CASE
A 59-year-old man with hypothyroidism and obesity was transferred from an outside hospital to our medical intensive care unit for management of acute hypoxemic respiratory failure due to coronavirus disease 2019 (COVID-19). One day prior to transfer, the patient required endotracheal intubation and mechanical ventilation, heavy sedation with neuromuscular blockade, and norepinephrine for hemodynamic support. His cardiac exam showed a regular rate and rhythm with 1+ peripheral pulses in all extremities and a capillary refill time of 2 to 3 s. Breath sounds were diminished bilaterally. Laboratory studies were notable for acute kidney injury (creatinine, 1.91 mg/dl [reference interval, 0.40 to 1.20 mg/dl]; blood urea nitrogen [BUN], 117 mg/dl [reference interval, 7 to 20 mg/dl]) and leukocytosis (white blood cells, 16.76 k/μl [reference interval, 4.3 to 11.3 k/μl]). A chest radiograph demonstrated diffuse bilateral patchy airspace opacities without focal consolidation (Fig. 1A). The patient was given one dose each of ceftriaxone and azithromycin for community-acquired pneumonia the day before transfer, but these were discontinued due to a low suspicion for a secondary bacterial pneumonia. Treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia included dexamethasone and remdesivir. Due to clinical improvement, paralysis was stopped and sedation lifted, and the patient remained stable on his ventilator settings with improvement in his oxygenation and hemodynamics. Ventilator settings were weaned over several days and he no longer required norepinephrine.
FIG 1.
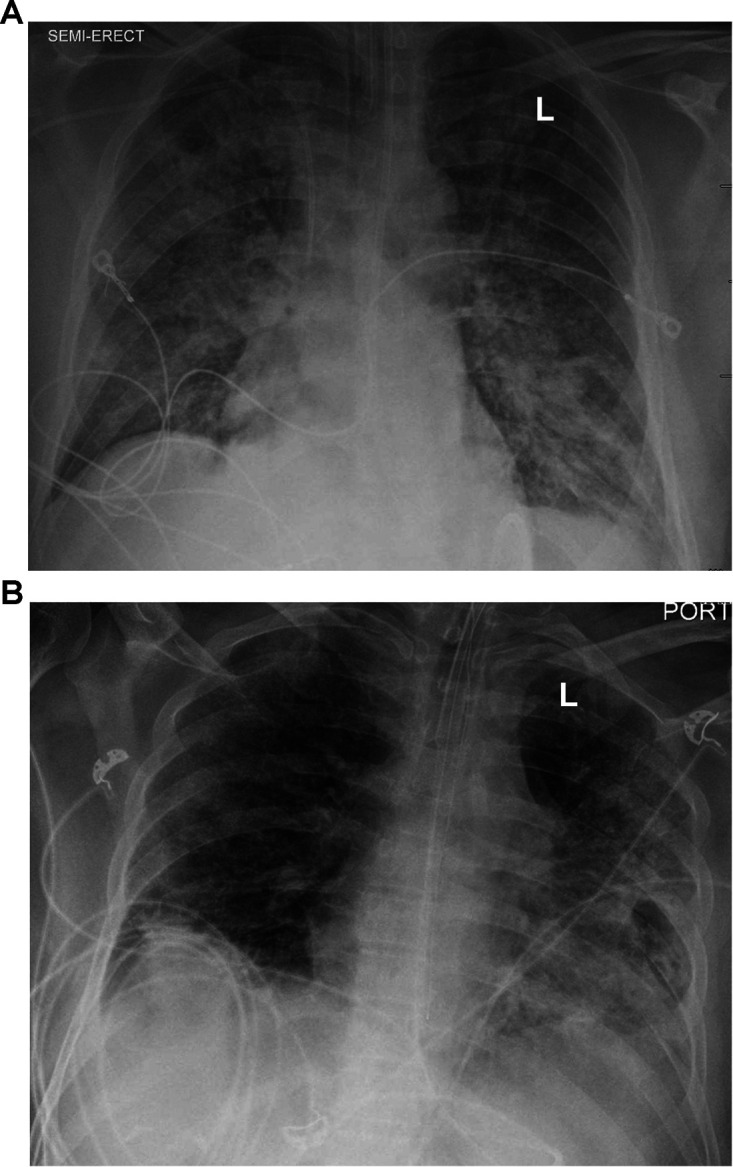
Chest radiographs during hospitalization. (A) Chest radiograph upon admission showing diffuse bilateral patchy airspace opacities without focal consolidation. (B) Chest radiograph on hospital day 20 showing more extensive consolidation in the left lung and suggesting that there may also have been a mild increase in patchy pulmonary opacities in the right lung.
Unfortunately, on hospital day 13, the patient acutely worsened, with deterioration of his mental status, low-grade fevers, redevelopment of a mild leukocytosis, and hypotension requiring vasoactive medications. Thick, tan secretions were suctioned from his endotracheal tube. Blood and tracheal aspirate cultures were sent, and empirical antibiotics (vancomycin and piperacillin-tazobactam) were started. Direct Gram stain of the tracheal aspirate demonstrated 3+ polymorphonuclear (PMN) leukocytes and 4+ Gram-positive rods (Fig. 2A). After 48 h, small (<0.5 mm in diameter), gray, nonhemolytic colonies (4+) grew on a Columbia sheep blood agar plate incubated at 35°C with 5% CO2 (Fig. 2B). Normal respiratory flora (3+) was also observed. The predominant isolate was identified as the lipophilic organism Corynebacterium accolens by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonik GmbH, Bremen, Germany) using the direct smear method with Biotyper software (8,468 spectra database). The MALDI score of the isolate was 2.02, with all database matches being C. accolens, which is consistent with a high-confidence species-level identification. Antimicrobial susceptibility testing (AST) was performed by adding 50 μl of a 0.5 McFarland suspension to 11 ml cation-adjusted Mueller-Hinton broth supplemented with 5% lysed horse blood (CAMHB-LHB). This suspension was used to inoculate custom broth microdilution AST panels (Sensititre; Thermo Fisher), followed by incubation at 35°C in ambient air for 24 to 48 h. The AST results available on hospital day 17 suggested that the isolate was susceptible to ceftriaxone (MIC = 0.5 μg/ml), doxycycline (MIC = 0.5 μg/ml), gentamicin (MIC ≤ 2 μg/ml), linezolid (MIC = 1 μg/ml), meropenem (MIC = 0.03 μg/ml), penicillin (MIC = 0.06 μg/ml), trimethoprim-sulfamethoxazole (MIC = 0.5/9.5 μg/ml), and vancomycin (MIC = 1 μg/ml) and resistant to erythromycin (MIC ≥ 8 μg/ml). The MIC for levofloxacin was ≤0.25 μg/ml with no interpretation. Repeat chest imaging on hospital day 20 demonstrated more extensive consolidation in the left lung and suggested that there may also have been a mild increase in patchy pulmonary opacities in the right lung (Fig. 1B). Based on clinical features (fevers, hypotension, worsening gas exchange, and purulent secretions), microbiological findings (predominance of C. accolens over normal respiratory flora and lack of other identifiable pathogens), laboratory data (leukocytosis), and imaging (progressive infiltrates), the clinical team attributed the ventilator-associated pneumonia (VAP) to C. accolens and treated it as a clinically significant isolate. Piperacillin-tazobactam was discontinued on hospital day 18, and vancomycin was continued until hospital day 25.
FIG 2.
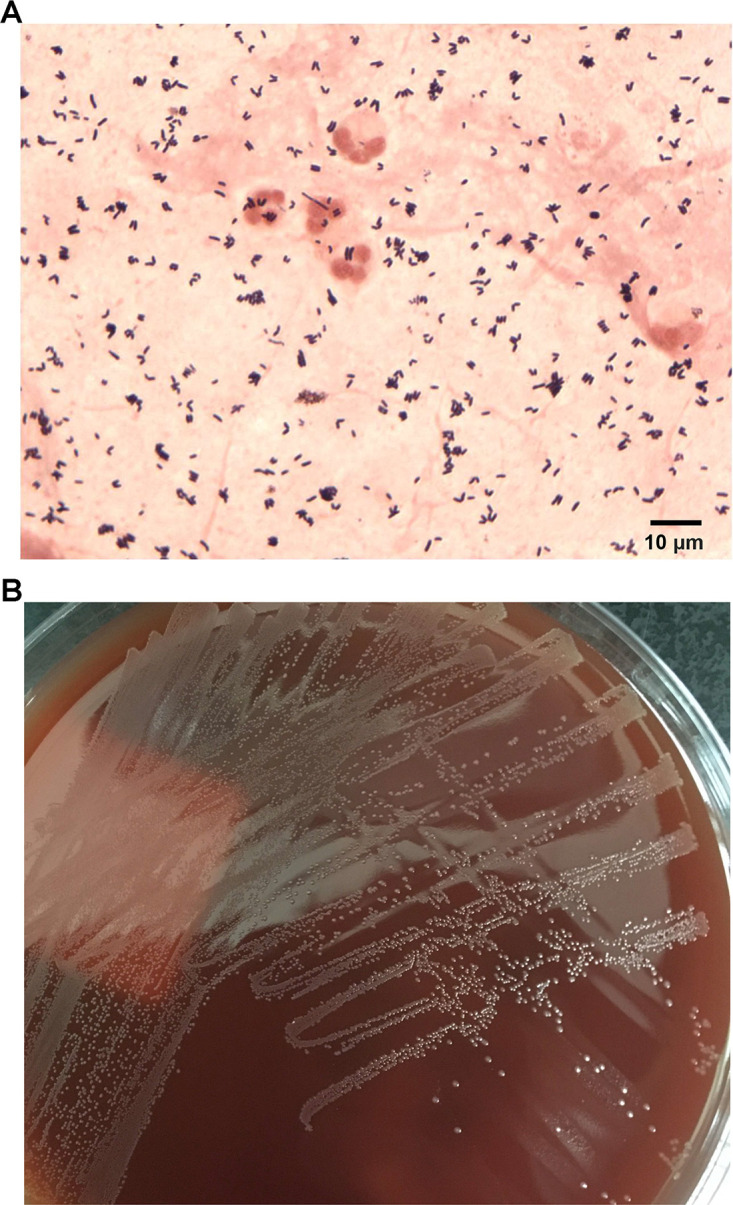
Microscopic and macroscopic morphology of Corynebacterium accolens. (A) Direct Gram stain of tracheal aspirate demonstrating coryneform Gram-positive rods and polymorphonuclear leukocytes. (B) A subculture of the C. accolens isolate, showing small, gray, nonhemolytic colonies on Columbia sheep blood agar after 48 h of incubation at 35°C with 5% CO2.
The patient’s hospital course was subsequently complicated by persistent shock, uremic encephalopathy, colonic pseudo-obstruction requiring surgical decompression, and lower gastrointestinal bleeding. After a failed trial of extubation, he underwent a percutaneously placed tracheostomy to aid in continued ventilator weaning and was transferred to our inpatient rehabilitation hospital. The patient continued to improve clinically, no longer requiring mechanical ventilation, and moved onto recovery at a skilled nursing facility.
DISCUSSION
Members of the genus Corynebacterium are aerobic, asporogenous, Gram-positive rods, consisting of at least 105 species (1). As suggested by the name, “coryne” meaning “club” in ancient Greek, Corynebacterium spp. often exhibit club-like morphology and are commonly found in clusters with angular V-shaped arrangements or palisades (Fig. 2A). More than 50 species are considered medically relevant, and nearly all are catalase-positive and nonmotile, with most also being oxidase-negative (1). The most widely recognized pathogen is Corynebacterium diphtheriae, which causes diphtheria, arguably the most prominent infectious disease caused by coryneform bacteria. Though generally considered as normal inhabitants of skin and mucous membranes, some nondiphtheritic corynebacteria have been reported to be associated with a diversity of human diseases (1–5). For example, Corynebacterium striatum is a frequently reported pathogen among the Corynebacterium species, which can cause bacteremia, pneumonia, and bronchitis, and may be multidrug resistant; Corynebacterium urealyticum is responsible for urinary tract infections, bacteremia, and wound infections; Corynebacterium ulcerans may carry the genes for diphtheria toxin and cause diphtheria-like disease and cutaneous infections; and Corynebacterium jeikeium can cause endocarditis and bacteremia (2, 3). Of note, some Corynebacterium species, such as C. accolens, C. jeikeium, and C. urealyticum, are fatty acid auxotrophs, requiring exogenous lipids for growth (3, 6). Isolates of these species exhibit improved growth on agar supplemented with additional lipids, such as Tween 80, and are referred to as “lipophilic” species (3, 6).
C. accolens was previously known as CDC Corynebacterium group G-1 and first isolated from clinical specimens by Neubauer et al. in 1991 (4, 5). As an inhabitant of the upper respiratory tract, C. accolens is one of the most common Corynebacterium species isolated from the nasal cavity of healthy people. Interestingly, it has been proposed to antagonize colonization by Streptococcus pneumoniae in this setting due to the release of antipneumococcal free fatty acids from host lipids through the action of an extracellular lipase, which is likely involved in acquisition of the fatty acids it requires for growth (6, 7). Despite this potentially beneficial role in the nasopharynx, C. accolens is increasingly recognized as being medically relevant and has been isolated from a variety of human clinical specimens, including wound drainage, endocervix, blood, and valvular vegetations (2–5). Cases of abscess, granulomatous mastitis, pelvic osteomyelitis, and both aortic and mitral valve endocarditis have been reported (2, 4, 8, 9). It may be challenging to distinguish between infections and colonization by C. accolens. Host immune status, clinical features, direct microbiological examination (e.g., Gram stain), specimen source, frequency of isolation, abundance, presence of other potential pathogens, and predominance in mixed cultures should be considered (4, 10). For example, repeated isolation of pure cultures of C. accolens from a sterile site may suggest that they are highly likely to be clinically significant (4). Nhan et al. (10) performed a retrospective study to evaluate the pathogenic role of Corynebacterium species in lower respiratory tract infections (LRTIs). Twenty-seven Corynebacterium isolates (17 C. pseudodiphtheriticum, 7 C. striatum, and 3 C. accolens isolates) from unique patients were recovered in significant quantities from respiratory specimens (sputum, endotracheal aspiration, bronchoalveolar lavage, or protected specimen brush). All 27 patients were clinically suspected of having LRTIs; 56% (15/27) were classified as being infected using CDC’s National Healthcare Safety Network criteria, and the remaining patients were considered colonized. Among the three C. accolens isolates, two were considered causative agents of VAP, and the other isolate was deemed a colonizer. Importantly, this study (10) demonstrated a significant association between true infection and hospital acquisition of the Corynebacterium isolates. These findings highlight the role of C. accolens as an opportunistic pathogen in LRTIs and VAP in high-risk populations, such as intensive care unit patients with underlying conditions (10). Interestingly, by using metagenomic next-generation sequencing, Mostafa et al. (11) found that SARS-CoV-2-positive patients had significantly decreased abundances of C. accolens isolates in nasopharyngeal specimens compared to those of SARS-CoV-2-negative patients. In our COVID-19 patient, high numbers of diphtheroids and PMNs were observed in the direct Gram stain of the tracheal aspirate, which is consistent with the C. accolens isolate being clinically significant.
Commercially available manual identification tests are widely used for identification of Corynebacterium spp., though studies have demonstrated that C. accolens isolates may be misidentified as Corynebacterium macginleyi with the API Coryne test and as Propionibacterium (Cutibacterium) acnes with the Vitek 2 ID-ANC card (4, 10). In contrast, the implementation of MALDI-TOF MS in clinical laboratories has provided a robust and cost-effective tool for rapid and accurate species-level identification of Corynebacterium spp. Suwantarat et al. (12) evaluated the performance of MALDI-TOF MS using direct on-plate extraction for identification of Corynebacterium spp. and found that this method was able to identify more Corynebacterium spp. than biochemical methods. Of note, probably due to the slow growth and tiny colonies on standard agar plates, lipophilic Corynebacterium species, such as C. accolens and Corynebacterium tuberculostearicum, may yield lower MALDI-TOF MS scores (12). The use of more biomass for MALDI-TOF MS might be helpful to increase the identification scores (12). In our case, however, the scores were sufficient to yield a confident identification of C. accolens.
Antimicrobial susceptibility testing of Corynebacterium spp. can be performed using a broth microdilution method following published guidelines (M45) from the Clinical and Laboratory Standards Institute (CLSI) (2, 13). This method involves inoculation of standardized bacterial suspensions into CAMHB-LHB (2.5% to 5% vol/vol) and incubation at 35°C in ambient air for 24 to 48 h (13). Of note, if lipophilic Corynebacterium spp. fail to grow sufficiently in CAMHB-LHB, supplementing with Tween 80 may be helpful (14). In our case, the isolate grew sufficiently under standard conditions and did not require supplementation. Interpretative criteria are presently available for 17 antimicrobial agents, but for vancomycin, daptomycin, and linezolid, only the “susceptible” category is defined due to the lack of data about resistant isolates (13). For nonsusceptible strains, it is recommended that the identification and AST be confirmed or the isolates be sent to a referral laboratory for confirmation (13). Current literature suggests that C. accolens isolates are susceptible to a broad range of antibiotics, including penicillins, ceftriaxone, gentamicin, vancomycin, and linezolid (3, 4, 10). However, susceptibility to erythromycin may be less reliable, as both susceptible (4) and resistant isolates (as seen in our case) have been observed (10).
In this case report, a patient with respiratory failure due to COVID-19 developed C. accolens-associated VAP. This case sheds light on the significance of detecting and treating the opportunistically pathogenic C. accolens in LRTIs in high-risk patients (2, 3). It also highlights the importance of accurate identification, AST, and consideration of the potential pathogenicity of non-diphtheriae Corynebacterium species.
SELF-ASSESSMENT QUESTIONS
-
1.
In which body site(s) can Corynebacterium accolens be frequently found in humans?
-
a.
Urinary tract
-
b.
Upper respiratory tract
-
c.
Gastrointestinal tract
-
d.
Genital tract
-
a.
-
2.
Which of the following phenotypic characteristics fit with identification of Corynebacterium accolens?
-
a.
Catalase positive and lipophilic
-
b.
Catalase negative and lipophilic
-
c.
Catalase positive and nonlipophilic
-
d.
Catalase negative and nonlipophilic
-
a.
-
3.
Which of the following diagnostic tests can yield the most reliable identification of Corynebacterium accolens?
-
a.
MALDI-TOF MS
-
b.
CAMP reaction
-
c.
Vitek 2 ID-ANC card
-
d.
Serologic tests
-
a.
Contributor Information
Mark A. Fisher, Email: mark.fisher@aruplab.com.
Carey-Ann D. Burnham, Washington University School of Medicine
REFERENCES
- 1.Bernard KA. 2019. Coryneform Gram‐positive rods, p 489–493. In Carroll KC, Pfaller MA (ed), Manual of clinical microbiology, 12th ed, vol 1. ASM Press, Washington, DC. [Google Scholar]
- 2.Bernard K. 2012. The genus corynebacterium and other medically relevant coryneform-like bacteria. J Clin Microbiol 50:3152–3158. 10.1128/JCM.00796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funke G, Bernard KA. 2015. Coryneform Gram-positive rods, p 474–503. In Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 11th ed, vol 1. ASM Press, Washington, DC. [Google Scholar]
- 4.Wong JS, Seaward LM, Ho CP, Anderson TP, Lau EO, Amodeo MR, Metcalf SC, Pithie AD, Murdoch DR. 2010. Corynebacterium accolens-associated pelvic osteomyelitis. J Clin Microbiol 48:654–655. 10.1128/JCM.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neubauer M, Šourek J, Rýc M, Boháček J, Mára M, Mňuková J. 1991. Corynebacterium accolens sp. nov., a gram-positive rod exhibiting satellitism, from clinical material. Syst Appl Microbiol 14:46–51. 10.1016/S0723-2020(11)80360-7. [DOI] [Google Scholar]
- 6.Bomar L, Brugger SD, Yost BH, Davies SS, Lemon KP. 2016. Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. mBio 7:e01725-15. 10.1128/mBio.01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou K, Sun F, Xu XL, Hao XK, Liu JY. 2020. Prevalences and characteristics of cultivable nasal bacteria isolated from preclinical medical students. J Int Med Res 48:300060520961716. 10.1177/0300060520961716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang LM, Brown H. 2007. Corynebacterium accolens isolated from breast abscess: possible association with granulomatous mastitis. J Clin Microbiol 45:1666–1668. 10.1128/JCM.02160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claeys G, Vanhouteghem H, Riegel P, Wauters G, Hamerlynck R, Dierick J, de Witte J, Verschraegen G, Vaneechoutte M. 1996. Endocarditis of native aortic and mitral valves due to Corynebacterium accolens: report of a case and application of phenotypic and genotypic techniques for identification. J Clin Microbiol 34:1290–1292. 10.1128/jcm.34.5.1290-1292.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nhan TX, Parienti JJ, Badiou G, Leclercq R, Cattoir V. 2012. Microbiological investigation and clinical significance of Corynebacterium spp. in respiratory specimens. Diagn Microbiol Infect Dis 74:236–241. 10.1016/j.diagmicrobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Mostafa HH, Fissel JA, Fanelli B, Bergman Y, Gniazdowski V, Dadlani M, Carroll KC, Colwell RR, Simner PJ. 2020. Metagenomic next-generation sequencing of nasopharyngeal specimens collected from confirmed and suspect COVID-19 patients. mBio 11:e01969-20. 10.1128/mBio.01969-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suwantarat N, Weik C, Romagnoli M, Ellis BC, Kwiatkowski N, Carroll KC. 2016. Practical utility and accuracy of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Corynebacterium species and other medically relevant coryneform-like bacteria. Am J Clin Pathol 145:22–28. 10.1093/ajcp/aqv006. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2016. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria—3rd ed. CLSI document M45. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Watts JL, Rossbach S. 2000. Susceptibilities of Corynebacterium bovis and Corynebacterium amylocolatum isolates from bovine mammary glands to 15 antimicrobial agents. Antimicrob Agents Chemother 44:3476–3477. 10.1128/AAC.44.12.3476-3477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


