Abstract
Background
Puerarin, an active compound of radix puerariae, is a major compound used in Chinese herbal medicines and it has been well known for its pharmacological effects, including antioxidant, anti‑inflammatory, neuroprotective and cardioprotective properties. The aim of the present study was to determine the role of puerarin (Pue) in complete Freund’s adjuvant (CFA)-induced trigeminal neuralgia (TN) and the effects of this compound on Sirt1 activity and on the progression of CFA-induced TN.
Methods
Mice were injected with CFA on the unilateral face to induce TN. A cell model of inflammation-associated TN was established by interleukin-1β (IL-1β; 10 ng/mL) and tumor necrosis factor-α (TNF-α; 50 ng/mL) stimulation of neurons. Reverse transcription-quantitative PCR and Western blot analyses were performed to analyze mRNA and protein expression levels in trigeminal ganglion and nerve cells. Terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) staining was used to determine nerve cell apoptosis following IL-1β/TNF-α or Pue treatment.
Results
Pue is a conceivable Sirtuin1 (Sirt1) activator used for the prevention of trigeminal nerve injury that attenuates CFA-induced TN and inflammatory cytokine-evoked overactivation of neuronal inflammation and apoptosis. Treatment of mice with inflammatory cytokines induced upregulation of cleaved caspase-3 protein expression, which was neutralized by Pue supplementation. Both in vivo and in vitro experiments led to the conclusion that Pue modulated Sirt1 activation and repressed transforming growth factor-β1 (TGF-β1) protein expression and drosophila mothers against decapentaplegic homolog3 (Smad3) phosphorylation in order to exert neuroprotection.
Conclusion
The findings suggested that Pue functioned as a potential Sirt1 activator to improve neuroinflammation-induced TN and neuronal apoptosis via the suppression of TGF-β1/Smad3 activity. The pharmacological activity of Pue provides a new perspective for the effective prevention and treatment of TN.
Keywords: apoptosis, inflammation, TGF-β1/Smad3, trigeminal neuralgia, Sirt1
Introduction
Trigeminal neuralgia (TN) is a common cerebral neuropathy, which is characterized by paroxysmal severe pain in the facial distribution of the trigeminal nerve.1 TN is associated with nervous lesions and the induction of inflammatory response.2,3 At present, pharmacological management is the mainstay for treatment of TN. For example, carbamazepine, baclofen, gabapentin, lidocaine and sumatriptan, are effective to alleviate pain in patients with TN.4–6 In addition, pro-inflammatory mediators have been shown to enhance hyperalgesia of orofacial tissues.7,8 Therefore, anti-inflammatory medications, such as TNF-α, IL-1β, and interleukin-6 (IL-6) receptor inhibitors, restrain neuronal hypersensitivity following nerve injury.2,9,10
Transforming growth factor-β (TGF-β) functions to regulate embryonic development, cell proliferation differentiation, migration, wound repair, immune system regulation. Smad3, a transcription factor, is a critical mediator of TGF-β signaling pathway.11 Sirtuin 1 (SIRT1), a NAD+ dependent deacetylase that has been reported to modify a number of transcription factors plays vital roles in TGF-β/Smad pathway inhibition.12 Earlier studies have shown that TGF-β has a protective role for TGF-b signaling against the pathological neural plasticity underlying neuropathic pain in animal models by targeting both neurons and glial cells.13–15
Puerarin (Pue) is an isoflavone derivative and a major bioactive component extracted from the traditional Chinese herbal medicine Pueraria lobata.16 Pue was originally identified as a cardioprotective agent.16,17 Moreover, the anti-inflammatory and analgesic properties of Pue have also been reported in diverse experimental animal models.18,19 For instance, Pue attenuates carrageenan and CFA-induced inflammatory pain.18 Pue relieves radicular pain of lumbar disc herniation by repressing the activation of microglia.19 However, the role of Pue in CFA-induced TN has not been investigated previously.
Previous studies have documented that multiple signaling pathways, including transient receptor potential vanilloid 1, calcitonin gene-related peptide, β1 subunit of Nav1.8, P2X3 receptor and extracellular signal regulated kinase, are implicated in puerarin-mediated remission of neuropathic pain.19–22 Sirt1 is a member of the sirtuin family and functions as a NAD+-dependent protein deacetylase in order to improve age-related diseases.23–25 Accumulating evidence has revealed that reduction of Sirt1 contributes to the development of neuropathic pain, accompanied with the deregulation of its downstream targets.26,27 CFA- induced downregulation of Sirt1 in the spinal cord, which was associated with inflammatory pain.28 The Sirt1 agonists, icariin and resveratrol (Res), block inflammatory response and the development of nociceptive sensitization.29,30 The present study, therefore, was designed to examine whether administration of Pue has anti-inflammatory effects in the mouse adjuvant TN model, induced using complete Freund’s adjuvant (CFA). In this study, percutaneous trigeminal nerve stimulation by CFA was performed to establish a mouse model with TN. Furthermore, the role of Pue and the changes in Sirt1 mRNA and protein expression levels were determined with regard to the progression of CFA-induced TN.
Materials and Methods
Animal Model
A total of 24 male C57BL/6J mice (8-week-old; 22±2 g; n = 6 in each group) were obtained from the Animal Laboratory Center of Zhongnan Hospital of Wuhan University and fed under standard laboratory conditions with a temperature-controlled environment (temperature: 25 ± 2°C; humidity: 60±5%) and an artificial 12-h light/dark cycle. The mice received CFA (Sigma-Aldrich; Merck KGaA) injection in the unilateral face as described previously31 and were fed within individual cages and divided into four groups as follows: Con group mice that received normal saline treatment; CFA group mice that received CFA subcutaneous injection on the unilateral face with three different sites (30 μL/site); CFA+ Pue group mice that received CFA injection combined with Pue (100 mg/kg/day; Sigma-Aldrich; Merck KGaA) supplementation; CFA+Res group mice that received CFA injection combined with Res (100 mg/kg/day; Sigma-Aldrich; Merck KGaA) supplementation. The effective concentration of Pue ranges from 50 mg/kg to 100 mg/kg, according to previous studies.18,32–34 After following Pue or Res treatment for 12 days, a total of 24 mice were immediately sacrificed by an intravenous injection of sodium pentobarbital (100mg/kg; Sigma-Aldrich; Merck KGaA) under resting state. The mice were monitored in special housing conditions before euthanasia when the mice suffered from anxiety, pain and distress. Animal health and behavior were monitored once daily. When the mice suffered from any one or more of the following: scratching, weight loss, eating disorder and rapid breathing, the mice should be immediately sacrificed. The euthanasia was confirmed by cardio-respiratory arrest. Trigeminal ganglions were collected from mice to evaluate gene and protein expression. The animal experiments were approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (Approval number: WHUA20180923a023). All experiments were conducted in accordance with the Institutional Committee for the Care and Use of Animals and guidelines from the Ethical Standards for Investigations of Experimental Pain in Conscious Animals.35
Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNA in trigeminal ganglions and nerve cells was extracted using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized using total RNA (2 μg) with moloney murine leukemia virus reverse transcriptase (Invitrogen;Thermo Fisher Scientific, Inc.). RT-qPCR was determined using an Applied Biosystems 7300 Real-Time PCR System (Thermo Fisher Scientific, Inc.) with the TaqMan Universal PCR Master Mix (Thermo Fisher Scientific, Inc.). mRNA expression levels were calculated using the 2−ΔΔCq method as described previously.36
Head Withdrawal Thresholds
The mouse head withdrawal thresholds were analyzed using an electronic von Frey anesthesiometer (IITC Life Science) every day in the period of the animal experiment as described previously.37
Cell Culture
Mouse nerve cells were dissociated from trigeminal ganglia and cultured in DMEM/F12 (Invitrogen; Thermo Fisher Scientific, Inc.) as described previously.38 In the inflammation-induced cell model of neuropathic pain, nerve cells were exposed to IL-1β (10 ng/mL) and TNF-α (50 ng/mL) or Pue (100 μM). Following 5 days of culture, the nerve cells were collected for molecular biology analysis.
TUNEL Assay
A TUNEL kit (Nanjing Jiancheng Bioengineering Institute; cat. no: G002-1-2) was used to evaluate cell apoptosis. The number of TUNEL-positive staining cells was counted using a fluorescence microscope (Olympus Corporation). TUNEL-positive cells in the different regions of each section were counted using a computer-based program.
Western Blot Analysis
Western blot analysis was performed as described previously.39 The following primary antibodies were used: Rabbit Anti-Sirt1 antibody (ab12193; Abcam), Rabbit Anti-Cleaved Caspase-3 antibody (ab2302; Abcam), Rabbit Anti-TGF-β1 antibody (ab92486; Abcam), Rabbit Anti-Smad3 antibody (ab40854; Abcam) Rabbit anti-phosphorylated-Smad3 antibody (ab52903; Abcam). The following secondary antibody was used: goat anti-rabbit IgG H&L (HRP) (ab205718; Abcam). Rabbit anti-β-actin antibody (ab179467; Abcam) was used as an internal reference.
Statistical Analysis
The data are presented as the mean ± standard deviation. The GraphPad Prism version 7.0 (GraphPad Software, Inc.) was used to perform statistical analysis. Intergroup differences were analyzed by one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s test or two-way, repeated-measures ANOVA. A P value less than 0.05 was considered statistically significant.
Results
Pue Attenuates CFA-Induced Pain of the Trigeminal Nerve
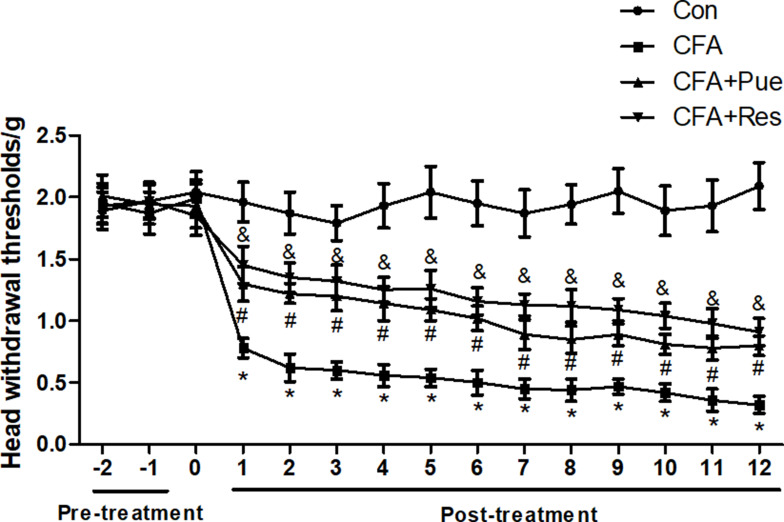
CFA-induced trigeminal neuropathic pain was evaluated by a behavioral test parameter and the head withdrawal threshold, which was inversely associated with pain. The head withdrawal threshold was significantly reduced in mice following CFA injection during the experimental period compared with that of the control group (Figure 1). However, administration of Pue or the Sirt1 agonist Res caused a dramatic increase in the value of the head withdrawal threshold in CFA-treated mice (Figure 1).
Figure 1.
Puerarin attenuates CFA-induced pain of the trigeminal nerve. Head withdrawal thresholds were analyzed using an electronic von Frey anesthesiometer every day in the period of animal experiments. *P<0.05, CFA group compared with the control group; #P<0.05, CFA+Pue group compared with the CFA group; &P<0.05, CFA+Res group compared with the CFA group.
Abbreviation: CFA, complete Freund’s adjuvant.
Pue Controls the Sirt1/TGF-β1/Smad3 Signaling Axis in the CFA-Induced Pain of the Trigeminal Nerve
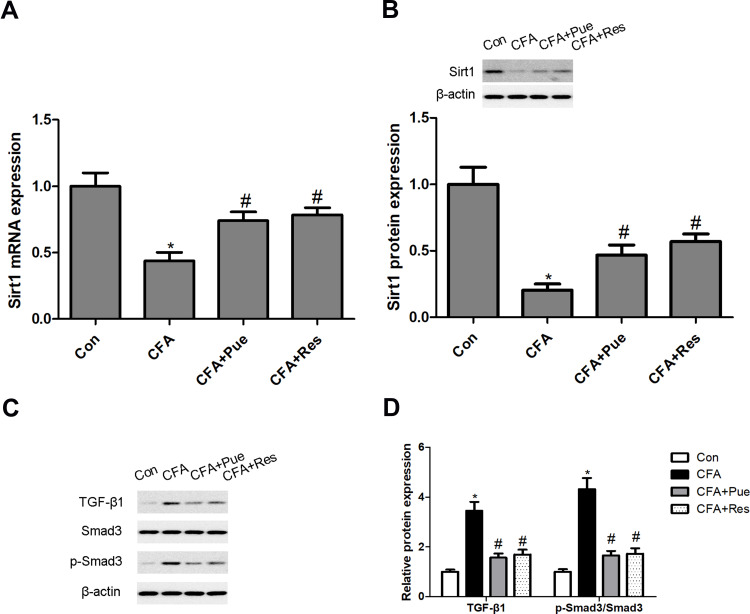
In the present study, a significant decrease in Sirt1 mRNA and protein levels was observed in the trigeminal ganglions from CFA-stimulated mice. However, both Pue and Res could activate Sirt1 expression to antagonize CFA-induced Sirt1 inactivation in the trigeminal ganglions (Figure 2A and B). In addition, the TGF-β1/Smad3 signaling pathway was explored, as a downstream target of Sirt1, in the pathogenesis of CFA-induced TN. Upregulation of TGF-β1 and phosphorylated Smad3 protein expression was induced by CFA in the trigeminal ganglions. However, the administration of Pue or Res could restrain CFA-activated TGF-β1/Smad3 signaling (Figure 2C and D).
Figure 2.
Puerarin regulates the Sirt1/TGF-β1/Smad3 signaling axis in the CFA-induced pain model of the trigeminal nerve. Sirt1 mRNA and protein levels in the trigeminal ganglions were determined using RT-qPCR (A) and Western blotting (B), respectively. TGF-β1, Smad3 and phosphorylated Smad3 levels were measured using Western blot analysis (C and D). *P<0.05, CFA group compared with the control group; #P<0.05, CFA+Pue or CFA+Res groups compared with the CFA group.
Abbreviations: Sirt1, Sirtuin1; CFA, complete Freund’s adjuvant; RT-qPCR, reverse transcription-quantitative PCR.
Pue Inhibits Inflammation in the Animal Model of CFA-Induced Pain of the Trigeminal Nerve
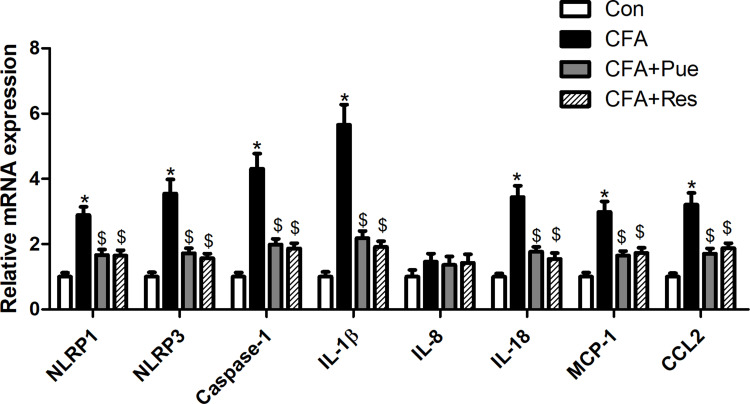
The inflammatory pathways, including the NLRP inflammasome and MCP-1/CCL2, were explored in the trigeminal ganglions from CFA-stimulated mice. The findings suggested that the mRNA levels of the NLRP inflammasome components, namely NLRP1, NLRP3 and caspase-1 and of the inflammatory cytokines, IL-1β and IL-18, were significantly upregulated in the trigeminal ganglions. In addition, MCP-1 and CCL2 mRNA levels were also increased in the trigeminal ganglions in response to CFA stimulation (Figure 3). However, CFA-induced inflammatory response in the trigeminal ganglions was mitigated by Pue or Res therapy.
Figure 3.
Puerarin inhibits inflammation in the animal model of CFA-induced pain of the trigeminal nerve. The mRNA levels of NLRP1, NLRP3, caspase-1, IL-1β, IL-8, IL-18, MCP-1 and CCL2 in the trigeminal ganglions were determined using RT-qPCR. *P<0.05, CFA group compared with the control group; $P<0.05, CFA+Pue or CFA+Res group compared with the CFA group.
Abbreviations: CFA, complete Freund’s adjuvant; RT-qPCR, reverse transcription-quantitative PCR.
Pue Inhibits Inflammatory Cytokine-Induced Nerve Cell Apoptosis and Inflammatory Response in vitro
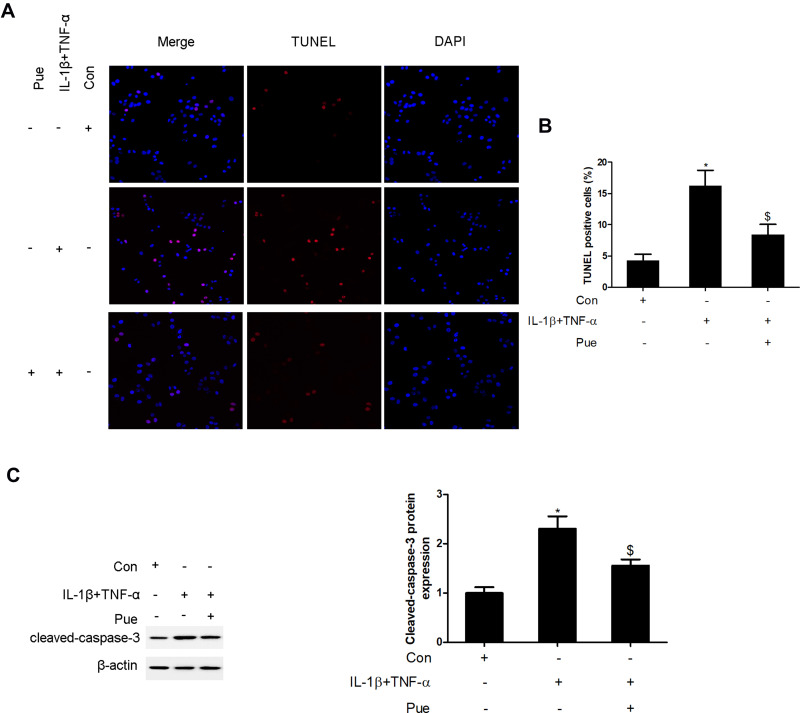
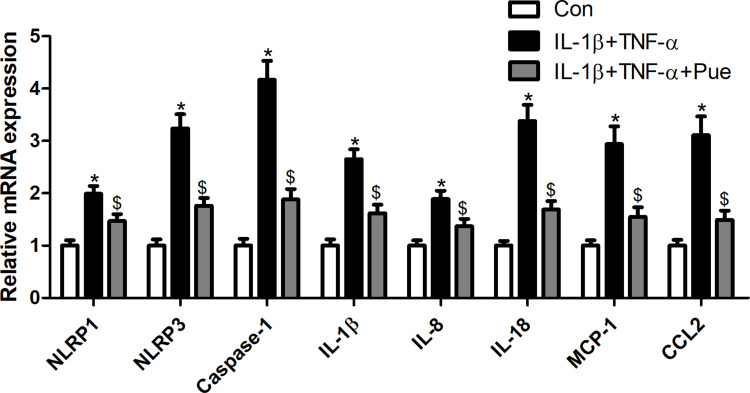
To further investigate the inflammation-associated neuropathic pain, the nerve cells were dissociated from the trigeminal ganglia. To establish an inflammation-associated cell model of nerve injury, the neurons were exposed to IL-1β (10 ng/mL) and TNF-α (50 ng/mL) for 24 h. IL-1β and TNF-α stimulation for 24 h led to a profound increase in the number of TUNEL-positive staining cells (Figure 4A and B). Moreover, the protein expression of cleaved-caspase-3 (Figure 4C) and the mRNA expression levels of specific inflammatory cytokines, including NLRP1, NLRP3, caspase-1, IL-1β, IL-8, IL-18, MCP-1 and CCL2 were increased (Figure 5). However, Pue treatment inhibited IL-1β and TNF-α-mediated cell apoptosis and inflammation.
Figure 4.
Puerarin inhibits inflammatory cytokine-induced nerve cell apoptosis in vitro. Following exposure of the nerve cells to IL-1β (10 ng/mL) and TNF-α (50 ng/mL) or puerarin (100 μM), the induction of cell apoptosis was analyzed using TUNEL staining (A and B); cleaved-caspase-3 levels were measured using Western blot analysis. (C) *P<0.05, IL-1β+TNF-α compared with the control group; $P<0.05, IL-1β+TNF-α compared with the IL-1β+TNF-α+puerarin group.
Figure 5.
Puerarin inhibits inflammation in nerve cells. Following exposure of nerve cells to IL-1β (10 ng/mL) and TNF-α (50 ng/mL) or puerarin (100 μM), NLRP1, NLRP3, caspase-1, IL-1β, IL-8, IL-18, MCP-1 and CCL2 mRNA levels were determined using RT-qPCR. *P<0.05, IL-1β+TNF-α compared with the control group; $P<0.05, IL-1β+TNF-α compared with the IL-1β+TNF-α+puerarin group.
Abbreviation: RT-qPCR, reverse transcription-quantitative PCR.
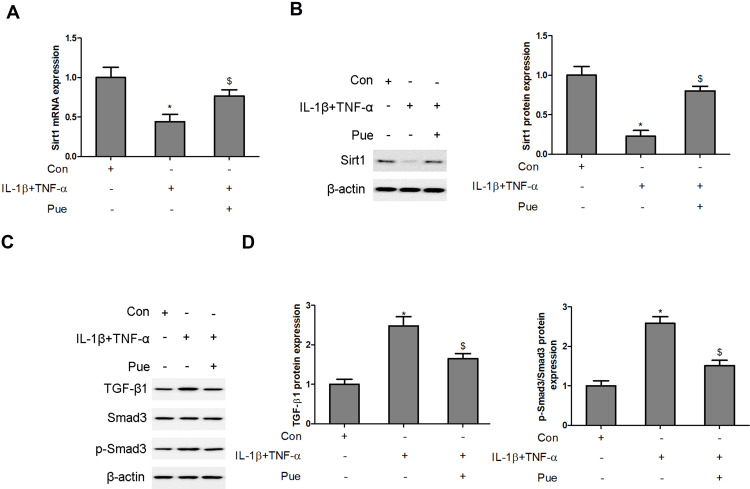
Pue Regulates the Sirt1/TGF-β1/Smad3 Signaling Axis in IL-1β and TNF-α-Stimulated Neurons
The activity of the Sirt1/TGF-β1/Smad3 signaling axis was evaluated in IL-1β- and TNF-α-stimulated neurons following co-treatment with puerarin. A significant decrease in Sirt1 mRNA and protein levels was observed in IL-1β and TNF-α-stimulated neurons (Figure 6A and B). However, Pue treatment activated Sirt1 expression in IL-1β and TNF-α-stimulated neurons. In addition, upregulation of TGF-β1 and phosphorylatedSmad3 protein expression was induced by IL-1β and TNF-αadministration in neurons. However, the administration of Pue hindered L-1β and TNF-α-activated TGF-β1/Smad3 signaling (Figure 6C and D).
Figure 6.
Puerarin regulates the Sirt1/TGF-β1/Smad3 signaling axis in IL-1β- and TNF-α-stimulated neurons. Following nerve cell exposure to IL-1β (10 ng/mL) and TNF-α (50 ng/mL) or puerarin (100 μM), Sirt1 mRNA and protein levels were determined using RT-qPCR (A) and Western blotting (B), respectively. The levels of TGF-β1, Smad3 and phosphorylated Smad3 were measured using Western blot analysis. (C and D) *P<0.05, IL-1β+TNF-α compared with the control group; $P<0.05, IL-1β+TNF-α compared with the IL-1β+TNF-α+puerarin group.
Abbreviations: Sirt1, Sirtuin1; RT-qPCR, reverse transcription-quantitative PCR.
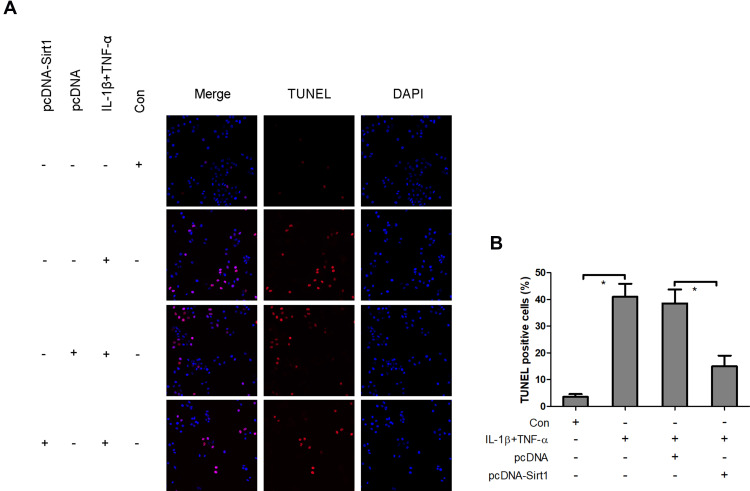
Overexpression of Sirt1 Blocks IL-1β and TNF-α-Induced Neuronal Apoptosis
The aforementioned findings strongly indicated that the reduction of Sirt1 expression levels was associated with IL-1β and TNF-α-mediated neuronal apoptosis. To assess whether Sirt1 was directly involved in neuronal apoptosis, pcDNA-Sirt1 expression plasmids were recombined and transfected into neurons to upregulate Sirt1 expression. TUNEL staining analysis revealed that IL-1β and TNF-α induced a significant increase in TUNEL positive staining neurons, which was impeded by pcDNA-Sirt1 transfection (Figure 7). This suggested that overexpression of Sirt1 could block IL-1β- and TNF-α-induced neuronal apoptosis in vitro.
Figure 7.
Overexpression of Sirt1 blocks IL-1β- and TNF-α-induced neuronal apoptosis. Following transfection of neurons with pcDNA-Sirt1 expression plasmids, the cells were treated with IL-1β and TNF-α and the induction of cell apoptosis was analyzed using TUNEL staining (A and B). *P<0.05.
Abbreviation: Sirt1, Sirtuin1.
Discussion
In the present study, multiple lines of evidence revealed that Pue attenuated CFA-induced TN and inflammatory cytokine-evoked overactivation of inflammation and neuronal apoptosis. Pue is a conceivable Sirt1 activator used for the prevention of trigeminal nerve injury. Both in vivo and in vitro experiments demonstrated that Pue modulated Sirt1 activation and repressed TGF-β1 protein expression and Smad3 phosphorylation to exert neuroprotection.
Currently, the molecular mechanism underlying the pathogenesis of neuropathic pain is complicated. To the best of our knowledge, neurovascular lesions are major causes that can predispose nerve root compression and subsequently trigger TN.40 Recent studies have shown that neuroinflammation contributes to the pathogenesis of TN.41,42 Excessive neuronal activity of trigeminal ganglion can be induced by peripheral inflammation via increasing the release of neurotransmitters and neuromodulators.43 At the cellular level, multiple inflammatory cells, such as white blood cells, neutrophils and monocytes, are apparently increased in patients with TN compared with the corresponding levels noted in normal subjects.42 In CFA-induced rodent models with TN and neuroinflammation, inflammatory cytokines and inflammation-associated signaling pathways are highly inducible in the trigeminal ganglion.44–47 In the present study, overactivation of inflammatory response was accompanied with TN and neuronal apoptosis. Sirt1 activity was decreased considerably in CFA-induced prosopalgic mice and IL-1β+TNF-α-stimulated neurons. Inversely, TGF-β1/Smad3 signaling was activated via upregulation of TGF-β1 protein expression and Smad3 phosphorylation in vivo and in vitro.
Sirt1 is widely expressed in the nervous system, including the dorsal root ganglion, the neuroepithelial layer and the trigeminal ganglion. This protein plays a crucial role in neural development and in the progression of pathological processes.48 Recently, Sirt1 was shown to participate in neuroprotection, wound healing and anti-inflammation.49,50 Sirt1 is profoundly inhibited in trigeminal sensory neurons that exhibit hyperglycemia-induced nerve injury.49 In a rat model of spared nerve injury, the production of inflammatory cytokines was inhibited by Sirt1.50 A growing number of evidence suggests that Sirt1 is a key gene and therapeutic target for improving neuropathic pain in various animal models.26,30 For example, Sirt1 activators mitigate hyperalgesia and spinal neuronal activation in diabetic neuropathic pain rats.26 In normal rats, Sirt1 knockdown distinctly increases pain behavior and spinal neuronal activation.26 The Sirt1 agonist, Res, attenuates paclitaxel-induced neuropathic pain via the activation of Sirt1.30 These findings suggest that activation of Sirt1 has a beneficial effect to prevent neuropathic pain. In the present study, Sirt1 levels were markedly decreased and associated with overactivation of the inflammatory response in the trigeminal ganglions of CFA-treated mice. Sirt1 levels were also declined in IL-1β and TNF-α-exposed neurons and were associated with IL-1β and TNF-α-induced neuronal apoptosis. Pharmacological evidence demonstrated that Pue could activate Sirt1 expression to improve TN, inflammation and neuronal apoptosis.
The TGF-β1/Smad3 signaling axis is considered an important signaling cascade to mediate the initiation and development of diverse diseases, including hepatic cirrhosis, cerebral ischemia-reperfusion injury and inflammation.51,52 TGF-β1 is a pleiotropic cytokine that participates in pathophysiological processes via the phosphorylation of Smad3.51 Previous evidence has shown that the TGF-β1/Smad3 signaling axis is controlled by Sirt1, whereas activation of Sirt1 inhibits TGF-β1 activity to regulate Smad3 phosphorylation.53,54 Consistent with these findings, previous studies indicated that downregulation of Sirt1 was accompanied with activation of TGF-β1 and an increase inSmad3 phosphorylation levels in the trigeminal ganglions of CFA-treated mice and in IL-1β- and TNF-α-exposed neurons, suggesting that abnormal Sirt1/TGF-β1/Smad3 signaling may be involved in neuroinflammation-induced neuropathic pain and neuronal apoptosis.53,55 However, Pue treatment enhanced Sirt1 activity, and suppressed TGF-β1/Smad3 in the current in vivo and in vitro experimental models.
In our study, we choose an assuring concentration of Pue (100 mg/kg) to protect against CFA-induced TN and inflammatory response in mice, according to previous studies.18,32–34 For example, Zhang et al reveal that Pue (100 mg/kg) attenuates neurological deficits in subarachnoid hemorrhage mice.32 Pue (80 mg/kg) inhibits inflammatory response in the cerebral cortex of Alzheimer’s disease mice.33 Puerarin (50 mg/kg) inhibits inflammation and oxidative stress in a mice model of dextran sulfate sodium-induced colitis.34 Puerarin (50 mg/kg) attenuates CFA-induced inflammatory pain.13,18 Our results indicate that Pue (100 mg/kg) inhibits CFA-induced TN and inflammatory response, but not with time-dependent within 12 days. These findings suggest that the effective concentration of Pue ranges from 50 mg/kg to 100 mg/kg to prevent pathological changes of tissues.
Conclusion
In conclusion, the current study suggested that Pue functioned as a potential Sirt1 activator to improve neuroinflammation-induced TN and neuronal apoptosis via the suppression of the TGF-β1/Smad3 activity. The pharmacological activity of Pue provides a new perspective for the effective treatment of TN.
Acknowledgment
This study was supported by Health Commission of Hubei Province Scientific Research Project (Grant No. WJ2021M168).
Abbreviations
Pue, puerarin; CFA, complete Freund’s adjuvant; TN, trigeminal neuralgia; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; TUNEL, Terminal-deoxynucleotidyl transferase mediated nick end labeling; Sirt1, Sirtuin1; TGF-β1, transforming growth factor-β1; Smad3, drosophila mothers against decapentaplegic homolog 3; IL-6, interleukin-6; Res, resveratrol.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Jones MR, Urits I, Ehrhardt KP, et al. A comprehensive review of trigeminal neuralgia. Curr Pain Headache Rep. 2019;23:74. doi: 10.1007/s11916-019-0810-0 [DOI] [PubMed] [Google Scholar]
- 2.Bista P, Imlach WL. Pathological mechanisms and therapeutic targets for trigeminal neuropathic pain. Medicines. 2019;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Launay PS, Reboussin E, Liang H, et al. Ocular inflammation induces trigeminal pain, peripheral and central neuroinflammatory mechanisms. Neurobiol Dis. 2016;88:16–28. doi: 10.1016/j.nbd.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 4.Kanai A, Suzuki A, Osawa S, Hoka S. Sumatriptan alleviates pain in patients with trigeminal neuralgia. Clin J Pain. 2006;22(8):677–680. doi: 10.1097/01.ajp.0000210917.18536.0d [DOI] [PubMed] [Google Scholar]
- 5.Bennetto L, Patel NK, Fuller G. Trigeminal neuralgia and its management. BMJ. 2007;334:201–205. doi: 10.1136/bmj.39085.614792.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71:1183–1190. doi: 10.1212/01.wnl.0000326598.83183.04 [DOI] [PubMed] [Google Scholar]
- 7.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis A, Bennett DLH. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:26–37. doi: 10.1093/bja/aet128 [DOI] [PubMed] [Google Scholar]
- 9.Kiguchi N, Kobayashi D, Saika F, Matsuzaki S, Kishioka S. Inhibition of peripheral macrophages by nicotinic acetylcholine receptor agonists suppresses spinal microglial activation and neuropathic pain in mice with peripheral nerve injury. J Neuroinflammation. 2018;15(1):96. doi: 10.1186/s12974-018-1133-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Shi XQ, Fan A, West B, Zhang J. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Mol Pain. 2018;14:1744806918764979. doi: 10.1177/1744806918764979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Zheng L, Yuan Q, et al. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018;6:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerr P, Palumbo-Zerr K, Huang J, et al. Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis. 2016;75:226–233. doi: 10.1136/annrheumdis-2014-205740 [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest. 2015;125:3226–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Echeverry S, Shi XQ, Haw A, Liu H, Zhang Z, Zhang J. Transforming growth factor-beta1 impairs neuropathic pain through pleiotropic effects. Mol Pain. 2009;5:16. doi: 10.1186/1744-8069-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lantero A, Tramullas M, Pílar-Cuellar F, et al. TGF-β and opioid receptor signaling crosstalk results in improvement of endogenous and exogenous opioid analgesia under pathological pain conditions. J Neurosci. 2014;34:5385–5395. doi: 10.1523/JNEUROSCI.4405-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28:961–975. doi: 10.1002/ptr.5083 [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Zhao M, Liang S, et al. The effects of puerarin on rat ventricular myocytes and the potential mechanism. Sci Rep. 2016;6:35475. doi: 10.1038/srep35475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ullah MZ, Khan AU, Afridi R, et al. Attenuation of inflammatory pain by puerarin in animal model of inflammation through inhibition of pro-inflammatory mediators. Int Immunopharmacol. 2018;61:306–316. doi: 10.1016/j.intimp.2018.05.034 [DOI] [PubMed] [Google Scholar]
- 19.Zhong Y, Huang YL, Hu YM, Zhu LR, Zhao YS. Puerarin alleviate radicular pain from lumbar disc herniation by inhibiting ERK-dependent spinal microglia activation. Neuropeptides. 2018;72:30–37. doi: 10.1016/j.npep.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Chen J, Wang R. Puerarin suppresses TRPV1, calcitonin gene-related peptide and substance P to prevent paclitaxel-induced peripheral neuropathic pain in rats. Neuroreport. 2019;30:288–294. doi: 10.1097/WNR.0000000000001199 [DOI] [PubMed] [Google Scholar]
- 21.Zhang XL, Cao XY, Lai RC, Xie MX, Zeng WA. Puerarin relieves paclitaxel-induced neuropathic pain: the role of Na(v)1.8 β1 subunit of sensory neurons. Front Pharmacol. 2018;9:1510. doi: 10.3389/fphar.2018.01510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu C, Xu W, Xu H, et al. Role of puerarin in the signalling of neuropathic pain mediated by P2X3 receptor of dorsal root ganglion neurons. Brain Res Bull. 2012;87:37–43. doi: 10.1016/j.brainresbull.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 23.Golestaneh N, Chu Y, Cheng SK, Cao H, Poliakov E, Berinstein DM. Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J Transl Med. 2016;14:344. doi: 10.1186/s12967-016-1101-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Liu X, Zhu W, et al. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front Aging Neurosci. 2014;6:103. doi: 10.3389/fnagi.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou CH, Zhang MX, Zhou SS, et al. SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats. Pain. 2017;158:130–139. doi: 10.1097/j.pain.0000000000000739 [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Fan J, Luo ZF, Yang Y, Xin WJ, Liu CC. Reduction of SIRT1 epigenetically upregulates NALP1 expression and contributes to neuropathic pain induced by chemotherapeutic drug bortezomib. J Neuroinflammation. 2018;15:292. doi: 10.1186/s12974-018-1327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Gu Y, Dai Q, He Y, Wang J. Spinal miR-34a regulates inflammatory pain by targeting SIRT1 in complete Freund’s adjuvant mice. Biochem Biophys Res Commun. 2019;516:1196–1203. doi: 10.1016/j.bbrc.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Gui Y, Zhang J, Chen L, et al. Icariin, a flavonoid with anti-cancer effects, alleviated paclitaxel-induced neuropathic pain in a SIRT1-dependent manner. Mol Pain. 2018;14:1744806918768970. doi: 10.1177/1744806918768970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Yang S, Wang L, et al. Resveratrol inhibits paclitaxel-induced neuropathic pain by the activation of PI3K/Akt and SIRT1/PGC1α pathway. J Pain Res. 2019;12:879–890. doi: 10.2147/JPR.S185873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Reyes M, Akerman S, Nguyen E, et al. Spontaneous behavioral responses in the orofacial region: a model of trigeminal pain in mouse. Headache. 2013;53:137–151. doi: 10.1111/j.1526-4610.2012.02226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Yang X, Ge X, Zhang F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed Pharmacother. 2019;109:726–733. doi: 10.1016/j.biopha.2018.10.161 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Kong WN, Chai XQ. Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice. Neural Regen Res. 2018;13:731–736. doi: 10.4103/1673-5374.230302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon YD, Lee JH, Lee YM, Kim DK. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed Pharmacother. 2020;124:109847. doi: 10.1016/j.biopha.2020.109847 [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4 [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 37.Bi RY, Meng Z, Zhang P, Wang XD, Ding Y, Gan YH. Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TMJ. PLoS One. 2017;12:e0178589. doi: 10.1371/journal.pone.0178589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tillu DV, Melemedjian OK, Asiedu MN, et al. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain. 2012;8:5. doi: 10.1186/1744-8069-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–2422. doi: 10.1080/15384101.2018.1526603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015;11:289–299. doi: 10.2147/TCRM.S37592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ericson H, Abu Hamdeh S, Freyhult E, et al. Cerebrospinal fluid biomarkers of inflammation in trigeminal neuralgia patients operated with microvascular decompression. Pain. 2019;160:2603–2611. doi: 10.1097/j.pain.0000000000001649 [DOI] [PubMed] [Google Scholar]
- 42.Yao Y, Chang B, Li S. Relationship of Inflammation With Trigeminal Neuralgia. J Craniofac Surg. 2020;31:e110–e113. doi: 10.1097/SCS.0000000000005879 [DOI] [PubMed] [Google Scholar]
- 43.Yin Y, Guo R, Shao Y, et al. Pretreatment with resveratrol ameliorate trigeminal neuralgia by suppressing matrix metalloproteinase-9/2 in trigeminal ganglion. Int Immunopharmacol. 2019;72:339–347. doi: 10.1016/j.intimp.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 44.Chen ML, Lin K, Lin SK. NLRP3 inflammasome signaling as an early molecular response is negatively controlled by miR-186 in CFA-induced prosopalgia mice. Brazilian J Med Biol Res = Rev Bras Pesqui Medicas e Biol. 2018;51:e7602. doi: 10.1590/1414-431x20187602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukács M, Warfvinge K, Kruse LS, et al. KYNA analogue SZR72 modifies CFA-induced dural inflammation- regarding expression of pERK1/2 and IL-1β in the rat trigeminal ganglion. J Headache Pain. 2016;17:64. doi: 10.1186/s10194-016-0654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aczél T, Kun J, É S, et al. Transcriptional alterations in the trigeminal ganglia, nucleus and peripheral blood mononuclear cells in a rat orofacial pain model. Front Mol Neurosci. 2018;11:219. doi: 10.3389/fnmol.2018.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Csáti A, Edvinsson L, Vécsei L, et al. Kynurenic acid modulates experimentally induced inflammation in the trigeminal ganglion. J Headache Pain. 2015;16:99. doi: 10.1186/s10194-015-0581-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa T, Wakai C, Saito T, et al. Distribution of the longevity gene product, SIRT1, in developing mouse organs. Congenit Anom. 2011;51:70–79. doi: 10.1111/j.1741-4520.2010.00304.x [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Zhao X, Wu X, Dai Y, Chen P, Xie L. microRNA-182 mediates Sirt1-induced diabetic corneal nerve regeneration. Diabetes. 2016;65(7):2020–2031. doi: 10.2337/db15-1283 [DOI] [PubMed] [Google Scholar]
- 50.Zeng Y, Shi Y, Zhan H, et al. Reduction of silent information regulator 1 activates interleukin-33/ST2 signaling and contributes to neuropathic pain induced by spared nerve injury in rats. Front Mol Neurosci. 2020;13:17. doi: 10.3389/fnmol.2020.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang G, Ge M, Han Z, et al. Wnt/β-catenin signaling pathway contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury and is possibly related to the transforming growth factorβ1/Smad3 signaling pathway. Biomed Pharmacother. 2019;110:420–430. doi: 10.1016/j.biopha.2018.11.143 [DOI] [PubMed] [Google Scholar]
- 53.Ma JQ, Sun YZ, Ming QL, Tian ZK, Yang HX, Liu CM. Ampelopsin attenuates carbon tetrachloride-induced mouse liver fibrosis and hepatic stellate cell activation associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int Immunopharmacol. 2019;77:105984. doi: 10.1016/j.intimp.2019.105984 [DOI] [PubMed] [Google Scholar]
- 54.Guan R, Wang J, Cai Z, et al. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol. 2020;28:101356. doi: 10.1016/j.redox.2019.101356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J, Jackson CW, Khoury N, Escobar I, Perez-Pinzon MA. Brain SIRT1 mediates metabolic homeostasis and neuroprotection. Front Endocrinol. 2018;9:702. doi: 10.3389/fendo.2018.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]