Abstract
Background and Objectives:
Based on laparoscopic views, we hypothesized that the involvement of the lateral compartment of the pelvis (LCP) by deep infiltrating endometriosis can be inferred by observing retraction of the obliterated umbilical artery (OUA) toward the abdominal cavity. We sought to verify the association between the retraction of the OUA and the presence of endometriosis in the ipsilateral LCP (parametrium, paracervix, or paracolpium).
Methods:
This preplanned, retrospective, cross-sectional study evaluated 76 women with deep endometriosis at a private referral center. Using magnetic resonance imaging, the retraction of OUA was represented by its distance from the rectus abdominis (four different measurements were used). The diameter of the OUA was also measured and considered. T2-weighted imaging of the pelvis were obtained in two planes (sagittal and axial) and from two reference points: the proximal angle of the artery (measurement 1) and a point immediately above (measurement 2). The measurements were assessed through an exploratory multivariate principal component analysis. The associations were tested using the bivariate, non-parametric statistical Mann-Whitney U test.
Results:
The presence of endometriosis of all LCP examined was 34.2% (95% confidence interval: 26.8–41.7) with the highest percentage in the paracervix. The retraction of the OUA was greater in women with endometriosis in the ipsilateral LCP for all four measurements and was statistically significant for three of them: Sagittal 1 (p = .011), Sagittal 2 (p = .015), Axial 1 (p = .021), and Axial 2 (p = .093). The OUA diameter was not associated with its retraction (p = .392).
Conclusion:
Retraction of the OUA toward the abdominal cavity is associated with the presence of endometriosis in the ipsilateral paracervix.
Keywords: Anatomy, Minimally invasive surgery, Pelvis, Endometriosis, Obliterated umbilical artery
INTRODUCTION
Deep infiltrating endometriosis involving the lateral compartment of the pelvis (LCP) constitutes a severe manifestation of endometriosis, often requiring surgical intervention.1 Surgically exploring the LCP to resect endometriotic lesions is a complex task with significant risk of functional complications.2–7
Due to a dynamic tissue remodeling process that occurs at involved sites,8 endometriotic lesions tend to attract adjacent structures9 (Figure 1A). Based on our observations during hundreds of laparoscopic surgeries for deep infiltrating endometriosis over nearly three decades, we hypothesized that the involvement of the LCP by deep endometriosis can be sufficiently severe so as to “retract” the ipsilateral obliterated umbilical artery (OUA) – also called the medial umbilical ligament – toward the abdominal cavity. Regarding Figure 1, Panel A shows a laparoscopic view of the anterior compartment of the pelvis with asymmetric retraction of the obliterated umbilical arteries in a woman with endometriosis infiltrating the right paracervix. Panel B shows the same patient assessed by MRI (axial mirrored view of the pelvis) with different distances between the obliterated umbilical arteries and the rectus abdominis, corresponding to the laparoscopic finding. If there is indeed an association between OUA retraction toward the abdominal cavity and LCP involvement by endometriotic lesions, and if this retraction can be identified visually during laparoscopic surgery, then surgeons could potentially use this anatomic finding as a “predictive sign” when considering whether to explore the LCP in search of endometriosis involvement that was not suspected pre-operatively. A PubMed search of the MEDLINE database conducted on March 20, 2021 by two of the authors (CPCJr and MFF) using the search string (“obliterated umbilical artery” OR “umbilical ligament”) AND (parametrium OR endometriosis OR lateral) yielded 29 publications. None addressed the association between OUA retraction and the presence of endometriosis in the LCP.
Figure 1.

Retraction of obliterated umbilical arteries toward abdominal cavity of a 34-year-old woman. Panel A: Laparoscopic View, Panel B: Magnetic resonance imaging. [Green arrows: obliterated umbilical artery; White arrows: rectus abdominis; Yellow bars: distance between the obliterated umbilical arteries and the rectus abdominis; Blue arrows: peritoneal retraction toward the deep endometriotic lesion of the anterior compartment involving the bladder, vesicouterine pouch, uterine serosa, and right round ligament (white dashed circle); U: uterus; I: ilium; R: rectosigmoid].
Magnetic resonance (MR) imaging is a valuable tool for the pre-operative evaluation of women with endometriosis.10,11 The positive and negative predictive values for the diagnosis of parametrial endometriosis are 90.9% and 97.2% respectively.12 Reviewing the MR images of women with a clinical diagnosis of endometriosis, we found that we could visualize the OUA, follow its course, and measure the distance from the OUA to the abdominal wall (rectus abdominis). Using pre-operative MR images, this study sought to verify an association between the presence of OUA retraction toward the abdominal cavity and the presence of endometriosis in the ipsilateral LCP (parametrium, paracervix, or paracolpium).
MATERIALS AND METHODS
This preplanned, interdisciplinary, observational, cross-sectional study evaluated retrospectively the MR images of 84 Brazilian women who were considering minimally invasive surgical resection of deep infiltrating endometriosis as treatment for pain refractory to medical management and/or infertility. The exclusion criteria were: (1) the inability to visualize deep infiltrating endometriosis with MR imaging (in the setting of suggestive symptoms), (2) previous hysterectomy, and (3) a uterus exceeding 150 mL (because a pilot study including 22 women suggested that large uteri make the assessment of the OUAs difficult when the patient is breathing spontaneously in the supine position inside the MR scanner).
The protocol was approved by an institutional review board. Written patient consent was obtained and placed on file at our institution. Procedures recommended in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement were followed.13
All cases were managed at the Crispi Institute for Minimally Invasive Surgery, Rio de Janeiro, RJ, Brazil (institutocrispi.com.br), a multidisciplinary endometriosis referral private center, which follows the guidelines of the American Society of Reproductive Medicine14 and the European Society of Human Reproduction and Embryology15 for the diagnosis and treatment of endometriosis. The diagnosis of endometriosis involves three steps: medical history, physical examination, and MR imaging. This study included the MR images that were performed at a single imaging center, Fonte Imagem Medicina Diagnóstica (fonteimagem.com.br) in Rio de Janeiro, RJ, Brazil, from January 1, 2018 to February 28, 2019.
As some patients in this series dropped out of the proposed surgical treatment for different reasons, we did not analyze laparoscopic images or anatomopathological reports of the parts removed, which will be the subject of a future study.
Anatomic landmarks
The two umbilical arteries arise from the anterior trunk of the internal iliac arteries and carry fetal blood to the placenta. After birth, these arteries are completely obliterated and are then recognized as OUAs. In fact, each OUA is the continuation of the ipsilateral internal iliac artery, which becomes completely obliterated shortly beyond the superior vesical artery branch. The two OUAs are important landmarks for locating or identifying the uterine artery and the paravesical space, and they have an intimate relationship with the LCP, including the parametrium and paracervix (Figure 2). Distal to the superior vesical artery branch, the OUAs run horizontally to the anterior abdominal wall along the lateral pelvic wall, and then turn craniomedially to the umbilicus.16 At this point, they are usually found as a remnant in the internal aspect of the anterior abdominal wall. In the Figure 2, Panel A shows the laparoscopic view of the left lateral compartment of the pelvis after lymphadenectomy and resection of deep endometriotic paracervical lesions. Panel B highlights the intimate anatomic relationship between the obliterated umbilical artery and several major posterolateral pelvic structures.
Figure 2.

Laparoscopic view of the left lateral compartment of the pelvis after lymphadenectomy and resection of deep endometriotic paracervical lesions. [P (green): paracervix; OUA (orange): obliterated umbilical artery; UA (purple): uterine artery; U (yellow): ureter; UL (pink): uterosacral ligament; C (white): cervix].
The parametrium is a cellular connective tissue located between the layers of the broad ligament. It is contiguous with the lateral margins of the uterus medially, with the vagina superiorly, with the parietal fascia laterally, with the cardinal ligaments inferiorly, and with the bladder anteriorly. The parametrium delineates the lateral edges of the uterus, while the paracolpium delineates the superior third of the vagina and extends laterally into the side walls of the pelvis. The term paracervix refers to the inferior aspect of the parametrium, enclosing the pericervical ring (Figure 3 - T2-weighted image obtained by a multiplanar reformation from a 3D cube sequence of the coronal plane). In this study, the LCP was considered to have endometriosis when endometriosis was visualized in the ipsilateral parametrium, paracervix, or paracolpium on MR imaging.
Figure 3.
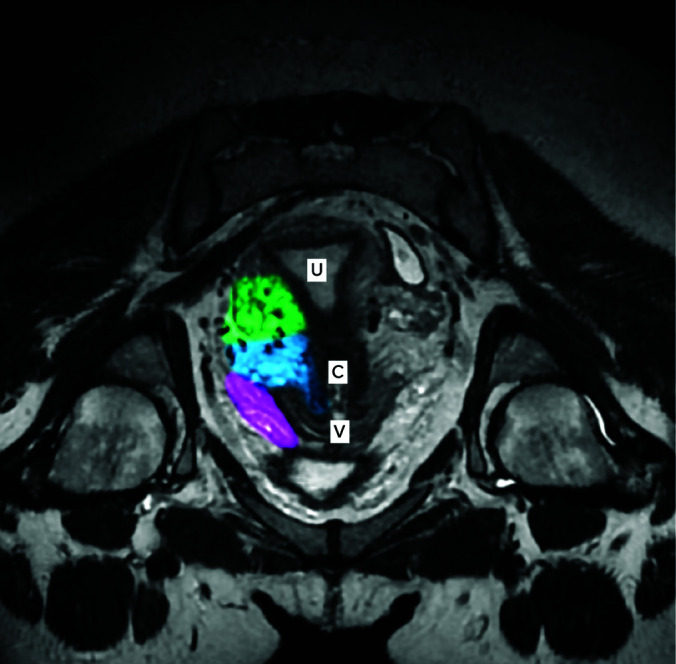
Longitudinal axis of a normal pelvis with a retroverted uterus (30-year-old woman). The right lateral compartment of the pelvis was highlighted and segmented in three colors: parametrium (green), paracervix (blue) and paracolpium (pink). [U: uterus; C: cervix; V: vagina].
MR Imaging
The MR images were performed with a closed-configuration superconducting 10.5-T system (MRI Signa Explorer 10.5 Tesla SV 250.0; GE-Healthcare®) using an eight-channel high-resolution surface phased-array torso coil.
The preparation protocol included bowel cleansing starting the day before the study with a low-residue diet, followed by fasting for four hours before the examination. Oral administration of sodium picosulfate (Gutalax®) was followed by an evacuant suppository administered no later than two hours before the examination. All MR images were performed with an empty bladder, vaginal distension with 20 ml of ultrasound gel, and with retrograde distension of the rectum and the sigmoid colon with 120 ml of a saline solution. The study was performed with the patient lying in the supine position (entry position: feet first). Scopolamine-N-butyl bromide (Buscopan® 20 mg), an antispasmodic agent, was administered intravenously just before the first image acquisition to reduce motion artifacts caused by bowel peristalsis and to improve lesion conspicuity.
The MR imaging protocol included T2-weighted imaging of the uterus in four planes: axial, sagittal, short, and long axis. Optionally, a 3-dimensional T2-weighted coronal sequence was also performed. T1-weighted imaging with and without fat suppression was performed in the sagittal plane and in the axial plane, both with and without intravenous gadolinium contrast. For the purposes of this study, we used only two T2-weighted sequences: sagittal and axial. The axial plane was angled 25 degrees from the horizontal line in order to be able to follow the course of the uterosacral ligaments.
The volume of the uterus was estimated using the Ellipsoid formula:
Volume = 0.523 x length x width x depth
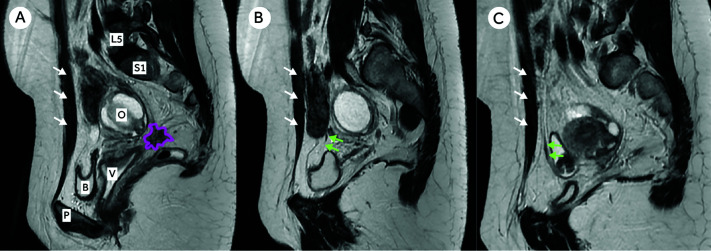
As this is an original study, different ways to assess the retraction of the OUA were contemplated and discussed by a multidisciplinary team comprised of two radiologists (ACCBS and LCB), two surgeons (CPCJr and CPC), and a quantitative methods expert (MFF). The retraction of each OUA was then measured as its distance from the rectus abdominis. The distance was measured in two planes (sagittal and axial) and from two reference points: the proximal angle of the artery (measurement 1) and a point immediately above (measurement 2). More specifically, measurement 1 was defined on the sagittal images as the point immediately after the OUA curves and ascends the anterior aspect of the pelvis (named Sagittal 1); and measurement 2 (named Sagittal 2) was made at a point no more than 10.0 cm above measurement 1 between the OUA’s parallel path and the rectus abdominis. For the two measurements in the axial plane (named Axial 1 and Axial 2), we used their corresponding slices made in the sagittal plane (Figures 1 and 4). The Figure 4 shows a MRI sagittal view of the pelvis of a 34-year-old woman showing endometriosis lesion in the right paracervix (colored in pink). The distance from the obliterated umbilical artery to the abdominal wall (rectus abdominis) was greater on the right than on the left, which had no endometriosis in the paracervix.
Figure 4.
Panel A, Distance from the obliterated umbilical artery to the rectus abdominis was greater on the right (Panel B) than on the left, which had no endometriosis in the paracervix (Panel C). [L5: 5th lumbar vertebrae; S1: 1st sacral vertebrae; O: right ovary; V: vagina; B: bladder; P: pubis; White arrows: Rectus Abdominis; Green arrows: obliterated umbilical artery].
Given the possibility that the caliber of the OUA may change as a result of the retraction or may even limit the retraction, we also measured its diameter. The largest diameter of the artery was measured only in the axial plane. All measurements were performed bilaterally. Altogether there were five measurements on each side: Sagittal 1, Sagittal 2, Axial 1, Axial 2, and Diameter.
The search for endometriosis in the LCP was made “blindly”, that is, before the measurements representing the retraction of the OUAs were made. The parametrium, paracervix, and paracolpium were assessed independently and considered to be affected by endometriosis when poorly marginated hypointense tissue, sometimes with internal hyperintense foci on T2-weighted image was observed extending into any of these anatomic sites (Figures 1 and 4). Also, we considered mild disease to be present on MR imaging as discrete blurring of the fat planes in these sites, usually contiguous with an endometriotic lesion, nodule, plaque, or ligament thickening and irregularity.
The same experienced radiologists (ACCBS and LCB) and the same gynecologists (CPCJr and CPC) assessed all the MRI images in order to attain a consensus about the measurements and the endometriosis diagnoses.
Sample size
The sample size calculation was performed contemplating bivariate analysis to compare two means and assumed an equal number of observations in the two groups. Preliminarily, a pilot study using the MR image studies of 22 women with endometriosis estimated the standard deviation for each measurement: Sagittal 1 (40.0 mm), Sagittal 2 (30.6 mm), Axial 1 (40.5 mm), Axial 2 (30.8 mm), and Diameter (10.1 mm). Then, assuming (conservatively) a pooled standard deviation of 6 units (variance 36), the study required a minimum sample size of 63 OUAs in each group (with and without endometriosis in the LCP) to achieve a power of 80% and a level of significance of 5% (two sided) in order to detect a true difference – of at least 3 units – between the means of the distance measurements.17 Finally, considering the low risk for the participants (only the breach of patient confidentiality), the impossibility of obtaining equal sample sizes in the two groups for all variables, and the possibility of a non-Gaussian data distribution (nonparametric statistics recommended), we included a total of 76 women (168 OUAs) to compare the groups with and without endometriosis in the LCP.
Statistics
An exploratory multivariate factor analysis was used to identify redundancy or duplication from a set of scale correlated variables. The five measurements (Sagittal 1, Sagittal 2, Axial 1, Axial 2, and Diameter) were initially subjected to a principal component extraction method using 1s as prior communality estimates (missing values not included). Then, a principal axis method was used to extract two components, followed by a varimax (orthogonal) rotation with Kaiser Normalization. The data distributions of the scale variables were assessed using the Shapiro-Wilk test (null hypothesis is normal distribution). The Nonparametric Mann-Whitney U test was used to compare two independent samples and the bivariate Spearman's rank correlation analysis (ρ) was used to express the strength of association between two ordinal variables. Charts and statistics were developed using IBM® SPSS® Statistics Standard Grad Pack 20 (IBM Corp., Armonk, NY, USA). Statistical results were considered significant when P < .05 (2-sided).
RESULTS
Of the 84 women enrolled in this study, 8 were excluded as detailed in Figure 5. The characteristics of the remaining 76 are presented in the Table 1; nulliparous young women predominant. Of these 76 women whose MR images were evaluated with special attention to the presence of endometriosis in the LCP, 19 women (25%) had bilateral endometriosis and 14 women (18.4%) had unilateral endometriosis - 7 (9.2%) on the left side and 7 (9.2%) on the right side; 43 women (56.6%) showed no endometriosis in the LCP.
Figure 5.
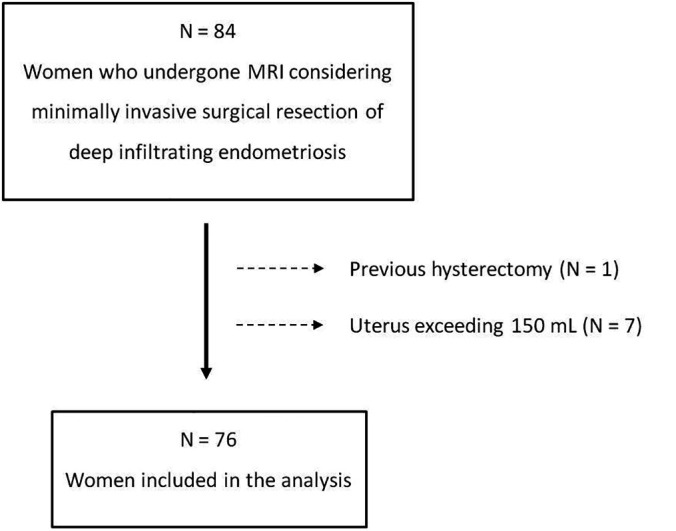
Flow diagram showing initial number of participants and those excluded.
Table 1.
Characteristics of The Sample (N = 76)
| Min | 25th Percentile | Median | 75th Percentile | Max | |
|---|---|---|---|---|---|
| Weight (Kg) | 45 | 57.3 | 64.5 | 79.0 | 103 |
| Height (m) | 1.51 | 1.57 | 1.62 | 1.67 | 1.73 |
| BMI (Kg.m-2) | 17.4 | 22.4 | 24.2 | 28.7 | 40.5 |
| Age (years) | 17 | 27 | 31 | 38 | 54 |
| Gesta (n) | 0 | 0 | 0 | 0 | 4 |
| Para (n) | 0 | 0 | 0 | 0 | 2 |
| Abortion (n) | 0 | 0 | 0 | 0 | 3 |
| Caesarean section (n) | 0 | 0 | 0 | 0 | 2 |
BMI, body mass index.
The retraction (distances) could not be reliably measured in some cases because of difficulty visualizing the OUA. Considering all the 152 assessed OUAs (76 women), the two proximal measurements (Sagittal 1 and Axial 1) had fewer missing values (9 and 6 missing values, respectively) than the distal measurements Sagittal 2 and Axial 2 (16 and 15 missing values, respectively). The diameter of the artery could not be measured at six sites (6 missing values). None of the five measurements (Sagittal 1, Sagittal 2, Axial 1, Axial 2, and Diameter) conformed to a normal distribution (Shapiro-Wilk test; P < .010).
The exploratory multivariate analysis was summarized in the Figure 6A. In the principal component analysis (orthogonal rotation) used to identify redundancy or duplication among a set of correlated variables, the circles represent the diameter of the OUA and the four different ways to measure the distance between the obliterated umbilical artery and the abdominal wall (Sagittal 1, Sagittal 2, Axial 1 and Axial 2). This graph of orthogonal components shows that the diameter of the obliterated umbilical artery appears to be independent of its retraction, which is represented by the 4 independent distance measurements between the obliterated umbilical artery and the abdominal wall (N =132; missing values not included). Combined, the two retained components accounted for 88.8% of the total variance. The measurements representing the retraction of the OUAs showed a very similar behavior loading mainly on the first component: Sagittal 1 (.916), Sagittal 2 (.946), Axial 1 (.912), and Axial 2 (.918). The second component was mainly loaded by the variable Diameter (.995), which was shown to occur independently of the other variables. Besides, under a Spearman's rank analysis, the bivariate correlation among all measurements used to assess the retraction of the OUA (Sagittal 1, Sagittal 2, Axial 1, Axial 2) was strong and statistically significant; the correlation coefficients (ρ) were Sagittal 1 x Sagittal 2: .889 (P < .001); Axial 1 x Axial 2: .868 (P < .001); Sagittal 1 x Axial 1: .777 (P < .001); Sagittal 2 x Axial 2: .808 (P < .001); Sagittal 1 x Axial 2: .732 (P < .001); and Sagittal 2 x Axial 1: .795 (P < .001). On the other hand, the diameter of the artery showed significant correlation with none of the measurements except Axial 1 (where the correlation was so weak it was probably by chance). The Spearman's rank correlation coefficients (ρ) between the OUA diameter and the retraction measurements were Sagittal 1: –.105 (p = .212); Sagittal 2: –.075 (p = .390); Axial 1: -.210 (p = .011); and Axial 2: –.105 (p = .220).
Figure 6.
Panel A shows the exploratory multivariate factor analysis. Panel B is a boxplot showing the four measurements (Quartiles) representing the distance between the obliterated umbilical artery and the abdominal wall (Rectus Abdominis), and the diameter of the obliterated umbilical artery.
Considering the 152 LCPs assessed by MR imaging, the prevalence of endometriosis was 34.2% (95% confidence interval [CI]: 26.8–41.7). The prevalence of endometriosis was highest in the paracervix at 33.6% (95% CI: 26.1 – 41.5), followed by 14.5% for the parametrium (95% CI: 9.2 – 20.8), and 5.3% for the paracolpium (95% CI: 2.0 – 9.2).
When the 152 LCPs were grouped as without endometriosis (N = 100) and with endometriosis (N = 52), there were statistically significant differences with respect to all the variables representing the retraction of the OUA, except Axial 2. All the medians of the measurements used to quantify the retraction of the OUA were greater in the group with endometriosis. The median (min-max) distance measurements in the cases without and with endometriosis in the LCP were, respectively, Sagittal 1: 7.1 (0.0–20.0) and 10.1 (2.1 – 15.1) mm; Sagittal 2: 5.0 (1.0 – 17.8) mm and 7.1 (1.6 – 14.7) mm; Axial 1: 5.7 (0.9 – 14.7) mm and 6.5 (1.6 – 17.3) mm; and Axial 2: 3.2 (0.9 – 14.4) mm and 4.9 (1.2 – 16.9) mm. The median diameters of the OUAs were 3.4 (1.8 – 6.5) mm without and 3.2 (1.5 – 50.6) mm with endometriosis; this difference was not statistically significant (Figure 6B). The nonparametric Mann-Whitney U test was used to compare the groups without and with ipsilateral endometriosis in the lateral pelvic compartment. Endometriosis in the lateral compartment means endometriosis in at least one of the main sites: parametrium, paracervix, or paracolpium. Small circles in the boxplot denote outliers.
The retraction of the OUA was greater when endometriosis infiltrated the ipsilateral paracervix. The difference was statistically significant for the measurements Sagittal 1 (p = .016), Sagittal 2 (p = .021), and Axial 1 (p = .024); but not significant for Axial 2 (p = .083). However, the differences were not statistically significant when comparing the groups without and with endometriosis in the parametrium (p = .624; .951; .607 and .256) or in the paracolpium (p = .501; .059; .173 and .065, respectively) separately.
DISCUSSION
This observational study gathered radiological evidence that supports the hypothesis that the OUA retracts toward the abdominal cavity when endometriosis is present in the LCP (parametrium, paracervix, or paracolpium). Besides, using both a multivariate and a bivariate approach, the findings show that this phenomenon is not associated with any significant changes in the diameter of the OUA.
The proximal distances measured by MRI (Sagittal 1 and Axial 1) were considered by the radiologists as the best to assess the retraction of the OUA, because they (1) were the easiest to obtain, (2) showed the least interference from other structures (and thus fewer missing values), and (3) the presence of endometriosis in the LCP seems to have a more evident retraction effect on the ipsilateral OUA close to the LCP than close to the umbilicus, when there is a natural tendency to approach the abdominal wall (Figure 1).
Despite the practical challenges in accurately delimiting the parametrium, the paracervix, and the paracolpium, the biological phenomenon of retraction of the OUA assessed in this study was in fact mainly represented by the paracervix, in which the prevalence of endometriosis was 33.6%. Actually, we believe that the low prevalence of endometriosis infiltrating the paracolpium (5.3%) and infiltrating the parametrium (14.5%) probably did not provide enough statistical power to identify a statistical difference when these sites were tested separately.
The limitations of the present study include (1) the use of MR imaging rather than the gold standard diagnostic method for endometriosis (histopathology); (2) the likely influence of adhesions; (3) the likely impact on the measurements taken from the MR imaging attributable to anatomic variations, such as the shape and size of the pelvis, the distribution of abdominal fat, or whether the uterus is large, anteverted, or had a large anterior projection; and (4) the dichotomization of the diagnosis of endometriosis, which did not take into account the size of the endometriotic lesions (i.e. there was no minimum size cut-off). The possibility of selection bias associated with access to care should be considered.
Several strengths of the study can be highlighted: (1) four independent distance measurements were used to represent the phenomenon of retraction of the OUA; (2) a robust non-parametric statistic test validated that the sample size was adequate to demonstrate the retraction phenomenon mathematically; (3) the interpretation of the MR imaging findings was a consensus process involving the same two gynecologists and the same two radiologists (all with extensive experience with endometriosis); and (4) the search for endometriosis in the LCPs was conducted “blindly”, that is, before measuring the distances used to quantify the retraction of each OUA.
A future study through laparoscopic vision to verify whether surgeons can, in fact, accurately identify this phenomenon is desirable. By displacing intra-abdominal structures that normally exert compression on the anterior abdominal wall, the Trendelenburg position and pneumoperitoneum used in laparoscopic pelvic surgeries together largely eliminate problems that hamper MR imaging evaluation and thus they facilitate direct visualization of the path of the OUA along the anterior abdominal wall (Figure 1). Thus, surgeons should consider the “retraction sign” when weighing whether to dissect or not dissect the LCPs in pursuit of parametrial extension of endometriotic lesions not suspected pre-operatively.
CONCLUSION
The presence of endometriosis in the paracervix is associated with ipsilateral retraction of the OUA toward the abdominal cavity.
Footnotes
Disclosure: none.
Funding sources: none.
Conflict of interests: none.
Informed consent: Dr. Claudio Peixoto Crispi Jr. declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Claudio Peixoto Crispi, Jr, Crispi Institute of Minimally Invasive Surgery, Rio de Janiero, Brazil..
Claudio Peixoto Crispi, Crispi Institute of Minimally Invasive Surgery, Rio de Janiero, Brazil..
Alice Cristina Coelho Brandão Salomão, Fonte Imagem Medicina Diagnóstica, Rio de Janeiro, Brazil..
Luciana Camara Belem, Fonte Imagem Medicina Diagnóstica, Rio de Janeiro, Brazil..
Fernanda de Paula Crispi, Crispi Institute of Minimally Invasive Surgery, Rio de Janiero, Brazil..
Marlon de Freitas Fonseca, Crispi Institute of Minimally Invasive Surgery, Rio de Janiero, Brazil.; Department of Women’s Health - Fernandes Figueira National Institute for Women, Children and Youth Health - Oswaldo Cruz Foundation, Rio de Janeiro, Brazil.
References:
- 1.Mabrouk M, Raimondo D, Arena A, et al. Parametrial endometriosis: the occult condition that makes the hard harder. J Minim Invasive Gynecol. 2019;26(5):871–876. [DOI] [PubMed] [Google Scholar]
- 2.Ballester M, Santulli P, Bazot M, Coutant C, Rouzier R, Daraï E. Preoperative evaluation of posterior deep-infiltrating endometriosis demonstrates a relationship with urinary dysfunction and parametrial involvement. J Minim Invasive Gynecol. 2011;18(1):36–42. [DOI] [PubMed] [Google Scholar]
- 3.de Resende JA, Júnior, Cavalini LT, Crispi CP, de Freitas Fonseca M. Risk of urinary retention after nerve-sparing surgery for deep infiltrating endometriosis: a systematic review and meta-analysis. Neurourol Urodyn. 2017;36(1):57–61. [DOI] [PubMed] [Google Scholar]
- 4.de Resende Júnior JAD, Crispi CP, Cardeman L, Buere RT, Fonseca MF. Urodynamic observations and lower urinary tract symptoms associated with endometriosis: a prospective cross-sectional observational study assessing women with deep infiltrating disease. Int Urogynecol J. 2018;29(9):1349–1358. [DOI] [PubMed] [Google Scholar]
- 5.Hernández Gutiérrez A, Spagnolo E, Zapardiel I, et al. Post-operative complications and recurrence rate after treatment of bowel endometriosis: comparison of three techniques. Eur J Obstet Gynecol Reprod Biol X. 2019;4:100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crispi CP, Jr, Crispi CP, de Paula Crispi F, Cardeman L, Salomao ACCB, de Freitas Fonseca M. Endometriosis infiltrating the pelvic floor muscles with histopathological correlation-A case report. J Obstet Gynaecol Res. 2019;45(10):2116–2120. [DOI] [PubMed] [Google Scholar]
- 7.Crispi CP, Jr, Crispi CP, Brandão Salomão ACC, Joaquim CMV, de Oliveira BRS, Fonseca MF. Five-month follow-up assessing defecography and urodynamics after laparoscopic nerve-sparing colorectal resection for endometriosis. Case Rep Obstet Gynecol. 2020; 8830867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohler F, Sommer A, Wachter DL, et al. Tissue remodeling and nonendometrium-like menstrual cycling are hallmarks of peritoneal endometriosis lesions. Reprod Sci. 2013;20(1):85–102. [DOI] [PubMed] [Google Scholar]
- 9.Crispi CP, de Souza CA, Oliveira MA, et al. Endometriosis of the round ligament of the uterus. J Minim Invasive Gynecol. 2012;19(1):46–51. [DOI] [PubMed] [Google Scholar]
- 10.Bourgioti C, Preza O, Panourgias E, et al. MR imaging of endometriosis: spectrum of disease. Diagn Interv Imaging. 2017;98(11):751–767. [DOI] [PubMed] [Google Scholar]
- 11.Brandão AC, Silva AO. Diseases of the female pelvis: advances in imaging evaluation. Magn Reson Imaging Clin N Am. 2013. May;21(2):447–469. [DOI] [PubMed] [Google Scholar]
- 12.Bazot M, Jarboui L, Ballester M, Touboul C, Thomassin-Naggara I, Daraï E. The value of MRI in assessing parametrial involvement in endometriosis. Hum Reprod. 2012;27(8):2352–2358. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 14.Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935. [DOI] [PubMed] [Google Scholar]
- 15.Dunselman GA, Vermeulen N, Becker C, et al. European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412. [DOI] [PubMed] [Google Scholar]
- 16.Yamaoka T, Kurihara K, Kido A, Togashi K. Four “fine” messages from four kinds of “fine” forgotten ligaments of the anterior abdominal wall: have you heard their voices? Jpn J Radiol. 2019;37(11):750–772. [DOI] [PubMed] [Google Scholar]
- 17.Dhand NK, Khatkar MS. Statulator: an online statistical calculator. Sample size calculator for comparing two independent means. Available at: http://statulator.com/SampleSize/ss2M.html. Accessed September 7, 2020.