical team conducted follow-up sessions of the patients until their discharge and their caregivers were instructed on how to properly manage the gastrostomy cannula to avoid further complications.
Statistical Analysis
Continuous variables were expressed by the mean ± S.D. and compared by Student’s t test or median and range and compared with the Mann–Whitney U test. Univariate Cox models were used to identify factors associated with early mortality (four weeks) after the PEG placement. All factors with P < .20 after the univariate analysis were included in the multivariate model to evaluate their independent effects. A backward stepwise procedure was used for the final Cox model with factors with P < .05. In both univariate and multivariate analysis Cox models, the data was reported with hazard ratios (HR) and 95% confidence interval (CI). Proportional mortality curves were plotted as Kaplan-Meier estimates. Two-tailed P values of less than 0.05 were considered statistically significant.
The data was analyzed using the R Project Statistical Computing software version 40.00.1 (www.r-project.org). This study was approved by the Institution Review Board number 662886170.3.0000.5192 and all the Health Insurance Portability and Accountability Act (HIPAA) compliant mechanisms were followed.
RESULTS
Study Population
A total of 277 patients were included in our study (Figure 1), out of which 160 (58%) were female and the mean age was 73.3 ± 15.7 years. Of the total, 93 patients (33.6%) had diabetes mellitus and 165 (59.6%) had blood hypertension. The baseline characteristics of the patients who were submitted to the PEG tube placement are shown in Table 1. The indications for the PEG placement were chronic neurologic dysphagia in 247 (89.5%) patients, and tumors and other diseases in 29 (10.5%). Two weeks before the procedure, 175 patients (63.4%) were in the Intensive Care Unit (ICU), 119 (43.1%) patients had a tracheostomy, 22 (7.9%) patients had an orotracheal tube, and 13 (4.7%) were submitted to dialysis. The differences between groups according to mortality are in Table 1.
Figure 1.

Flowchart of the patients selection.
Table 1.
Patients Characteristics
| n (%) | Total (n = 277) | <4 Weeks (n = 36) | >4 Weeks (n = 240) | P |
|---|---|---|---|---|
| Female | 160 (58) | 19 (52.8) | 141 (58.7) | 0.49* |
| Hypertension | 165 (59.6) | 21 (58.3) | 144 (59.7) | 0.87* |
| Diabetes mellitus | 93 (33.6) | 8 (22.2) | 85 (35.3) | 0.12* |
| Dialysis§ | 13 (4.7) | 4 (11.1) | 9 (3.7) | 0.052* |
| Orotracheal tube§ | 22 (8) | 3 (8.3) | 19 (8) | 0.93* |
| ICU hospitalization§ | 175 (63.4) | 30 (83.3) | 145 (60.4) | 0.008* |
| Tracheostomy§ | 119 (43.1) | 19 (52.8) | 100 (41.7) | 0.209* |
| Age, years*** | 73.3 ± 15.7 | 79.4 ± 14.3 | 77 ± 16 | 0.37 |
| Hemoglobin*** | 10.5 ± 2 | 9.4 ± 1.7 | 10.6 ± 2 | 0.002** |
| BUN*** | 58.8 ± 37.8 | 69.7 ± 36.2 | 57.1 ± 37.9 | 0.028** |
| Albumin (n = 122)*** | 3 ± 0.5 | 3 ± 0.6 | 3 ± 0.5 | 0.37† |
| Potassium (n = 223)*** | 4.3 ± 0.6 | 4.3 ± 0.6 | 4.2 ± 0.6 | 0.47† |
| CRP (n = 197)*** | 1464 ± 1464.8 | 413 ± 1492.2 | 1639 ± 4241.2 | 0.21† |
χ2, Pearson’s test; **, Mann–Whitney U test; ***, mean ± standard deviation; †, Student’s t test; §, preoperative period; ICU, intensive care unit; CRP, C reactive protein; BUN, blood urea nitrogen.
PEG-Related Complications
Among the participants, 59 patients had PEG-related complications. Early complications after PEG insertion were: tube avulsion in 35 (12.7%) patients, followed by dermatitis in 13 (4.7%), abscess in six (2.2%), peritonitis in four (1.4%), and bleeding in one (Table 2).
Table 2.
Clinical Outcomes and Complications of Percutaneous Endoscopic Gastrostomy
| n (%) | Total (n = 276) | <4 Weeks (n = 36) | >4 Weeks (n = 240) | P |
|---|---|---|---|---|
| Dialysis* | 21 (7.6) | 7 (19.4) | 14 (5.8) | 0.004 |
| Tracheostomy* | 106 (38.4) | 20 (55.6) | 86 (35.8) | 0.023 |
| Orotracheal tube* | 14 (5.1) | 5 (14) | 9 (3.8) | 0.01 |
| ICU hospitalization* | 119 (43.1) | 22 (61.1) | 97 (40.4) | 0.019 |
| Abscess | 6 (2.2) | 1 (2.8) | 5 (2.1) | 0.79 |
| Peritonitis | 4 (1.4) | 0 (0) | 4 (1.7) | 0.43 |
| Avulsion | 35 (12.7) | 3 (8.3) | 32 (13.3) | 0.40 |
| Dermatitis | 13 (4.7) | 0 (0.0) | 13 (5.4) | 0.153 |
| Bleeding | 1 (0.4) | 0 (0.0) | 1 (0.4) | 1.000 |
| Buried Bumper syndrome | 15 (5.4) | 1 (2.78) | 14 (5.83) | 0.451 |
*, Postoperative period.
Up to two weeks after the PEG insertion, 119 (43.1%) had been in the ICU, 106 (38.4%) had a tracheostomy tube, 21 (7.6%) underwent dialysis for renal insufficiency, and 14 (5.1%) were intubated (none were reintubations). There was no death related directly to PEG placement and the patients did not need other surgical procedures.
Mortality
The proportional mortality probability at 30 days was 13%. A total of 36 patients died in the time leading up to four weeks (Figure 2).
Figure 2.
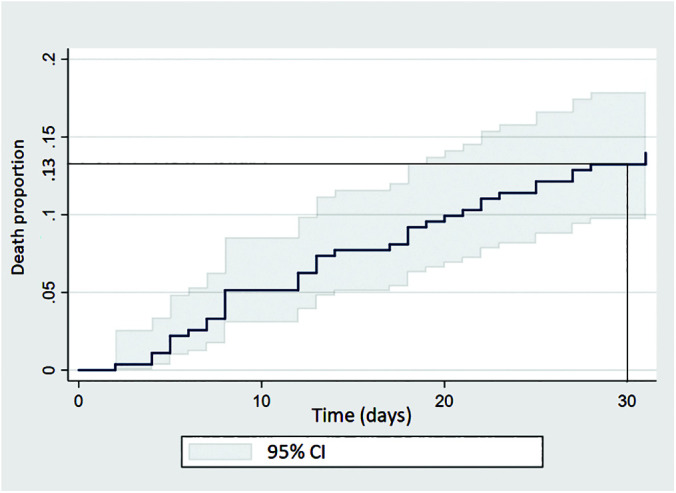
Kaplan-Meier graph showing four-week mortality.
Variables Associated with Mortality up to Four Weeks
Nine preoperative and three postoperative variables that are considered relevant for predicting survival at four weeks were tested using univariate analysis. As shown in Table 3, hemoglobin, BUN, and ICU internment were considered significantly associated with mortality up to four weeks. Postoperative dialysis, tracheostomy, and ICU internment were also associated to mortality in four weeks.
Table 3.
Univariate Analysis of Factors Associated with Survival at Four Weeks
| Variables | HR (CI 95%) | P |
|---|---|---|
| Preoperative dialysis | 1.33 (0.4–3.9) | 0.598 |
| Preoperative ICU hospitalization | 2 (1.5–2.6) | <0.001 |
| Preoperative endotracheal intubation | 1.05 (0.3–3.5) | 0.930 |
| Preoperative tracheostomy | 1.06 (0.5–2.07) | 0.848 |
| Hemoglobin | 0.9 (0.8–0.9) | <0.001 |
| BUN | 1.005 (1.002–1.008) | <0.001 |
| Albumin | 1.09 (0.5–2.3) | 0.801 |
| Potassium | 0.99 (0.6–1.6) | 0.996 |
| CRP | 1.00 (0.99–1.00) | 0.692 |
| Postoperative dialysis | 3.11 (1.9–4.9) | <0.001 |
| Postoperative ICU hospitalization | 1.6 (1.3–2.1) | <0.001 |
| Postoperative tracheostomy | 1.7 (1.3–2.2) | <0.001 |
HR, hazard Ratio; BUN, blood urea nitrogen; CRP, C reactive protein; ICU, intensive care unit.
Variables Independently Associated with Mortality up to Four Weeks
A multivariate analysis was performed for all variables by the Cox regression. Hemoglobin and preoperative ICU hospitalization were independently associated with mortality up to four weeks by the final model (Table 4).
Table 4.
Results of the Adjustments of the Initial and Final Cox Regression Models to Identify Risk Factors for Mortality of PEG in Patients
| Initial Model |
Final Model |
|||
|---|---|---|---|---|
| Variables | HR (CI 95%) | P | HR (CI 95%) | P |
| Postoperative dialysis | 2.74 (1.7–4.45) | <0.001 | 3.06 (1.91–4.89) | <0.001 |
| Preoperative ICU hospitalization | 1.7 (1.25–2.3) | 0.001 | 1.79 (1.36–2.36) | <0.001 |
| Postoperative ICU hospitalization | 1.14 (0.86–1.51) | 0.342 | ||
| Postoperative tracheostomy | 1.39 (1.07–1.82) | 0.013 | 1.45 (1.11–1.88) | 0.005 |
| Hemoglobin | 0.92 (0.86–0.99) | 0.041 | 0.91 (0.85–0.98) | 0.015 |
| BUN | 1 (0.99–1.00) | 0.127 | ||
BUN, blood urea nitrogen; ICU, intensive care unit; HR, hazard ratio.
DISCUSSION
Our study demonstrates that low hemoglobin and preoperative ICU hospitalization are factors associated with early mortality in patients submitted to the PEG tube insertion. Moreover, early mortality after the procedure was high (13%), in accordance with the literature, which reports 30-day mortality rates from 1.2 to 32.5%.
Anemia and ICU hospitalization prior to the procedure were independent risk factors for mortality. Our previous study with preliminary data and 150 patients revealed that hemoglobin lower than 10 mg/dL and preoperative ICU hospitalizations as predictors of mortality in eight weeks.23 This was the first study to demonstrate these factors as predictors, but we did not have a proper sample to evaluate mortality in four weeks at the time. The present study with almost double the patients allowed us to proper evaluate four-week mortality leading us to confirm that the same factors were associated with mortality.
Various studies have investigated factors with the risk of 30-day mortality in different populations. However, many did not exhibit an adequate mathematical analysis, and they identified heterogeneous factors that are grouped in two big categories: factors associated with chronic diseases and factors associated with advanced signs of malnutrition (hypoalbuminemia, anemia). Laboratory parameters such as serum albumin, C-reactive protein, serum sodium, BUN, and neutrophils were independently associated with mortality.4,13–18,20,21 Our previous study was the only one to discover low hemoglobin as a predictor of mortality. Age and malignancy were also risk factors described by some authors.3,5,16,19,21 Our sample was not enough to accurately study the effects of serum albumin, serum sodium, and C-reactive protein in early mortality due to the absence of these values in many patients: chiefly albumin and C-reactive protein.
Other authors reported early mortality after PEG but with no associated factors.24–29 In our cohort, surgical complications from the procedure were not predictors of mortality. Oh et al24 evaluated 116 patients with only three deaths (2.6%) in 30 days. PEG-related complications and mortality were not increased in the elderly group (≥ 65 years old) when compared to younger patients. Logistic regression in their cohort did not find any factor independently associated with mortality. Wirth et al9 performed a prospective multicenter observational study with 197 patients, where dysphagia and insufficient food intake were the main indications for PEG insertion and 9.6% of the patients had severe complication after the procedure. Mortality was higher in patients with severe complications caused by the procedures, such as peritonitis and severe wound infections.
Our study had no deaths from complications after the PEG placement. Despite the fact that these factors cannot be evaluated before the procedure, they were not associated with mortality. In our multivariate analysis, postoperative tracheostomy and dialysis were also independently associated with mortality. It was also found that patients who submitted to these interventions had higher risks of mortality. However, these factors can only be identified after the PEG placement and cannot aid in determining which patient would benefit from the surgical procedure.
The Timing to PEG
The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines for the PEG tube placement states that its indication should not be a terminal measure in patients with short life expectancy or advanced dementia.30 The European Society of Gastrointestinal Endoscopy (ESGE) also recommends refraining from PEG placement in patients with a life expectancy shorter than 30 days.31 Furthermore, it is important to select patients properly based on medical and ethical indications. Older patients with severe comorbidities have lower quality of life after the PEG insertion. Despite the plurality of parameters and variables associated with early mortality found described in the literature, the problem may be the timing when the procedure is indicated. An early indication prior to weight loss and catabolism may benefit patients as suggested by Dietrich and colleagues.32 The ESGE guidelines (2021) also recommends early PEG tubes in some patients with chronic degenerative diseases or some types of malignancy if patients present weight loss despite of oral nutrition.31 Performing the procedure when the patient is already malnourished and suffering from an advanced disease may be a severe mistake, contributing to early mortality. Studies have showed that increased levels of albumin are associated with longer survival.13–16 In our study, patients with anemia or those who had been in the ICU before the procedure were not in their best nutritional status when evaluated for the PEG insertion. The same might have happened in other cohorts with patients with low serum albumin, malignancy, and elevated inflammatory markers such as the C-reactive protein.
Strengths and Limitations
This study has several limitations. First, it is a retrospective cohort study with no comparison group. We could not evaluate all the data we wanted due to the lack of information in the medical records. Hospitalized patients have a higher risk of death after the PEG tube placement and our cohort was composed solely of these patients. Our study does not consider the locoregional characteristics of the population and as such, adds to the limitation of the analysis. Many patients were tended to at home with inadequate care for certain types of complications. Therefore, we cannot provide an external validation of our results. The strength of this study lies in our sample, the numbers of which practically doubled from our previous study. This larger sample with increased power could proportionate more reliable results.
CONCLUSION
In conclusion, our study discovered that anemia and previous ICU admission were predictors of mortality at four weeks after the percutaneous endoscopic gastrostomy insertion. These factors may guide physicians to discourage the indication for PEG. The indication for the PEG tube should be rigidly assessed in patients who are either in the ICU and suffer from low hemoglobin levels. It is of utmost importance that a protocol must be developed to address all patients who should benefit from the procedure based on their baseline characteristics.
Acknowledgments:
We thank Complexo Hospitalar Unimed Recife and Dr. Fernando Cruz for their collaboration with this manuscript.
Footnotes
Disclosure: none.
Funding: This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), under finance code 001.
Conflicts of interest: The authors declare no conflict of interest.
Informed consent: Dr. D. L. Lima declares that written informed consent was obtained from the patient’s for publication of this study/report and any accompanying images.
Contributor Information
Diego L. Lima, Department of Surgery, Montefiore Medical Center, The Bronx, New York, USA..
Luiz Eduardo C. Miranda, Department of Surgery, Oswaldo Cruz University Hospital, Recife, Brazil..
Marcel Rolland Ciro da Penha, Department of Surgery, Oswaldo Cruz University Hospital, Recife, Brazil.; Catholic University of Pernambuco, Recife, Brazil.
Raquel N. C. L. Lima, Pernambuco Health College, Recife, Brazil..
Dalmir Cavalcanti dos Santos, Pernambuco Health College, Recife, Brazil..
Matheus Stillner Eufrânio, Oswaldo Cruz University Hospital, University of Pernambuco..
Ana Clara G. Miranda, Oswaldo Cruz University Hospital, University of Pernambuco.
Leila Maria Moreira Beltrão Pereira, Pernambuco Institute of Liver. Oswaldo Cruz University Hospital, University of Pernambuco, Recife, Brazil..
References:
- 1.Chong VH, Vu C. Percutaneous endoscopic gastrostomy outcomes: can patient profiles predict mortality and weaning? Singapore Med J. 2006. May; 47(5):383–387. [PubMed] [Google Scholar]
- 2.Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872–875. [DOI] [PubMed] [Google Scholar]
- 3.Zopf Y, Maiss J, Konturek P, Rabe C, Hahn EG, Schwab D. Predictive factors of mortality after PEG insertion: guidance for clinical practice. JPEN J Parenter Enteral Nutr. 2011;35(1):50–55. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa M, Magalhaes J, Marinho C, Cotter J. Predictive factors of early mortality after percutaneous endoscopic gastrostomy placement: the importance of C-reactive protein. Clin Nutr Espen. 2016;14:19–23. [DOI] [PubMed] [Google Scholar]
- 5.Agudo Tabuenca A, Altemir Trallero J, Gimeno Orna JA, Ocón Bretón MJ. Mortality risk factors after percutaneous gastrostomy: who is a good candidate? Clin Nutr. 2019;38(2):856–861. [DOI] [PubMed] [Google Scholar]
- 6.Verhoef MJ, Van Rosendaal GM. Patient outcomes related to percutaneous endoscopic gastrostomy placement. J Clin Gastroenterol. 2001;32(1):49–53. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo F. A F, da Costa MC, Pelosi AD, Martins RN, Machado L, Francioni E. Predicting outcomes and complications of percutaneous endoscopic gastrostomy. Endoscopy. 2007;39(04):33–338. [DOI] [PubMed] [Google Scholar]
- 8.Varnier A, Iona L, Dominutti MC, et al. Percutaneous endoscopic gastrostomy: complications in the short and long-term follow-up and efficacy on nutritional status. Eura Medicophys. 2006;42(1):23–26. [PubMed] [Google Scholar]
- 9.Wirth R, Voss C, Smoliner C, Sieber CC, Bauer JM, Volkert D. Complications and mortality after percutaneous endoscopic gastrostomy in geriatrics: a prospective multicenter observational trial. J Am Med Dir Assoc. 2012;13(3):228–233. [DOI] [PubMed] [Google Scholar]
- 10.Sanders DS, Carter MJ, D'Silva J, James G, Bolton RP, Bardhan KD. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterology. 2000;95(6):1472–1475. [DOI] [PubMed] [Google Scholar]
- 11.Richter-Schrag HJ, Richter S, Ruthmann O, Olschewski M, Hopt UT, Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol. 2011;25(4):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisman DN, Levy AR, Gifford DR, Tamblyn R. Survival after percutaneous endoscopic gastrostomy among older residents of Quebec. J Am Geriatr Soc. 1999;47(3):349–353. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Im JP, Kim JW, et al. Small Intestine Research Group of the Korean Association for the Study of Intestinal Disease (KASID). Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: a multicenter, retrospective study. Surg Endosc. 2013;27(10):3806–3815. [DOI] [PubMed] [Google Scholar]
- 14.Lang A, Bardan E, Chowers Y, et al. Risk factors for mortality in patients undergoing percutaneous endoscopic gastrostomy. Endoscopy. 2004;36(6):522–526. [DOI] [PubMed] [Google Scholar]
- 15.Blomberg J, Lagergren J, Martin L, Mattsson F, Lagergren P. Complications after percutaneous endoscopic gastrostomy in a prospective study. Scand J Gastroenterol. 2012;47(6):737–742. [DOI] [PubMed] [Google Scholar]
- 16.Leeds J, McAlindon ME, Grant J, et al. Albumin level and patient age predict outcomes in patients referred for gastrostomy insertion: internal and external validation of a gastrostomy score and comparison with artificial neural networks. Gastrointest Endosc. 2011;74(5):1033–1039. e1-3. [DOI] [PubMed] [Google Scholar]
- 17.Kara O, Kizilarslanoglu MC, Canbaz B, et al. Survival after percutaneous endoscopic gastrostomy in older adults with neurologic disorders. Nutr Clin Pract. 2016;31(6):799–804. [DOI] [PubMed] [Google Scholar]
- 18.Gingold-Belfer R, Weiss A, Geller A, et al. Increasing serum albumin level shortly after gastrostomy tube insertion predicts longer survival in elderly patients with dementia. J Clin Gastroenterol. 2017;51(4):339–344. [DOI] [PubMed] [Google Scholar]
- 19.Poulose BK, Kaiser J, Beck WC, et al. Disease-based mortality after percutaneous endoscopic gastrostomy: utility of the enterprise data warehouse. Surg Endosc. 2013;27(11):4119–4123. [DOI] [PubMed] [Google Scholar]
- 20.Muratori R, Lisotti A, Fusaroli P, et al. Severe hypernatremia as a predictor of mortality after percutaneous endoscopic gastrostomy (PEG) placement. Dig Liver Dis. 2017;49(2):181–187. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Tamez S, Murakami A, et al. Survival of geriatric patients after percutaneous endoscopic gastrostomy in Japan. World J Gastroenterol. 2010;16(40):5084–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Light VL, Slezak FA, Porter JA, Gerson LW, McCord G. Predictive factors for early mortality after percutaneous endoscopic gastrostomy. Gastrointest Endosc. 1995;42(4):330–335. [DOI] [PubMed] [Google Scholar]
- 23.Miranda LE, Penha MRC, da Miranda ACG, et al. Risk factors associated with early mortality after percutaneous endoscopic gastrostomy in patients at a tertiary care center in Brazil: a retrospective single-center survival study. Arq Gastroenterol. 2019;56(4):412–418. [DOI] [PubMed] [Google Scholar]
- 24.Oh DJ, Kim B, Lee JK, Kang HW, et al. Can percutaneous endoscopic gastrostomy be carried out safely in the elderly? Geriatr Gerontol Int. 2016;16(4):481–485. [DOI] [PubMed] [Google Scholar]
- 25.Callahan CM, Haag KM, Weinberger M, et al. Outcomes of percutaneous endoscopic gastrostomy among older adults in a community setting. J Am Geriatr Soc. 2000;48(9):1048–1054. [DOI] [PubMed] [Google Scholar]
- 26.Laskaratos F-M, Walker M, Walker M, et al. Predictive factors for early mortality after percutaneous endoscopic and radiologically-inserted gastrostomy. Dig Dis Sci. 2013;58(12):3558–3565. [DOI] [PubMed] [Google Scholar]
- 27.Longcroft-Wheaton G, Marden P, Colleypriest B, Gavin D, Taylor G, Farrant M. Understanding why patients die after gastrostomy tube insertion: a retrospective analysis of mortality. JPEN J Parenter Enteral Nutr. 2009;33(4):375–379. [DOI] [PubMed] [Google Scholar]
- 28.Gundogan K, Yurci A, Coskun R, et al. Outcomes of percutaneous endoscopic gastrostomy in hospitalized patients at a tertiary care center in Turkey. Eur J Clin Nutr. 2014;68(4):437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baltz JG, Argo CK, Al-Osaimi AMS, Northup PG. Mortality after percutaneous endoscopic gastrostomy in patients with cirrhosis: a case series. Gastrointest Endosc. 2010;72(5):1072–1075. [DOI] [PubMed] [Google Scholar]
- 30.Löser C, Aschl G, Hébuterne X, et al. ESPEN guidelines on artificial enteral nutrition–percutaneous endoscopic gastrostomy (PEG). Clin Nutr. 2005;24(5):848–861. [DOI] [PubMed] [Google Scholar]
- 31.Arvanitakis M, Gkolfakis P, Despott EJ, et al. Endoscopic management of enteral tubes in adult patients: part 1: definitions and indications. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53(1):81–92. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich CG, Schoppmeyer K. Percutaneous endoscopic gastrostomy. Too often? Too late? Who are the right patients for gastrostomy? World J Gastroenterol. 2020;26(20):2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]


