Abstract
Cellular senescence has been found to have beneficial roles in development, tissue regeneration, and wound healing. However, in aging senescence increases, and the ability to properly repair and heal wounds significantly declines across multiple tissues. This age-related accumulation of senescent cells may cause loss of tissue homeostasis leading to dysregulation of normal and timely wound healing processes. The delays in wound healing of aging have widespread clinical and economic impacts, thus novel strategies to improve wound healing in aging are needed and targeting senescence may be a promising area.
Keywords: aging, senescence, wound healing, senolytics, senomorphics
INTRODUCTION
Aging is one of the strongest risk factors for developing wound complications and chronic wounds [1]. Thus, chronic wounds such as venous leg ulcers, diabetic foot ulcers, pressure ulcers, are more frequent in older patients [2], which imposes a large economic burden on healthcare [3]. As a result, the cost of caring for and managing non-healing ulcers or poorly managed wounds is estimated to be $25 billion per year [4], and care for chronic wounds in older adults costs about $10 billion annually [5]. Considering clinical and socioeconomic burdens of wounds in the aged population, research, and development of interventions to prevent chronic wounds and improve wound healing in the geriatric population becomes a significant priority.
AGING AND SENESCENCE
Aging is linked with multiple pathways of cellular dysfunction, including genomic instability, inflammation, stem cell dysfunction, and cellular senescence [6,7]. Senescent cells accumulate during aging [8] through a variety of mechanisms, including replicative and premature senescence. Telomere shortening is associated with replicative senescence because it results in critically short and dysfunctional telomeres, which cells detect as double-strand breaks [9]. When cells are exposed to oxidative stress and/or DNA damage as a result of physical or chemical agents, premature senescence occurs [10]. Senescent cells are characterized by cell cycle arrest, resistance to apoptosis, chromatin remodeling, morphological changes, such as flattened shape, cell enlargement, and a disrupted nuclear membrane [11,12]. These cells have a senescence-associated secretory phenotype (SASP) which influences surrounding cells and tissues [13,14].
Senescent cells play beneficial roles in the normal development, maintenance of tissue homeostasis, and have important physiological functions during aging, such as tumor suppression by halting cell cycle progression [15]. However, senescence has also been implicated as a major cause of age-related disease and aberrant tissue repair [16].
THE ROLE OF SENESCENCE IN NORMAL WOUND HEALING
Wound healing of the skin is characterized by three phases: inflammation, proliferation, and remodeling [17]. At different life stages, the wound healing process is altered across all phases and the speed of the wound healing process slows with aging [18]. In combination with malnutrition and accumulation of comorbidities, older age can permanently hinder the healing process leading to chronic non-healing wounds.
Senescence has been shown to play both physiological and pathological roles in the wound healing process. Senescent cells are present for a short period (between 3 and 12 days) in young mice in the wound site. Although there are important differences in murine and human wound healing, senescent cells have been detected in both wound models. These senescent cells restrict fibrosis [30] and promote divergence of myofibroblasts and therefore facilitate wound contraction and closure via SASP component PDGF-AA [20]. These short-lived senescent cells may initially enhance wound healing, but if allowed to persist, chronic senescence effects shift to a detrimental pro-inflammatory and proteolytic phenotype which limits regeneration [20,31]. Several senescence markers that arise during wound healing are cyclin-dependent kinase inhibitors (CDKI) such as p16INK4a and p21CIP1 which among other CDKI influence wound healing (Table 1).
Table 1.
Protein cyclin-dependent kinase inhibitors and their effect on wound healing.
| CDKIs | Effects on wound healing |
|---|---|
| reduces scarring [19] | |
| p15 | |
|
| |
| emerges early in response to cutaneous wound in young mice [20] promotes cutaneous wound healing by inducing CYR61 [21] activate responses in normal wound healing through Keratinocyte migration and re-epithelialization [22] |
|
| p16 | high expression in chronic wounds [23,24] |
|
| |
| delays the onset of the proliferation phase of wound healing [25] prolongs inflammation and scarring [26] delayed wound healing in aged mice [27] enhances wound healing and skin regeneration in elderly adults [27] promotes wound repair without jeopardizing tumor suppressive function [27] |
|
| p21 | high expression in chronic wounds [28] |
|
| |
| limits cell proliferation [29] | |
| p27 | maintains cell tissue homeostasis during adulthood [29] |
SENESCENCE FEATURES IN ABNORMAL WOUND HEALING
Delayed Wound Healing of Aging
The senescence response to wounding in aged organisms has not been fully characterized. Jiang et al found elevated and sustained p21 expression in aged (24 months old) mice after wounding which when downregulated with p21 siRNA improved aged wound healing rates [27]. Thus, sustained senescence after wounding may delay wound healing, and interventions that limit excessive senescence may be options for improving wound healing in aging.
Chronic Wounds in Aging
An increased presence of senescent cells has been hypothesized to contribute to the pathophysiology of chronic wounds which occur more frequently in older adults. Fibroblasts, harvested from human venous ulcers and pressure ulcers, grown in cell culture have features of senescence [32,33]. Chronic wounds can also harbor pathogenic microorganisms, which can contribute to senescence by increasing ROS production in keratinocytes and promoting inflammation [34]. The chronic ulcer milieu, which is high in pro-inflammatory factors, contributes to persistent wound senescence by exacerbating inflammation and, in addition, senescent chronic wound cells can promote senescence in neighboring cells through SASP [35]. A novel mechanistic link between aging and wound healing in diabetic wounds was recently identified in which intrinsically senescent macrophages were found to promote impaired wound healing in a diabetic (db/db) mice model [36,37].
THERAPEUTIC APPROACHES FOR SENESCENCE MANIPULATION TO IMPROVE WOUND HEALING IN AGING
Evidence for the variations between transient and chronic senescence is emerging, which has important implications for wound healing in aging. The evolving concept of transient vs. chronic senescence may provide significant new insight into the processes that occur during acute and pathological repair. Currently, transient senescence has been found to support tissue repair and wound healing whereas prolonged senescence has the opposite effect. Substances that specifically alter senescent cells offer a promising clinical tool for modulating delayed wound healing in aging and chronic wounds.
Senolytic substances selectively eliminate senescent cells and have the potential for the treatment of delayed wound healing and chronic wounds [38] (Figure 1). Xu et al. found that aged (20 months) mice improved their physical performance after receiving bi-weekly oral treatments of Dasatinib (D) and Quercetin (Q) for four months [39] and a single 3-day oral treatment of D+Q (Phase I clinical trial) was able to reduce senescence in diabetic patients' adipose tissue [40]. These findings imply that senolytic treatments can not only have immediate effects in target peripheral tissues but also overcome established tissue senescence. Senolytics could be considered in the treatment of human chronic wounds with elevated senescence levels. Senescence-oriented therapies may have the potential for the treatment of chronic wounds in the future.
Figure 1.
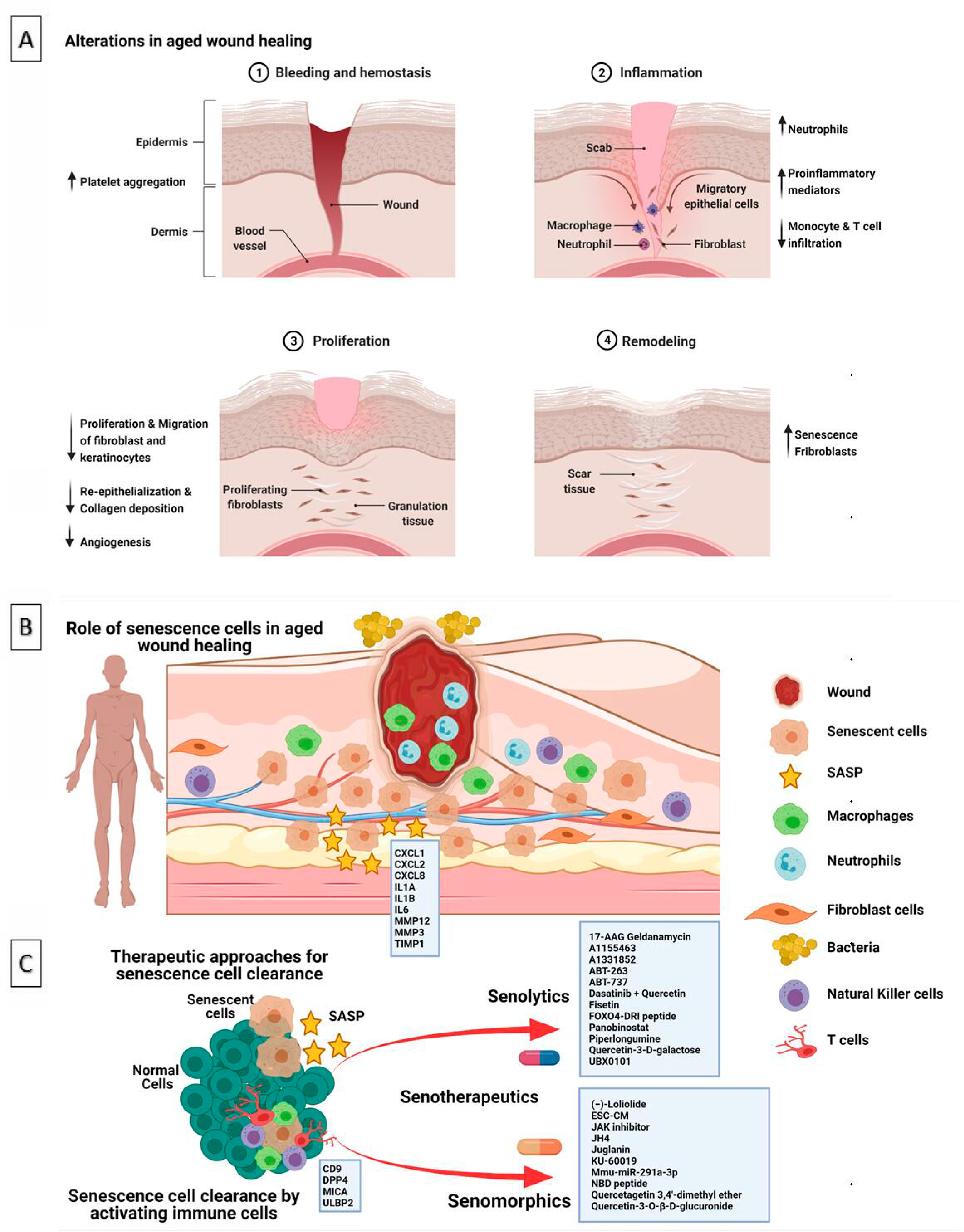
Role of aging, senescence in wound healing. A & B: Alterations and role of senescence in aged wound healing; C: Therapeutic approaches for senescence cell clearance. (Created in BioRender.com).
Senomorphics are an alternative approach to senescence modification that inhibits SASP or SASP components without killing senescent cells [41,42]. Senomorphics are agents that can convert senescent cell phenotypes to those of non-senescent cells by disrupting senescence-related signaling pathways and inflammation [43]. There are several classes of senomorphics that suppress senescence markers or their secretory phenotype without inducing apoptosis [44]. SASP inhibitors, such as metformin and rapamycin, have been found to accelerate diabetic mice wound healing and to increase the lifespan of mice, human skin fibroblasts, and keratinocytes [45–48]. Metformin and rapamycin demonstrated essential angiogenic and rejuvenative activities via the AMPK pathway in both young and elderly skin, and local administration of metformin has promising regenerative potential in healing cutaneous wound defects [49]. The disadvantage of senomorphics is that they must be administered continuously, necessitating improved safety profiles. Chronic treatment to suppress senescence or SASP may not be desirable if there are also beneficial features of senescence [41]. Also, one must consider that the components of SASP differ between senescent cell types and making targeting SASP factors more challenging. Understanding the biological significance of various senescent cell populations and their SASP components may improve senomorphic target specificity [50]. It should be noted that proving a drugʼs senolytic or senomorphic properties is challenging and should be approached with caution.
NATURAL COMPOUNDS THAT CAN MODIFY SENESCENCE AND WOUND HEALING PROCESS
The anti-SASP and/or senolytic activity of natural compounds are now being studied. Table 2 summarizes the effects of senolytic agents on senescent cells and wound healing. Despite the amount of in vivo and in vitro evidence on natural anti-senescence compounds, information on their safety and efficacy remains limited. Hormesis, as a health-promoting activity, can modulate the impact of natural or synthetic aging modulators as there is generally a favorable biological response to repeated low exposures of these compounds. However, the relatively high dosages necessary to induce biological effects, as well as their variable bioavailability, are two outstanding issues. Furthermore, no long-term human studies have shown the pharmacodynamics, pharmacokinetics, and hazardous consequences of an excess of natural antisenescence agents.
Table 2.
Effects of senolytic agents on senescent cells and wound healing.
| Senolytic agents | Role on senescence and wound healing | References |
|---|---|---|
| Quercetin | Promotes senescent cell clearance in healthy tissue. Combination of quercetin and dasatinib had significant effects on health span Promotes wound healing by enhancing fibroblast proliferation and decreasing fibrosis and scarring Promotes diabetic wound healing by altering macrophage polarization Demethylates the p16ink4a gene promoter |
[39,51–53] |
| Epigallocatechin Gallate | Decreased the level of acetylated p53 Promotes wound healing through targeting notch signaling |
[54,55] |
| Resveratrol | Protect human lung fibroblasts from senescence caused by a high glucose environment Enhances healing by improving cell proliferation and migration |
[56,57] |
| ABT263 | Clears senescent cells induced by DNA damage in the lung Removes senescent cells induced by p53 activation in the epidermis via transgenic p14(ARF) Involvement of apoptosis-related signal pathway in the senescent cell elimination process |
[58,59] |
| KU-60019 | Promotes cutaneous wound healing in aged mice by inhibiting ATM kinase | [60] |
| Mmu-miR-291a-3p | Promotes excisional skin wound healing in aged mice by targeting the TGFBR2/p21 pathway | [61] |
CONCLUSIONS
Delays in wound healing of aging have widespread clinical and economic impacts and novel strategies to improve wound healing in aging are needed. The evidence for the role of senescence in wound healing is growing, however, how senescence impacts wound healing of aging remains to be determined. Elevated baseline senescence and defective senescent clearance mechanisms that occur in aging may be initial targets to improve healing. Developing methods to promote transient senescence response during wound healing may prove to be a beneficial therapeutic approach to accelerate wound healing in aging. Senescent cell removal and SASP reduction are potential therapeutic strategies for improving wound healing during aging.
FUNDING
DSR is supported by grants from the National Institute on Aging (R03AG067983) and Boston Claude D. Pepper Older Americans Independence Center (P30AG031679).
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: a mini-review. Gerontology. 2013;59(2):159–64. [DOI] [PubMed] [Google Scholar]
- 2.Margolis DJ, Bilker W, Knauss J, Baumgarten M, Strom BL. The incidence and prevalence of pressure ulcers among elderly patients in general medical practice. Ann Epidemiol. 2002. Jul 1;12(5):321–5. [DOI] [PubMed] [Google Scholar]
- 3.Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Models Mech. 2014. Nov 1;7(11):1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound repair Regen. 2009. Nov;17(6):763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould LJ, Abadir PM, White-Chu EF. Age, frailty, and impaired wound healing. In Principles and practice of geriatric surgery. New York (US): Springer; 2020. p. 465–82. [Google Scholar]
- 6.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013. Jun 6;153(6):1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birch-Machin MA, Bowman A. Oxidative stress and ageing. Br J Dermatol. 2016. Oct;175:26–9. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J. Aging, cellular senescence, and cancer. Ann Rev Physiol. 2013. Feb 10;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campisi J, Di Fagagna FD. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007. Sep;8(9):729–40. [DOI] [PubMed] [Google Scholar]
- 10.Debacq-Chainiaux F, Ben Ameur R, Bauwens E, Dumortier E, Toutfaire M, Toussaint O. Stress-Induced (Premature) Senescence. In: Rattan S, Hayflick L, editors. Cellular Ageing and Replicative Senescence. Healthy Ageing and Longevity. Cham (Switzerland): Springer; 2016. May 9. [Google Scholar]
- 11.Rattanavirotkul N, Kirschner K, Chandra T. Induction and transmission of oncogene-induced senescence. Cell Mol Life Sci. 2020. Sep 16;78:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillispie GJ, Sah E, Krishnamurthy S, Ahmidouch MY, Zhang B, Orr ME. Evidence of the Cellular Senescence Stress Response in Mitotically Active Brain Cells—Implications for Cancer and Neurodegeneration. Life. 2021. Feb;11(2):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014. Jan 15;28(2):99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He S, Sharpless NE. Senescence in health and disease. Cell. 2017. Jun 1;169(6):1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Deursen JM. The role of senescent cells in ageing. Nature. 2014. May;509(7501):439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHugh D, Gil J. Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol. 2018. Jan 2;217(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005. Nov 1;15(11):599–607. [DOI] [PubMed] [Google Scholar]
- 18.Guo SA, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010. Mar;89(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995. Mar 1;108(3):985–1002. [DOI] [PubMed] [Google Scholar]
- 20.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014. Dec 22;31(6):722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010. Jul;12(7):676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natarajan E, Omobono JD II, Jones JC. and Rheinwald JG. 2005, November. Co-expression of p16INK4A and laminin 5 by keratinocytes: a wound-healing response coupling hypermotility with growth arrest that goes awry during epithelial neoplastic progression. J Invest Dermatol Symp Proc. 2005;10(2):72–85. [DOI] [PubMed] [Google Scholar]
- 23.Zieske JD. Expression of cyclin-dependent kinase inhibitors during corneal wound repair. Prog Retin Eye Res. 2000. May 1;19(3):257–70. [DOI] [PubMed] [Google Scholar]
- 24.Bitar MS, Abdel-Halim SM, Al-Mulla F. Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes. Am J Physiol Endocrinol Metab. 2013. Oct 15;305(8):E951–63. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Hu C, Zhang Y, Li L, Wang Z. Expression of cyclin-dependent kinase inhibitors, p21cip1 and p27kip1, during wound healing in rats. Wound Repair Regen. 2001. May;9(3):205–12. [DOI] [PubMed] [Google Scholar]
- 26.Bond JS, Duncan JA, Sattar A, Boanas A, Mason T, OʼKane S, et al. Maturation of the human scar: an observational study. Plast Reconstr Surg. 2008;121:1650–8. [DOI] [PubMed] [Google Scholar]
- 27.Jiang D, de Vries JC, Muschhammer J, Schatz S, Ye H, Hein T, et al. Local and transient inhibition of p21 expression ameliorates age-related delayed wound healing. Wound Repair Regen. 2020. Jan;28(1):49–60. [DOI] [PubMed] [Google Scholar]
- 28.Zieske JD. Expression of cyclin-dependent kinase inhibitors during corneal wound repair. Prog Retin Eye Res. 2000. May 1;19(3):257–70. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996. May 31;85(5):707–20. [DOI] [PubMed] [Google Scholar]
- 30.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010. Jul;12(7):676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schafer MJ, Haak AJ, Tschumperlin DJ, LeBrasseur NK, Targeting Senescent Cells in Fibrosis: Pathology, Paradox, and Practical Considerations. Curr Rheumatol Rep. 2018. 20(1):3. [DOI] [PubMed] [Google Scholar]
- 32.Mendez MV, Stanley A, Park HY, Shon K, Phillips T, Menzoian JO. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg. 1998. Nov 1;28(5):876–83. [DOI] [PubMed] [Google Scholar]
- 33.Vande Berg JS, Rose MA, Haywood-Reid PL, Rudolph R, Payne WG, Robson MC. Cultured pressure ulcer fibroblasts show replicative senescence with elevated production of plasmin, plasminogen activator inhibitor-1, and transforming growth factor-beta1. Wound Repair Regen. 2005;13(1):76–83. [DOI] [PubMed] [Google Scholar]
- 34.Grange PA, Chéreau C, Raingeaud J, Nicco C, Weill B, Dupin N, et al. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009. Jul 24;5(7):e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson HN, Upson SE, Banyard KL, Knight R, Mace KA, Hardman MJ. Reduced iron in diabetic wounds: An oxidative stress-dependent role for STEAP3 in extracellular matrix deposition and remodeling. J Invest Dermatol. 2019. Nov 1;139(11):2368–77. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson HN, Hardman MJ. Wound senescence: a functional link between diabetes and ageing? Exp Dermatol 2020;30:68e73. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson HN, Clowes C, Banyard KL, Matteuci P, Mace KA, Hardman MJ. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J Invest Dermatol. 2019. May 1;139(5):1171–81. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson HN, Hardman MJ. Senescence in wound repair: emerging strategies to target chronic healing wounds. Front Cell Dev Biol. 2020;8:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018. Aug;24(8):1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hickson LJ, Prata LG, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019. Sep 1;47:446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myrianthopoulos V, Evangelou K, Vasileiou PV, Cooks T, Vassilakopoulos TP, Pangalis GA, et al. Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol Ther. 2019. Jan 1;193:31–49. [DOI] [PubMed] [Google Scholar]
- 42.Zhu YI, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015. Aug;14(4):644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim EC, Kim JR. Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB Rep. 2019. Jan;52(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niedernhofer LJ, Robbins PD. Senotherapeutics for healthy ageing. Nat Rev Drug Discov. 2018. May;17(5):377. [DOI] [PubMed] [Google Scholar]
- 45.Han X, Tao Y, Deng Y, Yu J, Sun Y, Jiang G. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol Med Rep. 2017. Dec 1;16(6):8691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Carter CS Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horvath S, Lu AT, Cohen H, Raj K. Rapamycin retards epigenetic ageing of keratinocytes independently of its effects on replicative senescence, proliferation and differentiation. Aging. 2019. May 31;11(10):3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sodagam L, Lewinska A, Wnuk M, Rattan SI. Chronic exposure to rapamycin and episodic serum starvation modulate ageing of human fibroblasts in vitro. Biogerontology. 2017. Oct;18(5):841–54. [DOI] [PubMed] [Google Scholar]
- 49.Anisimov VN. Metformin and rapamycin are master-keys for understanding the relationship between cell senescent, aging and cancer. Aging. 2013. May;5(5):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basisty N, Kale A, Patel S, Campisi J, Schilling B. The power of proteomics to monitor senescence-associated secretory phenotypes and beyond: toward clinical applications. Expert Rev Proteomics. 2020. Apr 2;17(4):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durani LW, Jaafar F, Tan JK, Tajul Arifin K, Mohd Yusof YA, Wan Ngah WZ, et al. Targeting genes in insulin-associated signalling pathway, DNA damage, cell proliferation and cell differentiation pathways by tocotrienol-rich fraction in preventing cellular senescence of human diploid fibroblasts. Clin Ter. 2015. Jan 1;166(6):e365–73. [DOI] [PubMed] [Google Scholar]
- 52.Fu J, Huang J, Lin M, Xie T, You T. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J Surg Res. 2020. Feb 1;246:213–23. [DOI] [PubMed] [Google Scholar]
- 53.Tan S, Wang C, Lu C, Zhao B, Cui Y, Shi X, Ma X. Quercetin is able to demethylate the p16INK4a gene promoter. Chemotherapy. 2009;55(1):6–10. [DOI] [PubMed] [Google Scholar]
- 54.Han DW, Lee MH, Kim B, Lee JJ, Hyon SH, Park JC. Preventive effects of epigallocatechin-3-O-gallate against replicative senescence associated with p53 acetylation in human dermal fibroblasts. Oxid Med Cell Longev. 2012Nov 18;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang YW, Zhu QQ, Yang XY, Xu HH, Sun B, Wang XJ, Sheng J. Wound healing can be improved by (—)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. FASEB J. 2019. Jan;33(1):953–64. [DOI] [PubMed] [Google Scholar]
- 56.Zhang N, Li Z, Xu K, Wang Y, Wang Z. Resveratrol protects against high-fat diet induced renal pathological damage and cell senescence by activating SIRT1. Biol Pharm Bull. 2016. Sep 1;39(9):1448–54. [DOI] [PubMed] [Google Scholar]
- 57.Kaleci B, Koyuturk M. Efficacy of resveratrol in the wound healing process by reducing oxidative stress and promoting fibroblast cell proliferation and migration. Dermatol Ther. 2020. Nov;33(6):e14357. [DOI] [PubMed] [Google Scholar]
- 58.Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016. Apr 6;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang H, Chen C, Chen H, Duan X, Li J, Zhou Y, et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging. 2020. Jul 15;12(13):12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang HT, Park JT, Choi K, Kim Y, Choi HJ, Jung CW, et al. Chemical screening identifies ATM as a target for alleviating senescence. Nat Chem Biol. 2017. Jun;13(6):616. [DOI] [PubMed] [Google Scholar]
- 61.Bae YU, Son Y, Kim CH, Kim KS, Hyun SH, Woo HG, et al. Embryonic Stem Cell–Derived mmu-miR-291a-3p Inhibits Cellular Senescence in Human Dermal Fibroblasts Through the TGF-β Receptor 2 Pathway. J Gerontol A 2019. Aug 16;74(9):1359–67. [DOI] [PubMed] [Google Scholar]


