ABSTRACT
Metronidazole resistance in clinical Clostridioides difficile is often described as unstable, since resistant strains reportedly appear susceptible following freezer storage or brief passage. This has presented a conundrum for adopting susceptibility testing to accurately evaluate the connection between metronidazole resistance and decreased clinical efficacy of metronidazole in patients with C. difficile infections (CDIs). We discovered that supplementation of microbiological media with the metalloporphyrin heme is crucial for detection of metronidazole-resistant C. difficile using the agar dilution susceptibility testing method. Known metronidazole-resistant strains appeared susceptible to metronidazole in media lacking heme. Similarly, these resistant strains exhibited increased susceptibility to metronidazole when tested on heme-containing agars that were exposed to room light for more than 1 day, likely due to heme photodecomposition. In parallel experiments, resistance was reproducibly detected when heme-containing agars were either prepared and used on the same day or protected from light and then used on subsequent days. Notably, heme did not influence the susceptibilities of drug-susceptible strains that were of the same ribotype as the resistant strains. These findings firmly show that the consistent detection of metronidazole-resistant C. difficile is dependent upon heme and its protection from light. Studies are warranted to determine the extent to which this heme-associated metronidazole-resistant phenotype affects the clinical efficacy of metronidazole in CDI and the underlying genetic and biochemical mechanisms.
KEYWORDS: porphyrin, photodecomposition, antibiotic resistance, porphyrin
INTRODUCTION
Clostridioides difficile infection (CDI) is a leading cause of health care-associated diarrhea and mortality in developed countries. This is evident in the United States, where CDI caused approximately 30,600 and 20,500 in-hospital deaths in 2011 and 2017, respectively (1). The high health care burden of CDI coincided with the spread of epidemic strains, particularly epidemic ribotype 027 that tends to cause severe and recurrent disease (2, 3). Since the 1980s, oral metronidazole has been a preferred therapeutic for mild to moderate CDI, because it is inexpensive compared to vancomycin and fidaxomicin and shows potent activity against anaerobic C. difficile (4). In 2013 to 2015, metronidazole monotherapy accounted for approximately 58% of prescriptions for CDI in the United States (5). Interestingly, there has been an unexplainable decrease in metronidazole efficacy, which was accompanied by the emergence of epidemic strains. Accordingly, the 2017 the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) clinical practice guideline does not recommend metronidazole as a first-line monotherapy for adult CDI but still recommends intravenous metronidazole in combination with oral vancomycin for severe CDI (6). However, it often takes years before guideline changes become standard clinical practice; within 18 months of the guideline being published in the United States, there was only a 3% reduction in metronidazole use (7).
Since metronidazole is commonly prescribed in the real-world clinic (7), it is important to determine whether the dissemination of metronidazole-resistant strains is driving treatment failures (8–10). Metronidazole-resistant C. difficile is defined by the EUCAST breakpoint of >2 μg/ml or CLSI breakpoint of ≥32 μg/ml (11, 12). However, key issues with susceptibility testing contribute to the lack of knowledge of whether metronidazole-resistant strains in fact hamper treatment. Indeed, antibiotic susceptibility testing of anaerobes is not routine in hospital laboratories (13), and metronidazole-resistant C. difficile is not reliably detected by the commonly used, less laborious Epsilometer test (Etest) method (14). Moreover, in C. difficile, the metronidazole-resistant phenotype is presumed to be unstable due to inconsistent susceptibility testing results or the loss of resistance following freezer storage (15). This observation is at odds with reports of stable metronidazole resistance in other bacterial pathogens and protozoans, for which metronidazole is an indicated therapy (16). Metronidazole is a nitroheterocyclic prodrug that is activated by one-electron reduction in cellular reactions catalyzed by oxidoreductases (16, 17). This generates reactive species such as a nitroimidazole anion that kills cells by damaging DNA and proteins and depleting thiols (16). In general, metronidazole resistance mechanisms are conserved across species, resulting from the acquisition of mutations or horizontal gene determinants that prevent drug activation or detoxify reactive species (16, 17).
While we were evaluating metronidazole resistance in C. difficile, we initially experienced inconsistent MICs. However, unlike past studies (15), we hypothesized that the problem was likely due to nongenetic factors, since metronidazole resistance mechanisms are stable in other anaerobic bacteria and protozoans (16, 17). This led us to review each step in our agar dilution susceptibility testing method to discover that heme is critical for reproducibly identifying metronidazole-resistant C. difficile. Here, we document this discovery and best practice approaches that improve the detection of metronidazole-resistant C. difficile.
MATERIALS AND METHODS
Strains.
C. difficile strains and their sources are listed in Table S1 in the supplemental material. For susceptibility testing, C. difficile CD196 (ribotype 027) (18) was an antibiotic-susceptible control, while previously reported metronidazole-resistant strains were of different ribotype backgrounds (14, 18). All strains were grown routinely in prereduced brain heart infusion (BHI) broth or agar at 37°C in a Whitley A35 anaerobic workstation (Don Whitley Scientific) for 24 h. Experiments with open C. difficile cultures were performed in a class II, type A2 biological safety cabinet or in the A35 workstation; autoclaving and 10% (vol/vol) bleach were used as decontamination for cultures and work surfaces.
Microbiological media and chemicals.
For susceptibility testing, the following media were used: BHI supplemented with or without 5 mg/liter porcine hemin (Alfa Aesar, catalog number [no.] A11165), Wilkins-Chalgren agar (Oxoid, catalog no. CM0619), and brucella medium base (Oxoid, catalog no. CM0169). Additional chemicals used were bilirubin (Alfa Aesar, catalog no. AAA17522-03), biliverdin (Alfa Aesar, catalog no. AAJ65226-MC), protoporphyrin IV (Sigma, catalog no. P8293), iron(II) sulfate heptahydrate (Acros, catalog no. AC201392500), iron(III) chloride hexahydrate (Sigma, catalog no. F2877), hemoglobin (Sigma, catalog no. H7379), and vitamin K1 (Alfa Aesar, catalog no. AAAL10575-06). According to the experiment, chemicals were added to molten BHI agar or brucella agar.
Determination of MICs by agar dilution method.
(i) General method.
MICs were determined using agars containing doubling dilutions of antibiotic (0.125 to 32 μg/ml). Agars were reduced for 3 h before being inoculated with 2 μl of overnight cultures (104 to 105 CFU/spot) using a semiautomated 96-well benchtop pipettor. Plates were incubated for 24 h, and the MIC was recorded as the lowest concentration of antibiotic inhibiting visible growth. Agar MICs were determined using two or more biological replicates, as indicated in Results.
(ii) Optimized method.
After recognizing that heme was required and that light affected metronidazole susceptibility tests, MICs were routinely evaluated from agars prepared on the same day as the test. Agar plates were also loosely covered with aluminum foil during anaerobic incubation.
(iii) Differences with CLSI-recommended methodology.
Vitamin K1 was only used in the discovery-oriented experiments in BHI agars; it was removed after determining it was not required to detect metronidazole resistance. BHI is not the recommended medium for anaerobic susceptibility testing by CLSI, who recommends brucella agar supplemented with vitamin K1, heme, and sheep blood. The brucella agar medium base was used to confirm findings from BHI and Wilkins-Chalgren agars that heme was required for detection of metronidazole-resistant C. difficile. MICs were obtained after incubation for 24 h, as opposed to the recommended 48 h, as C. difficile grows well under the incubation conditions used in our A35 anaerobic workstation.
Determination of metronidazole MICs with Etest strips.
C. difficile strains were cultured for 24 h on BHI agars. The inoculate was prepared by resuspending culture from the agars in prereduced phosphate-buffered saline to an optical density of 1.0, corresponding to ∼108 CFU/ml. BHI agars containing hemin (5 μg/ml) were inoculated with 200 μl of culture and allowed to dry for 1 h in the anaerobic chamber. The metronidazole Etest strip was then applied, and plates incubated for 24 h. The MIC was recorded at the base of the inhibition ellipse adjacent to the test strip.
Growth kinetics.
Metronidazole was 2-fold serially diluted in prereduced BHI broth in 96-well plates and reduced for 3 h. Strains were grown to mid-log phase (optical density at 600 nm [OD600] of ∼0.2), and 100 μl was added to the prereduced BHI to give a total volume 200 μl. Growth was automatedly recorded in a Tecan Infinite M200 microplate reader at OD600 at hourly intervals. Three biological replicates per strain (7/34, IT1001, CD196, and ATCC 43596) were used.
RESULTS AND DISCUSSION
Initial discovery that heme is required to detect metronidazole resistance.
Below, we chronicle the series of observations, results, and scientific rationales that led us to discover that heme is required for metronidazole susceptibility testing of C. difficile. Our initial studies used clinical strains known to be resistant or susceptible to metronidazole; the resistant strains were isolated in Europe and are from the culture collection of Mark Wilcox at the University of Leeds, UK (18, 19). Unless otherwise stated, agar MICs were conducted in BHI agars (see Materials and Methods). C. difficile grows well in BHI medium, which is not recommended by CLSI for anaerobic susceptibility testing but it is a commonly used medium for C. difficile research (20, 21).
From the onset, we observed discordance in the MICs (Table 1) obtained with BHI agars that were prepared, stored, and used 3 days later (day 3) compared to those that were prepared and used on the same day (day 0), as follows. On day 0, we made duplicate BHI agars (i.e., lot 1 agars) containing metronidazole (0.125 to 32 μg/ml) and supplemented with vitamin K1 (10 μg/ml) and 5 μg/ml of hemin (as the source of heme and here referred to as heme). Half of the batch of agar plates were reduced for 3 h in a Whitley A35 anaerobic chamber. The remaining half of agar plates were stored, until day 3, in transparent bags in a standard chromatography refrigerator with a glass door unprotected from light. The reduced plates were inoculated with the six metronidazole-resistant clinical strains (Table 1) that were grown in BHI broth, and MICs were recorded after 24 h of incubation. Initially, on day 0, all six clinical strains appeared to be metronidazole resistant (MICs = 8 to 16 μg/ml), compared to the susceptible control C. difficile CD196 (MIC = 0.5 μg/ml). Resistance was defined according to the EUCAST breakpoint (>2 μg/ml) to identify strains with different susceptibilities to wild-type CD196; these test strains would be considered susceptible or showing reduced susceptibility based on the CLSI breakpoint of ≥32 μg/ml for resistant strains and ≤8 μg/ml for sensitive strains. We repeated the experiment 3 days later, by reducing the remaining stored plates from lot 1 and inoculating them with fresh overnight cultures. However, the 3-day-old agar plates failed to detect metronidazole resistance (MICs = 1 μg/ml against resistant strains) (Table 1). Because these results were typical of an unstable phenotype, we reperformed the susceptibility testing from scratch using fresh BHI medium (i.e., lot 2 agars), with fresh bacteria cultured from the spore stocks at −80°C. Again, all six strains showed resistance to metronidazole (MICs = 8 to 16 μg/ml) when tested on the same day that the agars were made, but they appeared susceptible (MICs = 1 μg/ml against resistant strains) (Table 1) when tested on 3-day-old agars.
TABLE 1.
Discordant metronidazole MICs occur depending on the age of the test agar plates
| Strain (ribotype) | MIC (μg/ml)a |
||||
|---|---|---|---|---|---|
| Lot 1 agar |
Lot 2 agar |
||||
| Day 0 | Day 3 | Day 0 | Day 3 | ||
| 17/27 (RT001)b | 16 | 1 | 16 | 1 | |
| 72/25 (RT001)b | 8 | 1 | 8 | 1 | |
| 7/34 (RT106)b | 8 | 1 | 8 | 1 | |
| 71/01 (RT106)b | 16 | 1 | 16 | 1 | |
| 70/76 (RT027)b | 8 | 1 | 8 | 1 | |
| 70/75 (RT027)b | 16 | 1 | 16 | 1 | |
| CD196 (RT027)c | 0.5 | 0.5 | 0.5 | 0.5 | |
Susceptibility to metronidazole was measured according to the age of premade agar plates. Results are based on three biological replicates tested on BHI agars.
Metronidazole resistant.
Metronidazole-susceptible control; 3-day-old agars failed to detect metronidazole resistance, as reflected by susceptible MICs.
To uncover causes of the above-described conflicting MICs, we hypothesized that during the 3-day storage, the medium additives vitamin K1 or hemin became degraded, as they are light-sensitive components (22–24). We based this premise on the following scientific rationales. First, susceptibility test results can be affected by medium components and storage. For example, increased MICs for tetracyclines occur when the antibiotic is oxidized in aerated medium that is stored for more than 1 week (25, 26), in a reaction that may be driven by UV or visible light (27). Second, because the control strain CD196 remained susceptible on 3-day plates, it suggested that metronidazole was not degraded, which is in keeping with the drug being stable at 2°C to 8°C (we are also unaware of reports that metronidazole is unstable in agars). Third, since metronidazole resistance mechanisms are still very unclear in clinical C. difficile (16, 28), we speculated that these strains might have evolved ways to utilize heme or vitamin K1 in oxidative stress responses to metronidazole. For example, in bacteria, heme has various roles as an iron source and cofactor to redox-associated proteins that perform various functions (29); it was recently shown that heme can be hijacked by C. difficile HsmA protein to reduce oxidative damage (30). Regarding vitamin K1, its reduced form can protect lipids from reactive oxygen species (31, 32). Thus, to test the importance of heme or vitamin K1 to the metronidazole resistance phenotype, we determined MICs in BHI agars supplemented with either vitamin K1 (10 μg/ml) or heme (5 μg/ml). As shown in Table 2, heme but not vitamin K1 enabled detection of the metronidazole-resistant strains (MICs = 8 to 16 μg/ml in heme agars versus 1 μg/ml in agars with vitamin K1). This suggested that heme could be a central factor for variability in metronidazole susceptibility test results. Vitamin K1 was therefore removed from all further experiments. All experiments used three biological replicates. Furthermore, all strains in Table 2 were susceptible to vancomycin (EUCAST breakpoint of >2 μg/ml), with vancomycin MICs of ≤2 μg/ml that were not influenced by heme, using agars made on the day of the tests.
TABLE 2.
Detection of metronidazole resistance depends on addition of heme to agars
| Strain (ribotype) | MIC (μg/ml)a |
||
|---|---|---|---|
| Heme | Vitamin K1 | BHI only | |
| 17/27 (RT001)b | 16 | 1 | 1 |
| 72/25 (RT001)b | 8 | 1 | 1 |
| 7/34 (RT106)b | 8 | 1 | 1 |
| 71/01 (RT106)b | 16 | 1 | 1 |
| 70/76 (RT027)b | 8 | 1 | 1 |
| 70/75 (RT027)b | 16 | 1 | 1 |
| CD196 (RT027)c | 0.5 | 0.5 | 0.5 |
Effect of medium supplement on metronidazole susceptibility; supplementation of BHI agar with heme (5 μg/ml) was associated with detection of metronidazole resistance, unlike BHI supplemented with vitamin K1 (10 μg/ml) or BHI only. Results are based on three biological replicates.
Metronidazole resistant.
Metronidazole-susceptible control.
Validation that heme is needed to detect metronidazole-resistant C. difficile.
To substantiate that detection of the metronidazole resistance phenotype required heme, we analyzed how agar dilution MICs are affected by (i) the concentration of heme and (ii) the exposure of heme-containing agars to light. (iii) We also evaluated Etests MICs in heme-containing BHI media and (iv) agar dilution MICs using brucella medium base. These experiments used a wider panel of strains, including others reported to be metronidazole susceptible (e.g., BAA-1875 [ribotype 078]) or resistant (e.g., IT1001 and IT1002 [ribotype 010]) (14, 18).
(i) Metronidazole resistance is heme dose dependent.
Agar MICs were determined using BHI agars. Figure 1 shows that decreasing concentrations of heme (from 5 to 0.16 μg/ml) decreased metronidazole MIC values against resistant strains. At 2.5 μg/ml of heme, most strains were susceptible (MICs ≤ 2 μg/ml), apart from IT1001 and IT1002 that only appeared susceptible (MIC = 2 μg/ml) at 0.31 μg/ml of heme. We term this resistance phenotype heme-associated metronidazole resistance. Collectively, these results indicate that the standard concentration of heme (5 μg/ml) could reproducibly detect different metronidazole-resistant strains.
FIG 1.
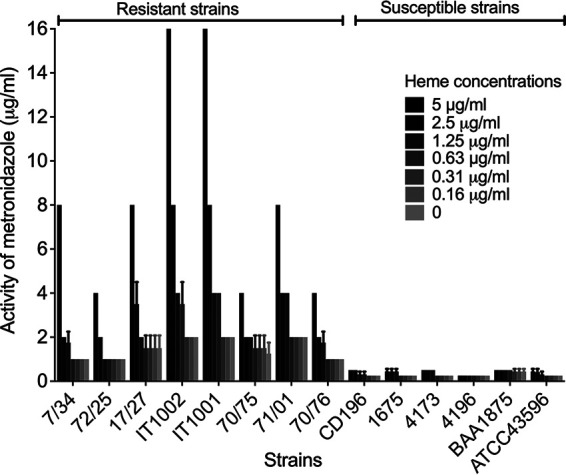
Correlation between concentration of heme and activity of metronidazole (MICs) against resistant and susceptible C. difficile strains. Brain heart infusion (BHI) agars containing drug were supplemented with heme at various concentrations, as shown. Results are based on four biological replicates and plotted as mean MICs with standard deviations for each strain.
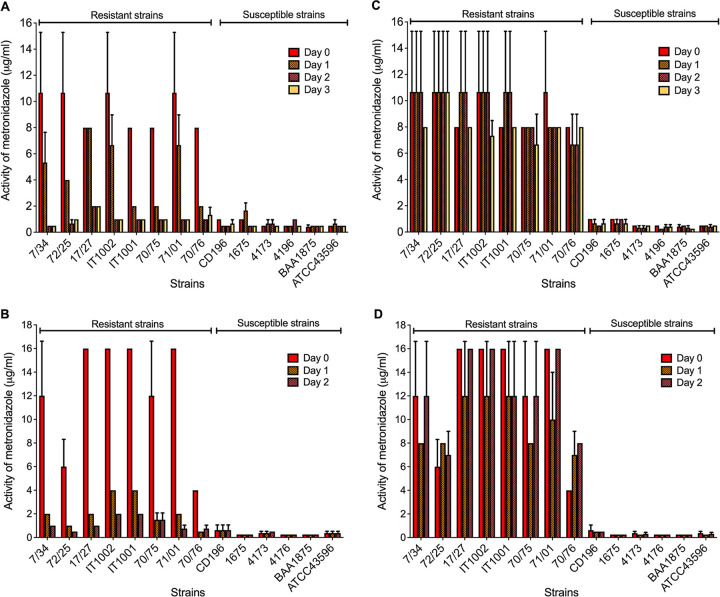
(ii) Exposure of heme agars to light produces erratic metronidazole MICs.
Heme strongly absorbs light and undergoes photodecomposition in oxygen, producing iron-oxo heme complexes and derivatives from porphyrin degradation (33–35). We reasoned that exposure of heme agars to light would misclassify metronidazole-resistant strains due to light inactivation of heme. MICs were therefore measured over 3 days with BHI agars that were all prepared on day 0 and either left exposed to room light or wrapped with standard aluminum foil to protect them from light. The plates were stored in a chromatography refrigerator. For light-exposed agars, over the three test days, metronidazole MICs began to vary by day 1, and all resistant strains appeared susceptible on agars exposed to light for 3 days (Fig. 2A). Conversely, foil-wrapped plates consistently detected metronidazole-resistant strains (Fig. 2C). As expected, we did not detect resistance using the negative-control agars that lacked heme.
FIG 2.
Effect of exposing agars to light on the activity of metronidazole (MICs) against resistant and susceptible C. difficile strains. Plots show that exposing heme-containing agars to light prevents reliable detection of metronidazole-resistant strains. (A and B) Brain heart infusion (BHI) agars; (C and D) Wilkins-Chalgren (WC) agars; plates exposed to light for the indicated number of days (A and C) compared to those that were foil wrapped (B and D). Results for BHI and WC are based on three and four biological replicates, respectively, and plotted as the mean MICs with standard deviations. Over consecutive days, the metronidazole MICs against resistant strains are comparable to those at day 0 for foil-wrapped plates.
We next performed susceptibility tests using Wilkins-Chalgren agar, a former reference medium (12) that is suggested to be a more reliable medium for metronidazole susceptibility testing of C. difficile (36). It was shown that Wilkins-Chalgren agar is better at detecting metronidazole-resistant C. difficile (36), but the underlying scientific rationale is lacking. Interestingly, the dehydrated agar base for Wilkins-Chalgren medium contains heme that is 5 mg/liter when reconstituted. Hence, we tested if light exposure would also affect identification of metronidazole resistance on Wilkins-Chalgren agars. Metronidazole-resistant strains were also misidentified when metronidazole-containing Wilkins-Chalgren agars were stored in light compared to that for corresponding agars that were foil wrapped (Fig. 2B and D). These results clearly suggest that storage of heme-containing agars in room light is a significant source for inconsistent metronidazole MICs, which is a factor that can be easily overlooked.
(iii) Evaluation of Etests in heme-containing BHI agars.
Etests on BHI agars with hemin showed lower metronidazole MICs than those from agar dilution tests, as follows: strain 70/76 had an Etest MIC of 1.5 μg/ml versus 8 μg/ml by the agar dilution method, the Etest MIC against strain 17/27 was 3 to 4 μg/ml versus 8 to 16 μg/ml by the agar dilution method, and the Etest MIC against strain CD196 was 0.064 to 0.094 μg/ml versus 0.5 to 1 μg/ml by the agar dilution method; results are from biological triplicates. Based on the Etest, strain 70/76 would be misclassified as metronidazole susceptible. We suspect that the lower metronidazole Etest MICs might be driven by the outcome of two key dynamic variables, i.e., the rate of metronidazole diffusion from Etest strips and its cellular uptake versus the rate in which heme is extracted from the agar by C. difficile.
(iv) Agar dilution MICs on brucella agars.
Brucella medium base was used to further assess metronidazole MICs with or without heme (5 μg/ml). As shown in Table 3, the metronidazole-resistant strains exhibited resistance (MICs = 4 to 16 μg/ml), compared to the EUCAST breakpoint, for brucella agars supplemented with heme. However, the strains appeared susceptible on corresponding brucella agars without heme. Conversely, the antibiotic-susceptible strain ATCC 700057 was susceptible under either condition. Vitamin K1 was not added to the brucella agars.
TABLE 3.
Metronidazole MICs with or without heme using brucella agars
| Strain (ribotype) | MIC (μg/ml)a |
|
|---|---|---|
| Without heme | With heme | |
| 7/34 (RT106)b | 1 | 8 |
| 71/01 (RT106)b | 1 | 16 |
| 72/25 (RT001)b | 1 | 8 |
| 17/27 (RT001)b | 0.5 | 8–16 |
| IT1002 (RT010)b | 1 | 4–8 |
| IT1001 (RT010)b | 1 | 8 |
| 70/75 (RT027)b | 1 | 8–16 |
| 70/76 (RT027)b | 0.5 | 8–16 |
| CD196 (RT027)c | 0.25 | 0.5 |
| 1675 (RT027)c | 0.25 | 0.25 |
| 4173 (RT027)c | 0.5 | 0.50 |
| 4196 (RT027)c | 0.25 | 0.5 |
| BAA1875 (RT078)c | 0.5 | 0.5 |
| ATCC 43596 (RT012)c | 0.5 | 1 |
| ATCC 700057 (RT038)c | 0.25 | 0.25 |
MICs are from three biological replicates and shown as the range, where obtained. Brucella agars did not contain vitamin K1.
Metronidazole resistant.
Metronidazole susceptible.
Heme catabolites do not induce the metronidazole resistance phenotype and heme does not enhance growth.
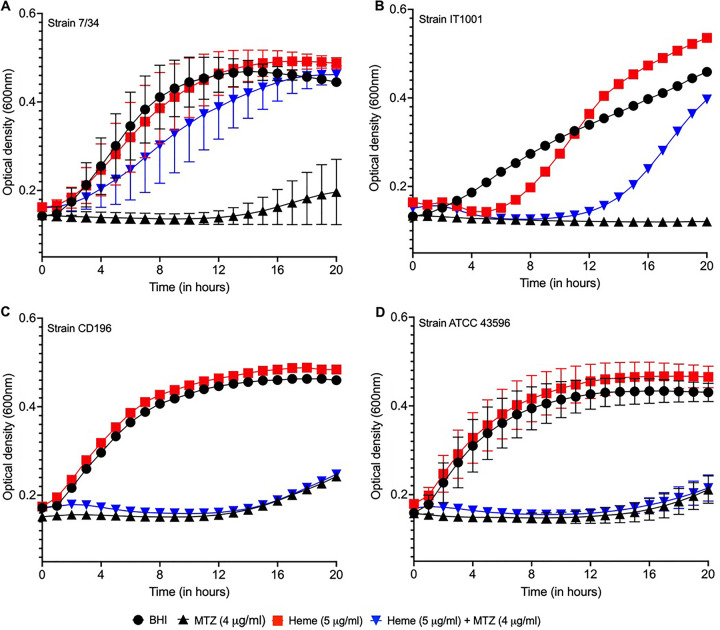
We also tested if iron and other heme catabolites influence metronidazole resistance and whether heme impacts C. difficile growth rates. Various bacterial pathogens degrade heme to obtain the micronutrient iron and produce tetrapyrroles that may scavenge reactive species (37). Because it is not known how C. difficile catabolizes heme, we selected known catabolites (iron, porphyrin, bilirubin, or biliverdin) to gauge if they modulate metronidazole susceptibility at equimolar concentrations of 8 μm based on the molarity of hemin at 5 μg/ml; all MICs were determined on BHI agars. None of the selected catabolites supported detection of a metronidazole resistance phenotype (Table 4). Conversely, resistance was evident in BHI containing hemoglobin (Table 4). It might be tempting to speculate that metronidazole-resistant C. difficile uses heme to improve growth to recover in the presence of metronidazole. However, as shown in Fig. 3, heme did not enhance the growth of resistant or susceptible strains; this is in keeping with heme not being a preferred iron source for C. difficile (38). Growth was only observed for the resistant strains in metronidazole, when heme was added to the media (Fig. 3). Hence, it is plausible that metronidazole-resistant strains might use undegraded heme as a biological cofactor.
TABLE 4.
Effect of heme catabolites on metronidazole MICs
| Strain (ribotype) | MIC (μg/ml)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| BHI | Heme | Hb | BLV | BLB | PPIX | FeCl3 | FeSO4 | |
| 17/27 (RT001)b | 0.50 | 32 | 8 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| 70/76 (RT027)b | 0.50 | 8 | 4 | 1 | 0.5 | 0.25 | 0.50 | 1 |
| CD196 (RT027)c | 0.25 | 0.25 | 0.50 | 0.25 | 0.25 | 0.5 | 0.50 | 0.25 |
| BAA-1875 (RT078)c | 0.25 | 0.25 | 0.25 | 0.50 | 0.50 | 0.12 | 0.25 | 0.50 |
MICs of metronidazole with heme or catabolites were determined on BHI agars. Results are based on three biological replicates. Only BHI medium with heme (hemin or Hb [hemoglobin]) supported detection of metronidazole-resistant C. difficile. BHI agars were supplemented with catabolites at 8 μM, equivalent to 5 μg/ml of hemin. BLV, biliverdin; BLB, bilirubin; PPIX, protoporphyrin IX; FeCl3, iron chloride; FeSO4, iron sulfate.
Metronidazole resistant.
Metronidazole susceptible.
FIG 3.
Effect of heme on the growth of metronidazole-resistant and -susceptible C. difficile in brain heart infusion (BHI) broth in the presence and absence of metronidazole. (A and B) Metronidazole-resistant 7/34 and IT1001 only grew in metronidazole (4 μg/ml) when heme was added. (C and D) Heme did not rescue the inhibition of metronidazole-susceptible CD196 and ATCC 43596. In the absence of drug, heme did not enhance the growth of the tested strains. Growth curves are plotted as the means from three biological replicates with standard errors of the means; error bars for CD196 and IT1001 are not seen, as they are shorter than the size of the symbol in their plots.
Heme-associated metronidazole resistance is widespread.
We expanded our susceptibility tests to various ribotypes of C. difficile clinical strains (n = 64) that were isolated in different geographic regions (see Table S1 in the supplemental material). The panel included 10 previously reported ribotype 027 metronidazole-resistant strains from Israel (10), isogenic ribotype 027 metronidazole-susceptible (CD26A54_S) and -resistant (CD26A54_R) strains from Canada (39), and uncharacterized strains of various ribotypes from across the United States (i.e., from the Texas Medical Center, n = 9, and the CDC panel at BEI Resources, n = 42). MICs were from two biological replicates on BHI agars with or without heme and compared to EUCAST and CLSI breakpoints (Table S1).
Previously reported metronidazole-resistant isolates (n = 10) from Israel all required heme to exhibit resistance (i.e., MIC of 8 μg/ml with heme versus 0.5 μg/ml without heme). Strikingly, CD26A54_S and CD26A54_R both displayed heme-associated resistance to metronidazole (MICs of 8 to 16 μg/ml and 1 μg/ml, with and without heme, respectively). Because CD26A54_S was originally thought to be metronidazole susceptible according to Etests, Lynch et al. (39) passaged it in metronidazole to obtained CD26A54_R. Based on our results, CD26A54_S was misclassified. Thus, the reported mutations in CD26A54_R, not found in CD26A54_S, may either be second-step or laboratory-derived mechanisms of metronidazole resistance (these include mutations in pyruvate ferredoxin oxidoreductase, ferric uptake regulator, and the oxygen-independent coproporphyrinogen III oxidase) (16, 39). Heme-associated metronidazole-resistant strains from the CDC strain panel were primarily ribotype 027 (n = 7). Regarding strains from Houston, Texas, both 014/20 and 027 ribotypes showed heme-associated resistance. In all cases, heme was required to identify metronidazole-resistant strains. These findings firmly suggest that an unknown mechanism of heme-associated metronidazole resistance is widely disseminated among genetically related and unrelated strains (i.e., as defined by ribotyping).
Concluding remarks.
The increased use of metronidazole in response to higher rates of CDI caused by epidemic C. difficile likely enhanced the selection pressure for the spread of metronidazole-resistant C. difficile. It is therefore unsurprising that over the past 2 decades, decreased clinical response rates have been observed in patients treated with metronidazole (40). Attempts to correlate metronidazole resistance with clinical outcomes in CDI patients have failed (41, 42), partly due to shortcomings in the Etest approach to identify metronidazole-resistant strains (14, 36) and the perception that they have an unstable phenotype (15). Significantly, our work shows that metronidazole resistance in C. difficile is unlikely to be unstable, but detection of the phenotype requires undegraded heme in agar media and its protection from ambient room light during storage. Therefore, metronidazole susceptibility tests should either be conducted on the same day that heme agar plates are prepared or the heme agar plates should be light protected during storage for their subsequent use (e.g., 3 days of storage in this study). Our observations are largely based on MICs from BHI agar that is not recommended by the CLSI for anaerobic susceptibility testing. Similarly, Etests were performed on BHI agars, which is a non-manufacturer-supported medium. Nonetheless, corresponding findings in the recommended brucella medium base gives credence to agar dilution MICs obtained with BHI agar. This indicates that the integrity of heme is the deciding factor for detecting metronidazole-resistant C. difficile; this feature should be considered in the workflow of individual laboratories performing metronidazole susceptibility testing.
Importantly, the heme-associated metronidazole resistance phenotype occurred in strains of different ribotypes from various geographic locations, while counterpart metronidazole-susceptible strains did not exhibit the phenotype. Our findings are supported by a recent complementary study by Boekhoud et al. (43), also reporting that heme distinguishes metronidazole-resistant from -susceptible C. difficile strains of various ribotypes. Collectively, this could point to a novel mechanism that has evolved and is conserved across C. difficile ribotypes. We are not aware of other metronidazole-resistant bacteria or protozoans that adopt heme for resistance. It is also important to note that in the study by Boekhoud et al. (43), Etest MICs on brucella and BHI agars showed good correlation. However, in our study, Etest MICs tended to be lower than BHI agar dilution MICs. Further work will be required to access the suitability of Etests for detecting metronidazole-resistant C. difficile in agar plates that are protected from light or used on the same day.
The potential for treatment failure by metronidazole-resistant C. difficile may not only depend on the colonic concentrations of drug but also the level of free heme. We anticipate that metronidazole-resistant strains with heme-associated MICs of >2 to 16 μg/ml should have a survival advantage in colonic concentrations of metronidazole that is, on average, 9.3 ± 7.5 μg/g in watery stools in acute disease (44). Nonetheless, such strains would be regarded as susceptible based on the CLSI breakpoint (≥32 μg/ml). This suggests the need for studies to delineate what level of resistance (i.e., MICs) is required to cause poor treatment responses to metronidazole in CDI. Regarding heme, frank bleeding in CDI is not common, but patients with underlying inflammatory bowel disease are more prone to intestinal bleeding (45, 46) and might be more at risk for failing metronidazole therapy. However, hemoglobin has been shown to accumulate in the ceca of mice during CDI in response to epithelial damage and inflammation (47). Taken together, evaluations of clinical outcomes of metronidazole based on MICs will need to also consider the colonic concentrations of heme.
Although oral metronidazole is no longer a guideline-recommended first-line drug for adult CDI, it is still commonly used in practice. In addition, it is recommended and used intravenously for patients that cannot tolerate oral antibiotics and for fulminant CDI. Certainly, the emergence and undetected spread of metronidazole-resistant C. difficile challenge the status quo that prompt susceptibility testing for anaerobes is unwarranted. Furthermore, if metronidazole resistance impacts therapy and such strains are widespread, then it may be necessary to rethink the worth of recommended combination therapies of vancomycin with metronidazole. However, without routine susceptibility testing, it might be possible that vancomycin could suffer a similar fate to that of metronidazole, considering that vancomycin-resistant strains have emerged (48–50) and their therapeutic impacts are unknown.
ACKNOWLEDGMENTS
This work was in part funded by grants R56AI126881 and R01AI139261 to J.G.H. from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
The funders had no role in study design, data collection, interpretation of the findings, or in the writing and submission of the manuscript.
We are grateful for the receipt of clinical strains from Mark Wilcox, University of Leeds, United Kingdom; Michael Mulvey, Public Health Agency of Canada; Amos Adler, Tel Aviv University, Israel; Paola Mastrantonio, Istituto Superiore di Sanità, Italy; Dale Gerding, Loyola University Medical Center, United States; Scott Curry, University of Pittsburgh School of Medicine, United States; and BEI Resources, National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Footnotes
Supplemental material is available online only.
Contributor Information
Julian G. Hurdle, Email: jhurdle@tamu.edu.
Patricia J. Simner, Johns Hopkins
REFERENCES
- 1.Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, Wilson LE, Holzbauer SM, Phipps EC, Dumyati GK, Beldavs ZG, Kainer MA, Karlsson M, Gerding DN, McDonald LC, Emerging Infections Program Clostridioides difficile Infection Working Group. 2020. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 382:1320–1330. 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrella LA, Sambol SP, Cheknis A, Nagaro K, Kean Y, Sears PS, Babakhani F, Johnson S, Gerding DN. 2012. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis 55:351–357. 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao K, Micic D, Natarajan M, Winters S, Kiel MJ, Walk ST, Santhosh K, Mogle JA, Galecki AT, LeBar W, Higgins PD, Young VB, Aronoff DM. 2015. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clin Infect Dis 61:233–241. 10.1093/cid/civ254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelaez T, Alcala L, Alonso R, Rodriguez-Creixems M, Garcia-Lechuz JM, Bouza E. 2002. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother 46:1647–1650. 10.1128/AAC.46.6.1647-1650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novosad SA, Mu Y, Winston LG, Johnston H, Basiliere E, Olson DM, Farley MM, Revis A, Wilson L, Perlmutter R, Holzbauer SM, Whitten T, Phipps EC, Dumyati GK, Beldavs ZG, Ocampo VLS, Davis CM, Kainer M, Gerding DN, Guh AY. 2020. Treatment of Clostridioides difficile infection and non-compliance with treatment guidelines in adults in 10 US geographical locations, 2013–2015. J Gen Intern Med 35:412–419. 10.1007/s11606-019-05386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. 2018. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 66:e1–e48. 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy CJ, Buehrle D, Vu M, Wagener MM, Nguyen MH. 2021. Impact of revised Infectious Diseases Society of America and Society for Healthcare Epidemiology of America clinical practice guidelines on the treatment of Clostridium difficile infections in the United States. Clin Infect Dis 72:1944–1949. 10.1093/cid/ciaa484. [DOI] [PubMed] [Google Scholar]
- 8.Freeman J, Vernon J, Pilling S, Morris K, Nicholson S, Shearman S, Longshaw C, Wilcox MH, Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes Study Group. 2018. The ClosER study: results from a three-year pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes, 2011–2014. Clin Microbiol Infect 24:724–731. 10.1016/j.cmi.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Thorpe CM, McDermott LA, Tran MK, Chang J, Jenkins SG, Goldstein EJC, Patel R, Forbes BA, Johnson S, Gerding DN, Snydman DR. 2019. U.S.-based national surveillance for fidaxomicin susceptibility of Clostridioides difficile-associated diarrheal isolates from 2013 to 2016. Antimicrob Agents Chemother 63:e00391-19. 10.1128/AAC.00391-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler A, Miller-Roll T, Bradenstein R, Block C, Mendelson B, Parizade M, Paitan Y, Schwartz D, Peled N, Carmeli Y, Schwaber MJ. 2015. A national survey of the molecular epidemiology of Clostridium difficile in Israel: the dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn Microbiol Infect Dis 83:21–24. 10.1016/j.diagmicrobio.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 11.European Committee on Antimicrobial Susceptibility Testing. 2021. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf.
- 12.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing. CLSI document M100, 30th edition. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Schuetz AN. 2014. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Clin Infect Dis 59:698–705. 10.1093/cid/ciu395. [DOI] [PubMed] [Google Scholar]
- 14.Moura I, Spigaglia P, Barbanti F, Mastrantonio P. 2013. Analysis of metronidazole susceptibility in different Clostridium difficile PCR ribotypes. J Antimicrob Chemother 68:362–365. 10.1093/jac/dks420. [DOI] [PubMed] [Google Scholar]
- 15.Pelaez T, Cercenado E, Alcala L, Marin M, Martin-Lopez A, Martinez-Alarcon J, Catalan P, Sanchez-Somolinos M, Bouza E. 2008. Metronidazole resistance in Clostridium difficile is heterogeneous. J Clin Microbiol 46:3028–3032. 10.1128/JCM.00524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande A, Wu X, Huo W, Palmer KL, Hurdle JG. 2020. Chromosomal resistance to metronidazole in Clostridioides difficile can be mediated by epistasis between iron homeostasis and oxidoreductases. Antimicrob Agents Chemother 64:e00415-20. 10.1128/AAC.00415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisson G, Goodwin A, Raudonikiene A, Hughes NJ, Mukhopadhyay AK, Berg DE, Hoffman PS. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother 46:2116–2123. 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherian PT, Wu X, Yang L, Scarborough JS, Singh AP, Alam ZA, Lee RE, Hurdle JG. 2015. Gastrointestinal localization of metronidazole by a lactobacilli-inspired tetramic acid motif improves treatment outcomes in the hamster model of Clostridium difficile infection. J Antimicrob Chemother 70:3061–3069. 10.1093/jac/dkv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman J, Vernon J, Morris K, Nicholson S, Todhunter S, Longshaw C, Wilcox MH, Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium difficile Ribotypes' Study Group. 2015. Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin Microbiol Infect 21:248.e9–248.e16. 10.1016/j.cmi.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Edwards AN, Suarez JM, McBride SM. 2013. Culturing and maintaining Clostridium difficile in an anaerobic environment. J Vis Exp 21:e50787. 10.3791/50787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorg JA, Dineen SS. 2009. Laboratory maintenance of Clostridium difficile. Curr Protoc Microbiol Chapter 9:Unit9A.1. 10.1002/9780471729259.mc09a01s12. [DOI] [PubMed] [Google Scholar]
- 22.Billion-Rey F, Guillaumont M, Frederich A, Aulagner G. 1993. Stability of fat-soluble vitamins A (retinol palmitate), E (tocopherol acetate), and K1 (phylloquinone) in total parenteral nutrition at home. JPEN J Parenter Enteral Nutr 17:56–60. 10.1177/014860719301700156. [DOI] [PubMed] [Google Scholar]
- 23.S KS, Bautista JF, Watson RA. 1991. Metalloporphyrin photochemistry with matrix isolation. J Am Chem Soc 113:6111–6114. 10.1021/ja00016a029. [DOI] [Google Scholar]
- 24.Bartocci C, Scandola F, Ferri A, Carassiti V. 1980. Photoreduction of hemin in alcohol-containing mixed solvents. J Am Chem Soc 102:7067–7072. 10.1021/ja00543a030. [DOI] [Google Scholar]
- 25.Hope R, Warner M, Mushtaq S, Ward ME, Parsons T, Livermore DM. 2005. Effect of medium type, age and aeration on the MICs of tigecycline and classical tetracyclines. J Antimicrob Chemother 56:1042–1046. 10.1093/jac/dki386. [DOI] [PubMed] [Google Scholar]
- 26.Bradford PA, Petersen PJ, Young M, Jones CH, Tischler M, O'Connell J. 2005. Tigecycline MIC testing by broth dilution requires use of fresh medium or addition of the biocatalytic oxygen-reducing reagent oxyrase to standardize the test method. Antimicrob Agents Chemother 49:3903–3909. 10.1128/AAC.49.9.3903-3909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang ST, Lee SY, Wang SH, Wu CY, Yuann JP, He S, Cheng CW, Liang JY. 2019. The influence of the degradation of tetracycline by free radicals from riboflavin-5'-phosphate photolysis on microbial viability. Microorganisms 7:500. 10.3390/microorganisms7110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boekhoud IM, Hornung BVH, Sevilla E, Harmanus C, Bos-Sanders I, Terveer EM, Bolea R, Corver J, Kuijper EJ, Smits WK. 2020. Plasmid-mediated metronidazole resistance in Clostridioides difficile. Nat Commun 11:598. 10.1038/s41467-020-14382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giardina G, Rinaldo S, Johnson KA, Di Matteo A, Brunori M, Cutruzzola F. 2008. NO sensing in Pseudomonas aeruginosa: structure of the transcriptional regulator DNR. J Mol Biol 378:1002–1015. 10.1016/j.jmb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Knippel RJ, Wexler AG, Miller JM, Beavers WN, Weiss A, de Crecy-Lagard V, Edmonds KA, Giedroc DP, Skaar EP. 2020. Clostridioides difficile senses and hijacks host heme for incorporation into an oxidative stress defense system. Cell Host Microbe 28:411.e6–421.e6. 10.1016/j.chom.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vervoort LM, Ronden JE, Thijssen HH. 1997. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem Pharmacol 54:871–876. 10.1016/S0006-2952(97)00254-2. [DOI] [PubMed] [Google Scholar]
- 32.Ambrożewicz E, Muszyńska M, Tokajuk G, Grynkiewicz G, Žarković N, Skrzydlewska E. 2019. Beneficial effects of vitamins K and D3 on redox balance of human osteoblasts cultured with hydroxyapatite-based biomaterials. Cells 8:325. 10.3390/cells8040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahu S, Goldberg DP. 2016. Activation of dioxygen by iron and manganese complexes: a heme and nonheme perspective. J Am Chem Soc 138:11410–11428. 10.1021/jacs.6b05251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryu WH, Gittleson FS, Thomsen JM, Li J, Schwab MJ, Brudvig GW, Taylor AD. 2016. Heme biomolecule as redox mediator and oxygen shuttle for efficient charging of lithium-oxygen batteries. Nat Commun 7:12925. 10.1038/ncomms12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moan J, Berg K. 1991. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol 53:549–553. 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 36.Baines SD, O'Connor R, Freeman J, Fawley WN, Harmanus C, Mastrantonio P, Kuijper EJ, Wilcox MH. 2008. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother 62:1046–1052. 10.1093/jac/dkn313. [DOI] [PubMed] [Google Scholar]
- 37.Nobles CL, Green SI, Maresso AW. 2013. A product of heme catabolism modulates bacterial function and survival. PLoS Pathog 9:e1003507. 10.1371/journal.ppat.1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cernat RC, Scott KP. 2012. Evaluation of novel assays to assess the influence of different iron sources on the growth of Clostridium difficile. Anaerobe 18:298–304. 10.1016/j.anaerobe.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Lynch T, Chong P, Zhang J, Hizon R, Du T, Graham MR, Beniac DR, Booth TF, Kibsey P, Miller M, Gravel D, Mulvey MR, Canadian Nosocomial Infection Surveillance Program (CNISP). 2013. Characterization of a stable, metronidazole-resistant Clostridium difficile clinical isolate. PLoS One 8:e53757. 10.1371/journal.pone.0053757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, Gelone SP, Broom C, Davidson DM, Polymer Alternative for CDI Treatment (PACT) Investigators. 2014. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 59:345–354. 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 41.Venugopal AA, Riederer K, Patel SM, Szpunar S, Jahamy H, Valenti S, Shemes SP, Khatib R, Johnson LB. 2012. Lack of association of outcomes with treatment duration and microbiologic susceptibility data in Clostridium difficile infections in a non-NAP1/BI/027 setting. Scand J Infect Dis 44:243–249. 10.3109/00365548.2011.631029. [DOI] [PubMed] [Google Scholar]
- 42.Al-Nassir WN, Sethi AK, Nerandzic MM, Bobulsky GS, Jump RL, Donskey CJ. 2008. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis 47:56–62. 10.1086/588293. [DOI] [PubMed] [Google Scholar]
- 43.Boekhoud IM, Sidorov I, Nooij S, Harmanus C, Bos-Sanders I, Viprey V, Spittal W, Clark E, Davies K, Freeman J, Kuijper EJ, Smits WK, COMBACTE-CDI Consortium. 20April2021. Haem is crucial for medium-dependent metronidazole resistance in clinical isolates of Clostridioides difficile. J Antimicrob Chemother 10.1093/jac/dkab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolton RP, Culshaw MA. 1986. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 27:1169–1172. 10.1136/gut.27.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saidel-Odes L, Borer A, Odes S. 2011. Clostridium difficile infection in patients with inflammatory bowel disease. Ann Gastroenterol 24:263–270. [PMC free article] [PubMed] [Google Scholar]
- 46.Khanna S, Shin A, Kelly CP. 2017. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 15:166–174. 10.1016/j.cgh.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Knippel RJ, Zackular JP, Moore JL, Celis AI, Weiss A, Washington MK, DuBois JL, Caprioli RM, Skaar EP. 2018. Heme sensing and detoxification by HatRT contributes to pathogenesis during Clostridium difficile infection. PLoS Pathog 14:e1007486. 10.1371/journal.ppat.1007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tickler IA, Goering RV, Whitmore JD, Lynn AN, Persing DH, Tenover FC, Healthcare Associated Infection Consortium. 2014. Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013. Antimicrob Agents Chemother 58:4214–4218. 10.1128/AAC.02775-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snydman DR, McDermott LA, Jacobus NV, Thorpe C, Stone S, Jenkins SG, Goldstein EJ, Patel R, Forbes BA, Mirrett S, Johnson S, Gerding DN. 2015. U.S.-based national sentinel surveillance study for the epidemiology of Clostridium difficile-associated diarrheal isolates and their susceptibility to fidaxomicin. Antimicrob Agents Chemother 59:6437–6443. 10.1128/AAC.00845-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen WJ, Deshpande A, Hevener KE, Endres BT, Garey KW, Palmer KL, Hurdle JG. 2020. Constitutive expression of the cryptic vanGCd operon promotes vancomycin resistance in Clostridioides difficile clinical isolates. J Antimicrob Chemother 75:859–867. 10.1093/jac/dkz513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download JCM.00585-21-s0001.pdf, PDF file, 0.1 MB (116.4KB, pdf)