Abstract
Background and Objectives:
The rise in cesarean deliveries, has led to increase in maternal complications in subsequent pregnancies such as abnormal placental implantation, uterine rupture, hemorrhage and, less commonly, cesarean scar pregnancies (CSP). Our objective was to describe patient characteristics following a combined medical and surgical treatment approach to first trimester cesarean scar pregnancies.
Methods:
This was a case series approved by the Institutional Review Board of cesarean scar pregnancies over a two-year period at a single academic institution. The study included five patients with diagnosed cesarean scar pregnancies opting for pregnancy termination with the desire for fertility preservation. Medical treatment involved intra-gestational sac injection of lidocaine followed by systemic injection of methotrexate. At a minimum of two months later, surgical resection of cesarean scar pregnancy and repair of the uterus was performed.
Results:
Median patient age was 36 (range 34 – 42) years, with 4 (3 – 10) prior pregnancies and 2 (1 – 3) prior cesarean deliveries. 40% (2/5) were Hispanic, 20% (1/5) Caucasian, 20% (1/5) African-American, and 20% (1/5) South Asian. After medical intervention, patients waited on average 4.6 ± 2.3 months before surgery. No post-intervention complications or recurrences occurred. Two patients had a subsequent pregnancy.
Conclusion:
This case series demonstrates an ideal management of cesarean scar pregnancy using combined medical and surgical approach in treating current ectopic pregnancy and repairing the uterine defect successfully without recurrence.
Keywords: Ectopic pregnancy, Cesarean delivery, Pregnancy termination
INTRODUCTION
Cesarean deliveries currently represent approximately 32% of deliveries in the United States and have increased 60% since 1996.1 With this rise in cesarean deliveries, there has been an increase in maternal complications in subsequent pregnancies such as abnormal placental implantation, uterine rupture, hemorrhage and, less commonly, cesarean scar pregnancies (CSP).2 A hysterotomy at the time of a cesarean delivery may potentially leave a uterine defect, or “niche”, with a reported prevalence of 24 – 70% after one cesarean delivery.3
A cesarean scar pregnancy is formed upon embryo implantation in the niche, followed by invasion of the placental tissue within, and often through, the myometrial walls. The current incidence of cesarean scar pregnancies is between 1/1800 to 1/2000 pregnancies, representing about 6% of ectopic pregnancies.4,5 Scar pregnancies are often misdiagnosed and mistaken for cervical ectopic pregnancy or threatened abortions6 and are associated with significant maternal morbidity including uterine rupture, hemorrhage, and death.2
Early diagnosis and treatment of the condition is essential. However, the “gold standard” modality for treatment of scar pregnancies is yet to be established. Since its original diagnosis in 1978, suggested medical treatments include expectant management, systemic and local injection of methotrexate (MTX), ultrasound guided aspiration or injection of abortifacients, and uterine artery embolization.4,5,7,8 Surgical options include dilation and curettage, hysteroscopy, open or laparoscopic resection and hysterectomy.4,6,9,10 A review of 112 cases of scar pregnancies demonstrated that surgical wedge resection with repair of uterus had the highest success rate in comparison with D&C, which was associated with high maternal morbidity.5 The first laparascopic repair of uterine scar defect (niche or uteroperitoneal or isthmocele) was reported in 2003.11,12 Our objective was to describe patient characteristics following a combined medical and surgical treatment approach to first trimester cesarean scar pregnancies.
MATERIALS AND METHODS
This case series was approved by the Institutional Review board, and cesarean scar pregnancies were diagnosed at a single academic institution and treated using a protocol of combined medical and surgical approaches as described below. Cases were collected over a span of two years from 2016 to 2018. Patients were diagnosed by positive serum beta human chorionic gonadotropin (hCG) and first trimester ultrasonography identifying an intrauterine pregnancy in the location of an anterior lower uterine segment scar with the following sonographic signs4,5,13: loss of myometrial wall thickness surrounding the gestational sac (Figure 1), low implantation of the gestational sac (Figure 2), empty uterine fundus (Figure 3), and Doppler evidence of vascular invasion into the myometrium (Figure 4).
Figure 1.
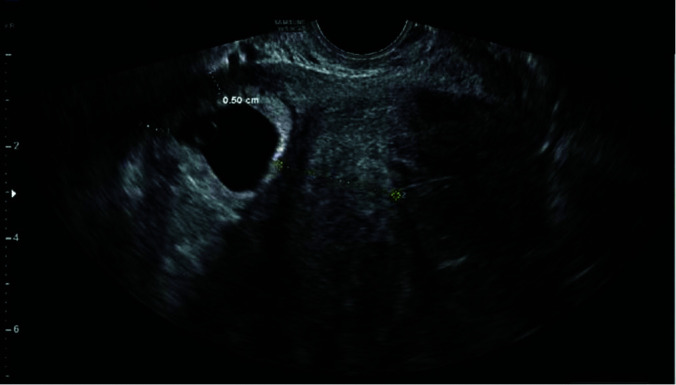
Low implantation of the gestational sac with loss of myometrial wall thickness anteriorly.
Figure 2.
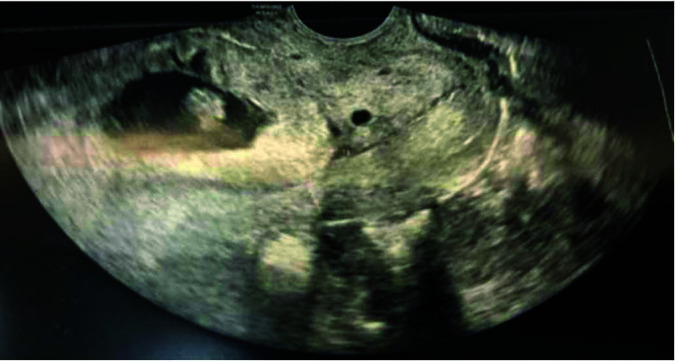
Low implantation of gestational sac, above the internal cervical os.
Figure 3.
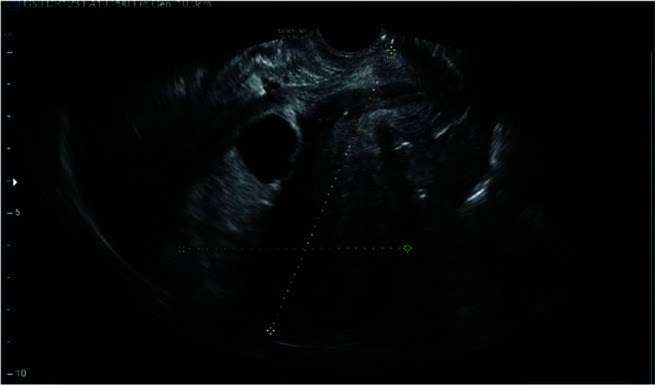
Cross-hair lines indicating an empty fundal cavity with gestational sac implanted in lower uterus.
Figure 4.
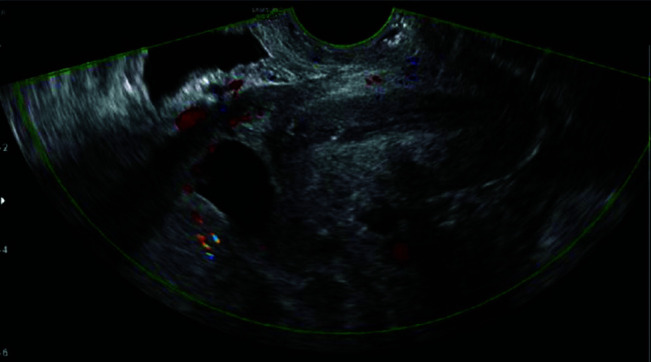
Doppler evidence of vascular invasion into the surrounding myometrium.
Upon diagnosis of cesarean scar pregnancy, patients were counseled regarding their options including medical, surgical, and expectant management in addition to risks and benefits of continuation of pregnancy. All patients opted for pregnancy termination with the desire for fertility preservation. Treatment began with transabdominal sonographic guided intra-gestational sac injection of 1 or 2% lidocaine depending on availability, 5 – 10 cc, until cardiac activity ceased (Figure 5). This was immediately followed by a single systemic intramuscular injection of 50 mg/m2 methotrexate. Patients were monitored outpatient with serial beta hCG levels to ensure continued downward trend with no plateau. Once medical therapy was completed, patients were advised to wait a minimum of 2 months prior to undergoing laparoscopic resection of the cesarean scar pregnancy with repair of uterine defect. During this time patients were advised to use barrier contraception. Prior to surgery, all patients had sonographically documented persistent products of conceptions within the uterine niche.
Figure 5.
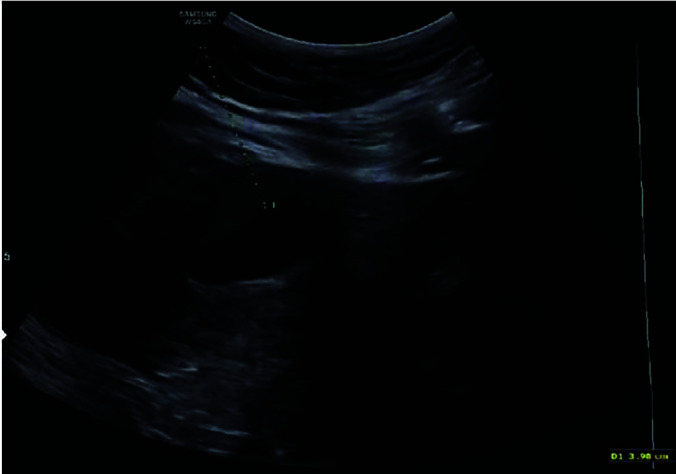
Initial treatment with sonographic mapping for ultrasound guided transabdominal intra-gestational sac injection of lidocaine.
The following describes our technique using robotic assisted laparoscopic resection of residual products of conception and repair of uterine defect in the treatment of cesarean scar pregnancies. Surgeries were completed on an outpatient basis under general endotracheal anesthesia after appropriate consents were obtained. Video hysteroscopy was performed to visualize the defect and identify the location of the pregnancy. Once identified, attempts were made to remove as much products of conception as possible with the resectoscope loop without energy (Figure 6). This was done to be able to better evaluate the uterine cavity. A HUMI® uterine manipulator (Copper Surgical, Trumbull, CT) was then placed. Exploratory laparoscopy was then performed using the da Vinci XI (Intuitive, Sayville, CA) robotic platform.14 Three robotic 8-mm ports were placed at the level of umbilicus for entry of the telescope, electrosurgical scissors, and bipolar forceps. One 12-mm assist trocar was placed in the right lower abdomen for introducing suture material and removal of specimen. The procedure started with lysis of any adhesions between the uterus and anterior abdominal wall (Figure 7). At this point, a bladder flap was developed using a combination of sharp and blunt dissection. To decrease the risk of bladder injury, the anterior leaf of the broad ligament was opened bilaterally, and entry was made into the “New Space”, as previously described by Nezhat et al.15 To identify the isthmocele margins, the fibrotic edge was removed, and well vascularized tissue was identified. Indocyanine green dye was injected intravenously and the robotic firefly feature was used to confirm the vascularity of the tissues periodically. Monopolar robotic scissors were used to resect the protruding pregnancy from the uterine defect. The specimen was then confined to a laparoscopic bag and sent to pathology. The edges of the uterine defect were further resected using sharp dissection to achieve a fresh vascular plane for optimal healing (Figure 8). The uterine defect was then repaired transversely using a two-layer closure with #0 barbed suture in a continuous non-locked fashion, avoiding the placement of intracavitary sutures (Figure 9). Chromopertubation was performed to ensure a water-tight repair and patency of the fallopian tubes. All surgeries were performed endoscopically without conversion to laparotomy. Blood loss was minimal, and all patients were discharged home the same day.
Figure 6.
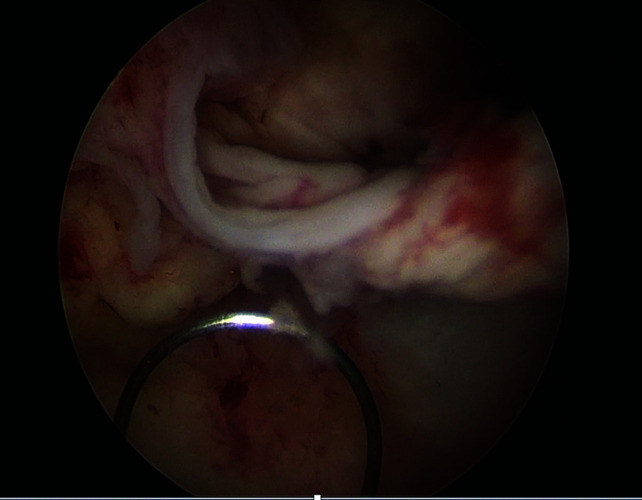
Hysteroscopic view of uterine cavity with retained products and use of cold loop electrode.
Figure 7.
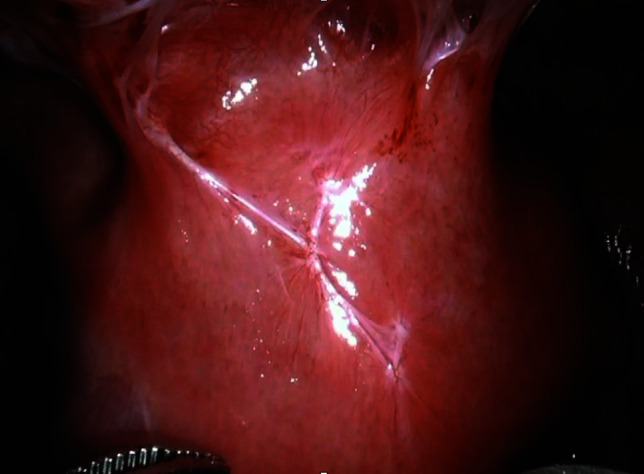
Laparoscopic view of anterior uterus prior to revision prior to lysis of adhesions.
Figure 8.
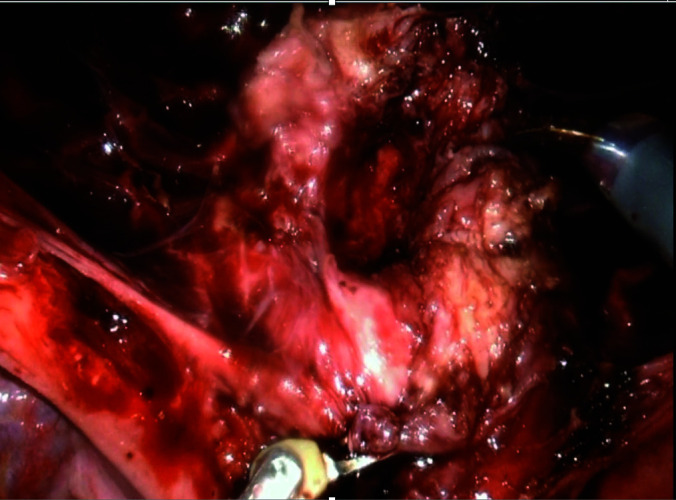
Laparoscopic view of uterus after resection of cesarean ectopic pregnancy with well vascularized edges.
Figure 9.
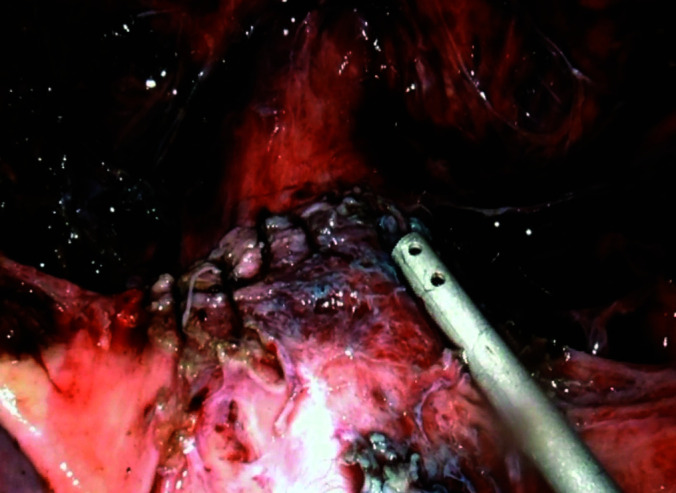
Laparoscopic view of uterus status post resection of cesarean ectopic pregnancy after water tight closure with barbed suture.
All medical and surgical interventions were performed by one maternal-fetal medicine specialist (MC) and one advanced gynecologic laparoscopic surgeon (FN), respectively. Patients’ charts were reviewed for demographic, obstetric, medical, and surgical history. Descriptive statistics were used to evaluate data with mean ± SD for normally distributed continuous data and median (range) for not normally distributed data.
RESULTS
Five cases were diagnosed with cesarean scar pregnancies and treated using an institutional protocol of combined medical and surgical approaches during the study period. After counseling, all patients opted for pregnancy termination with the desire for fertility preservation. The median age of the patient was 36 (range 34 – 42) years. The median number of prior pregnancies was 4 (range 3 – 10) and number of prior cesarean sections was 2 (range 1 – 3). Forty percent (2/5) of the patients were Hispanic, 20% (1/5) Caucasian, 20% (1/5) African American, and 20% (1/5) South Asian. After medical intervention with intra-sac lidocaine and systemic methotrexate, patients waited a mean duration of 4.6 ± 2.3 months) prior to proceeding with surgery (Table 1). Beta hCG levels prior to surgical procedure ranged from 0 – 582 mIU/mL.
Table 1.
Characteristics of Patients Undergoing Combined Medical and Surgical Treatment of Cesarean Scar Pregnancy
| ID | Age | Race | G | Prior CD | Prior CSP | Medical Treatment | Gestational Age at Medical Management* | Interval Months | Subsequent Pregnancy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Asian | 6 | 3 | 1 | 5 cc of 2% lidocaine | 6.1 | 2 | No |
| 2 | 40 | African American | 10 | 1 | 0 | 10 cc of 2% lidocaine | 8.1 | 2 | No |
| 3 | 36 | Hispanic | 4 | 3 | 0 | 5 cc of 2 % lidocaine | 8.2 | 6 | No |
| 4 | 34 | White | 3 | 1 | 0 | 5 cc of 1% lidocaine | 7.3 | 5 | Yes |
| 5 | 34 | Hispanic | 4 | 2 | 0 | 5 cc of 1% lidocaine | 6.5 | 8 | Yes |
G, gravidity; CD, cesarean delivery; CSP, cesarean scar pregnancy. *Gestational age expressed in weeks and days.
Full descriptive characteristics for the 5 cases are provided in Table 1. All of the patients underwent uneventful medical and surgical intervention as detailed above, with pathology confirmed chorionic villi in all resected tissues. Six weeks post-operatively, all patients had transvaginal sonographic assessment of the lower uterine segment, which demonstrated a well healed lower uterine segment without evidence of a defect (Figure 10). Patients were advised to wait a minimum of 3 – 4 months prior to an attempt to conceive. There were no complications post interventions and no recurrence of cesarean scar pregnancies thus far. Two patients have had a successful subsequent pregnancy with normal implantation.
Figure 10.
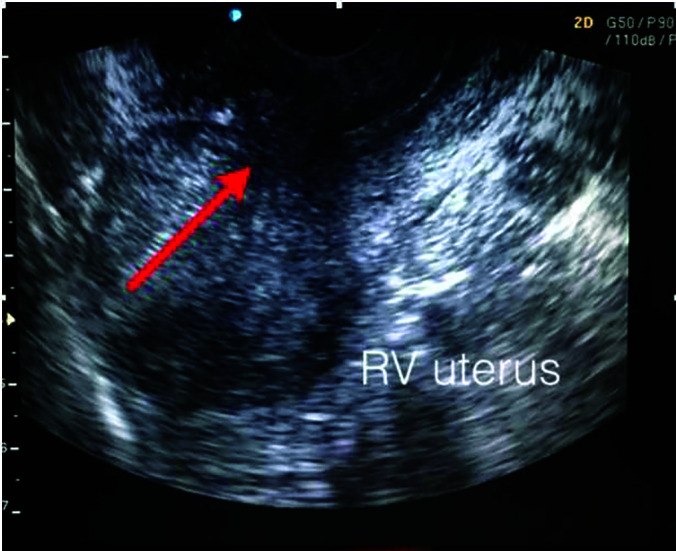
Arrow indicating area of uterine repair with no evidence of defect or niche.
DISCUSSION
Here we describe our combined medical and surgical treatment approach for cesarean scar pregnancies. We have found that treatment of the cesarean scar pregnancy as well as surgical repair of the niche defect decreases the recurrence rates, pelvic pain, and abnormal uterine bleeding. Among our patients, we did not observe recurrences, complications, failed treatment, or complaints of pelvic pain or abnormal uterine bleeding. We were also able to show successful pregnancy outcomes following our intervention.
Currently, there is a lack of gold standard treatment protocols for the management of these pregnancies. A systematic review of 52 studies describes 14 different approaches with the authors recommending the following five surgical procedures for successful treatment of a scar pregnancy: “[1] resection through a transvaginal approach, [2] laparoscopy, [3] uterine artery embolization in combination with dilatation and curettage and hysteroscopy, [4] uterine artery embolization in combination with dilatation and curettage, and [5] hysteroscopy”.9 We have had a favorable experience using a combination of both medical and surgical treatment approaches with a minimally invasive technique for resection of residual products of conception and repair of uterine defect.
The strength of our study is consistency, as all of the patients underwent the same successful medical and surgical procedures by the same two providers. In addition to the small sample size, a limitation to our study is differences in time interval between medical and surgical interventions among the 5 patients. Although we recommended waiting two months prior to proceeding with surgery, longer time intervals occurred due to patient preference. A minimum of two months was recommended to allow the medical therapy to terminate the pregnancy and decrease blood supply to the affected area and so decrease overall surgical complications and blood loss. We want to emphasize that our results provide the groundwork for a successful new way of treating cesarean scar pregnancies, using a combined approach. Nevertheless, high-quality prospective studies are needed to replicate our results.
Footnotes
Disclosure: None.
Funding: None.
Conflicts of Interest: The authors declare no conflict of interest.
Informed consent: Dr. Nezhat declares that written informed consent was obtained from the patient’s for publication of this study/report and any accompanying images.
Contributor Information
Eva Hoffmann, Department of Obstetrics and Gynecology, NYU Winthrop Hospital, NYU Long Island, School of Medicine, Mineola, New York, USA..
Sevan Vahanian, Department of Obstetrics and Gynecology, NYU Winthrop Hospital, NYU Long Island, School of Medicine, Mineola, New York, USA..
Vanessa T. Martinelli, Department of Obstetrics and Gynecology, NYU Winthrop Hospital, NYU Long Island, School of Medicine, Mineola, New York, USA..
Martin Chavez, Department of Obstetrics and Gynecology, NYU Winthrop Hospital, NYU Long Island, School of Medicine, Mineola, New York, USA..
Michael Mesbah, Department of Obstetrics and Gynecology, NYU Winthrop Hospital, NYU Long Island, School of Medicine, Mineola, New York, USA..
Farr R. Nezhat, Department of Obstetrics and Gynecology, NYU Winthrop Hospital, NYU Long Island, School of Medicine, Mineola, New York, USA.; Department of Obstetrics and Gynecology, Weill Cornell Medical College of Cornell University, New York, New York, USA.
References:
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2017. Natl Vital Stat Rep. 2018;67(8);1–50. [PubMed] [Google Scholar]
- 2.Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta & cesarean scar pregnancy: a review. Am J Obstet Gynecol. 2012;207;14. [DOI] [PubMed] [Google Scholar]
- 3.Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43(4):372–382. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol. 2003;21(3):220–227. [DOI] [PubMed] [Google Scholar]
- 5.Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Am Coll Obstet Gynecol. 2006;107(6):1373–1381. [DOI] [PubMed] [Google Scholar]
- 6.Lee CL, Wang CJ, Chao A, Yen CF, Soong YK. . Laparoscopic management of ectopic pregnancy in a previous caesarean section scar. Hum Reprod. 1999;14(5):1234–1236. [DOI] [PubMed] [Google Scholar]
- 7.Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus–an unusual cause of postabortal haemorrhage: a case report. S Afr Med J. 1978;53(4):142–143. [PubMed] [Google Scholar]
- 8.Petersen KB, Hoffmann E, Larsen CR, Nielsen HS. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016;105(4):958–967. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang Y, Huang L. Uterine artery embolization compared with methotrexate for the management of pregnancy implanted within a cesarean scar. Am J Obstet Gynecol. 2009;201:152.e1-3. [DOI] [PubMed] [Google Scholar]
- 10.Robinson RK, Dayal MB, Gindoff P, Frankfurter D. A novel surgical treatment for cesarean scar pregnancy: laparoscopically assisted operative hysteroscopy. Fertil Steril. 2009;92(4):1497.e13-16. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson MT, Osias J, Velasco A, Charles R, Nezhat C. Laparoscopic repair of uteroperitoneal fistula. JSLS. 2003;7:367–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Nezhat C, Falik R, Li A. Surgical management of niche, isthmocele, uteroperitoneal fistula, or cesarean scar defect: a critical rebirth in the medical literature. Fertil steril. 2017;107:69–70. [DOI] [PubMed] [Google Scholar]
- 13.Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: what is it and how is it treated? OBG Manag. 2016;28(4):32, 34,, 36,, 38,-39, 53. [Google Scholar]
- 14.Mahmoud MS, Nezhat F. Robotic-assisted laparoscopic repair of a cesarean section scar defect. J Minim Invasive Gynecol. 2015;22(7):1135–1136. [DOI] [PubMed] [Google Scholar]
- 15.Nezhat C, Grace L, Razavi GM, Mihailide C, Bamford H. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128(3):629–633. [DOI] [PubMed] [Google Scholar]


