Abstract
LSH, a homologue of the ISWI/SNF2 family of chromatin remodelers, is required in vivo for deposition of the histone variants macroH2A1 and macroH2A2 at specific genomic locations. However, it remains unknown whether LSH is directly involved in this process or promotes other factors. Here we show that recombinant LSH interacts in vitro with macroH2A1–H2B and macroH2A2–H2B dimers, but not with H2A.Z–H2B dimers. Moreover, LSH catalyzes the transfer of macroH2A into mono-nucleosomes reconstituted with canonical core histones in an ATP dependent manner. LSH requires the ATP binding site and the replacement process is unidirectional leading to heterotypic and homotypic nucleosomes. Both variants macroH2A1 and macroH2A2 are equally well incorporated into the nucleosome. The histone exchange reaction is specific for histone variant macroH2A, since LSH is not capable to incorporate H2A.Z. These findings define a previously unknown role for LSH in chromatin remodeling and identify a novel molecular mechanism for deposition of the histone variant macroH2A.
INTRODUCTION
Eukaryotic genomes are assembled in arrays of nucleosomes. Each nucleosome consists of about 147 bp of DNA that is wrapped around an octamer of core histones H2A, H2B, H3 and H4 (1–3). The organization, modification and composition of nucleosomes contributes fundamentally to the regulation of DNA accessibility in chromatin which has an impact on DNA based processes such as transcription, recombination and DNA repair (2,4).
The canonical core histones can be replaced with histone variants that are evolutionary conserved (3,5,6). The histone variant H2A.Z, for example, is enriched at regulatory elements and at promoter regions and regulates transcription, whereas macroH2A is frequently associated with repressed chromatin (5). Mammalian macroH2A is expressed from two genes, H2AFY2 and H2AFY, encoding macroH2A2 and macroH2A1 respectively, the latter forming two isoforms due to a variation in splicing (7,8). Human macroH2A1 or macroH2A2 share about 64% identity with canonical H2A in the N-terminus, termed the histone like domain (1–122 aa), but comprise additionally a nonhistone macro domain (161–372 aa) (7,9). MacroH2A renders nucleosomes more resistant to exonuclease digestion and makes them more stable in vitro (9–11). This promotes interaction between nucleosomes, furthers chromatin condensation and contributes to the more compacted nature of heterochromatin (8,12–14).
The chromatin incorporation of histone variants occurs at specific genomic regions and is generally not coupled to replication (3,5,6,8). The deposition and the removal of histone variants are regulated by specific chaperones or chromatin remodeling complexes (2,3,15). All Chromatin remodeling complexes contain a SWI2/SNF2 homologue of the SF2 helicase family (4,16,17). SNF2 family members share likeness in their ATPase domain which is required for remodeling function, and members are usually grouped into four or more subfamilies based on additional domains that confer specific functional properties (17–19). For example, the CHD subfamily comprises chromodomains, the SWI/SNF subfamily comprises a helicase SANT-associated (HSA) domain and a bromodomain, and the ISWI subfamily comprises a HAND-SANT-SLIDE (HSS) domain. These subfamilies are known for their ability to reposition nucleosomes. On the other hand, the INO80/SWR1 subfamily which contains an HSA domain is capable to conduct exchange of histone variants. For example, the 14-subunit SWR1 complex in yeast, comprises Swr1 as SNF2 ATPase of the INO80/SWR1 subfamily and has been shown to deposit the histone variant H2A.Z into chromatin in vivo and in vitro (15,20,21). The yeast INO80 complex, containing the SNF2 ATPase Ino80 of the INO80/SWR1 subfamily, promotes the reverse process and removes H2A.Z from chromatin (5). While several complexes have been identified that exchange H2A.Z with H2A, the mechanisms of macroH2A deposition is less explored and no dedicated chromatin remodeler or chaperones have been identified (5,8).
Mammalian LSH belongs to the class of SNF2 like chromatin remodeler, but does not contain any of the HSA, HSS, chromo- or bromo domains characteristic of other subfamily members (4,16,17,22). Mutation of LSH causes ICF4 syndrome a multiorgan diseases with increased childhood lethality (23). Mice with a deletion of LSH die perinatally and display multiple organ defects (24–27). LSH-deficient cells show increased susceptibility to exposure to ionizing radiation and the repair of double stranded breaks (28,29). Proliferating LSH deficient cells show increased signs of spontaneous DNA damage and a reduced ability to join double strand DNA breaks (26,30). In addition, LSH deficient cells are susceptible to replication stress and LSH plays a role in the protection of stalled replication forks (63). The yeast LSH homologue Irc5 also plays a part in maintenance of genomic stability and prevents DNA damage at stalled replication forks by promoting loading of cohesin (31). LSH is associated with heterochromatin and repression of retroviral elements and other repeat elements (32–34). LSH deletion alters CpG DNA methylation level and H3K27me3 distribution, two epigenetic marks linked to heterochromatin (34,35). LSH modulates chromatin accessibility in vivo (36,37). Recombinant LSH alone has no ability to reposition nucleosomes (28,38), in contrast to the Arabidopsis homologue DDM1 (39). However, LSH can induce nucleosome sliding in vitro in a complex with CDCA7 (38).
Despite the discovery of the histone variant macroH2A decades ago (7), the mechanism of its incorporation into chromatin remained unknown. A recent study demonstrated that the chaperone FACT is in vivo responsible for removal of macroH2A at transcribed regions in a process termed ‘pruning’ (8). Using an in vivo experimental system, we have recently demonstrated that LSH plays a role in the deposition of the histone variant macroH2A in the genome. Using chemical-induced proximity to rapidly recruit LSH to an engineered locus, we found that recruitment of LSH to a specific genomic location is followed by macroH2A1 and macroH2A2 deposition (40). The conserved ATP binding motif of LSH is required implying that ATP binding or ATP hydrolysis are required for LSH’s molecular function. Furthermore, deletion of LSH led to genome-wide reduction of macroH2A occupancy (40). These results indicate that LSH mediates loading of macroH2A into chromatin in vivo. However, it remained unknown whether this is a direct or indirect function of LSH and whether co-factors are required.
Here, we identify LSH as a histone variant exchange factor using an in vitro experimental approach. Our results reveal a novel primary molecular function of LSH and imply a linkage between the histone variant macroH2A and a human disease.
MATERIALS AND METHODS
Cell culture and establishment of stable cell lines
U2OS cells (ATCC) were transiently transfected with 3 × FLAG-LSH (WT)-IRES2-GFP, 3 × FLAG-LSH (K254A)-IRES2-GFP or GFP vectors by using TurboFect transfection reagent (Invitrogen). Subsequently, two continuous rounds of fluorescence-activated cell sorting (FACS) were conducted to collect GFP positive U2OS cells at 3 and 15 days after transfection to generate stable cell line. All U2OS cell lines were grown in high-glucose DMEM (Invitrogen) supplemented with 10% Fetal Bovine Serum (FBS, Omega Scientific) and 50 U/ml Penicillin-Streptomycin (Gibco).
Protein purifications
The synthetic full-length of human LSH (WT) or LSH (K254A) cDNA was subcloned by Gateway LR recombination (Thermo Fisher) into the vector pDest-636 which includes N-terminal His6 and MBP tags. The reaction was transformed into DH10B cells and colonies were selected on LB plates with Ampicillin. For construction of recombinant baculovirus, bacmid DNA was generated and Tni-FNL cells were transfected as previously described (41). After 72 h post-infection, Tni-FNL cell were collected and lysed in lysis buffer (20 mM HEPES, 300 mM NaCl, 1 mM TCEP and 1:100 v/v of protease inhibitor), and passed twice on an M-110EH-30 microfluidizer (Microfluidics) at 7000 psi to complete lysis. The lysate was clarified by centrifugation at 100 000 × g for 30 min at 4°C. The clarified samples were filtered (0.45 μm) and applied to a 20 ml IMAC HP column (GE Scientific) that was pre-equilibrated with LB + 50 mM imidazole (10% Buffer B) on a Bio-Rad NGC. Proteins were eluted with a 10 CV gradient of 10–100% Buffer B (LB + 500 mM imidazole). To remove the His6-MBP solubility tag, 5% (v/v) TEV protease was added to the pool and the mixture was dialyzed using a 10K MWCO dialysis membrane (Life Technologies). The dialyzed samples were loaded onto IMAC HP columns pre-equilibrated with LB. The bound protein was eluted by a 10 CV gradient of 0–10% Buffer B followed by a 10 CV gradient of 10–100% Buffer B. The final pool was dialyzed into LB and concentrated using a 10K MWCO spin concentrator (Amicon) at 4000 rpm, 4°C. The final protein concentration was determined, and proteins were stored at −80°C. Likewise, macroH2A1 and macroH2A2 with 3×Flag or 6×His tag together with H2B were individually expressed in the Escherichia coli expression system and purified as described above. For macroH2A1 and macroH2A2 histone variant, we used the histone like domain of macroH2A (1–122aa) as previously reported for in vitro studies (9,11). This domain shares about 64% homology with canonical H2A in the N-terminus (1–122 aa) (7). The expression of full length macroH2A1 and macroH2A2 in this system was not successful.
Reconstitution of mono-nucleosomes
Recombinant nucleosomes were reconstituted by salt gradient dialysis as described with modifications (42,43). Briefly, 5 μl histone dimers (20 μM) and 5 μl histone tetramers (10 μM) were mixed with 50 pmol biotin labeled or unlabeled sea urchin 5S rDNA (208 bp) in a total of 100 μl nucleosome assembly buffer (20 mM Tris–HCl pH 8.0, 2.0 M NaCl, 1 mM EDTA and 1 mM DTT). The mixture was allowed to equilibrate for 1 h at room temperature, and subsequently transferred into a dialysis unit (10 000 MWCO) and placed in the dialysis buffer (20 mM Tris–HCl pH 8.0, 1 mM EDTA and 1 mM DTT) containing 1.5, 1.0 and 0.6 M NaCl. Each dialysis step was carried out at 4°C for 2–3 h, with the exception of 0.6 M NaCl dialysis buffer which was performed overnight at 4°C. Reconstituted nucleosomes were further dialyzed against 20 mM Tris–HCl pH 8.0, 0.25 M NaCl and 1 mM DTT at 4°C for 2–3 h. Since the position of reconstituted nucleosomes using the 5S rDNA template can be influenced by temperature (44,45), we incubated the nucleosome at 37°C for an hour (42,46). MacroH2A-H2B dimers were reconstituted as previously described (42,43). H2A–H2B dimer (NEB), H2A.Z_Flag–H2B dimer (Diagenode) and H3–H4 tetramer (NEB) were commercially available.
Co-immunoprecipitation (Co-IP) assay and immunoblotting
About 2 × 107 U2OS cells expressing 3 × FLAG-LSH or 3 × FLAG-LSH (K254A) were used for Co-IP as described with modifications (40). Briefly, cell pellets were resuspended in IP lysis buffer (25 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 5% glycerol, 0.5 mM DTT, 0.1 mM PMSF and 1 × protease inhibitor cocktail) and incubated for 10 min on ice with periodic mixing. The lysates were cleared by centrifugation at 13 000 × g for 10 min at 4°C. Protein concentration of the supernatant fraction was measured by the BCA protein assay (Thermo Scientific). For each IP, 1 mg protein was treated with Pierce universal nuclease (Thermo Scientific), an endonuclease that degrades single-stranded, double-stranded, linear and circular DNA and RNA and is similar in performance to Benzonase™ Nuclease (EMD Merck), and precleaned by addition of Protein G Magnetic Beads (Bio-Rad). The precleaned sample was mixed with 4 μg Flag antibody (Invitrogen, MA1-91878) and incubated at 4°C overnight, followed by antibody-protein complex capture with Protein G magnetic beads. After extensive washing with IP lysis buffer and 1× PBS buffer, immunoprecipitated proteins were boiled in 1 × Laemmli sample buffer (Bio-Rad). U2OS cells expressing GFP served as a control group. IP samples were subsequently loaded onto acrylamide/bis gels and transferred to PVDF membranes after electrophoresis. Following blocking with 5% nonfat milk for 1 h, membranes were incubated at 4°C overnight with primary antibodies: anti-Flag (Cell Signaling, 14793S, 1:1000); anti-mH2A1 (Abcam, ab183041, 1:1000); anti-mH2A2 (Abcam, ab102126, 1:1000); anti-H2A.Z (Abcam, ab4174, 1:1000); anti-H2A (Abcam, ab18255, 1:1000). After incubation with HRP-conjugated secondary antibodies for 1 h at room temperature, ECL western blotting analysis system was used for signal detection.
In vitro Co-IP assay
For in vitro Co-IP experiments, 100 nM purified LSH (WT) or LSH (K254A) was mixed with 50 nM mH2A1_Flag-H2B dimer, mH2A2_Flag–H2B dimer, H2A.Z_Flag–H2B dimer or H2A–H2B dimer in 50 μl binding buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 0.5 mM EDTA, 0.1 μg/ml BSA, 5% glycerol, 0.5 mM DTT, 0.1 mM PMSF and 1× protease inhibitor cocktail), and incubated at 37°C for 1 h. Afterwards, samples were mixed with 4 μg polyclonal rabbit-antiserum raised against recombinant LSH (27) or 4 μg Flag antibody (Cell Signaling, 14793S) or 4 μg IgG (Millipore, 12–371) in 1 ml dilution buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 0.5 mM EDTA, 0.1 μg/ml BSA, 0.5 mM DTT, 0.1 mM PMSF and 1× protease inhibitor cocktail), and incubated at 4°C overnight. Protein G magnetic beads were added into the reaction mixtures and incubated at 4°C for 2 h. After incubation, beads were washed three times with washing buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.2% Triton X-100, 0.5 mM EDTA, 0.5 mM DTT, 0.1 mM PMSF and 1× protease inhibitor cocktail), and boiled in 1× Laemmli sample buffer. IP samples were subsequently loaded onto acrylamide/bis gels and subjected to western blotting as described above with primary antibodies: anti-LSH (27) (1:5000); anti-Flag (Invitrogen, MA1-91878, 1:1000); anti-H2A (Abcam, ab242365, 1:1000).
Electrophoretic mobility shift assay (EMSA)
An increasing concentration of purified LSH (WT) or LSH (K254A) recombinant protein (0–80 nM) were mixed with 15 nM canonical mono-nucleosomes or 208 bp 5S rDNA and incubated for 30 min at room temperature to allow complex formation. Glycerol was added for loading. Free and LSH-bound nucleosomes or DNA were loaded onto 1.3% agarose gels. All gels were run in 0.5× TBE buffer at room temperature and stained by SYBR Green (Invitrogen).
ATPase activity assay
For ATPase assay, purified 50 nM LSH WT or LSH (K254A) was mixed with 15 nM DNA, histone dimer, nucleosome or combinations in 200 μl ATPase buffer (25 mM Tris–HCl pH 7.5, 50 mM NaCl, 1.5 mM MgCl2, 1 mM ATP, 2% glycerol, 100 μg/ml BSA, 0.5 mM β-mercaptoethanol and 0.05 mM EDTA), and incubated at 25°C for 60 min. For kinetic analysis, 50 nM LSH WT recombinant protein was mixed with 15 nM DNA or H2A containing nucleosomes alone, or the combination of 15 nM H2A nucleosome with 15 nM mH2A_Flag-H2B dimer. Reactions without cofactors served as controls. Samples were incubated with ATP at concentrations ranging from 10 to 500 μM for 30 min. ATPase activities were measured using a Colorimetric ATPase Assay Kit (NOVUS) containing PiColorLock Gold reagent and purified Pi -free ATP to prevent background signals. The ATPase reaction rate was obtained from dividing the concentration of phosphate generated in the samples by the total reaction time. The kinetic parameters Vmax, Kcat and Km were determined by nonlinear fitting of the Michaelis-Menten curve over plotted values, and the data was produced by GraphPad Prism v. 8 software.
In vitro histone transfer assay
Histone transfer assay was performed as described with modifications (2). Briefly, immobilized mono-nucleosomes were reconstituted by salt dialysis onto a biotinylated 208 bp DNA fragment containing sea urchin 5S rDNA. To generate biotinylated DNA fragment, 10 μg DNA was incubated with 7.5 μl NEBuffer 2 (10×), 2 μl of 0.4 mM biotin-14-dATP (Invitrogen), 2 μl of dBTP mix (1 mM dGTP, 1 mM dCTP, 1 mM dTTP), 1 μl of DNA polI (Klenow, NEB), 5 mM MgCl2, 1.5 mM DTT and add H2O to the volume of 75 μl with incubation at room temperature for 30 min. Add 1.5 μl of 500 mM EDTA and incubate at 75°C for 20 min to stop reaction. DNA was precipitated by isopropanol and washed with 70% ethanol. The biotinylated mono-nucleosomes (50 nM) were immobilized onto 40 μl Dynabeads M-280 (Invitrogen). After washing to remove unbound mono-nucleosomes, beads were incubated with purified LSH (WT or K254A) protein (25–100 nM), 100 nM histone dimers, and 1 mM ATP in transfer buffer (25 mM HEPES–KOH, pH 7.6, 0.37 mM EDTA, 0.35 mM EGTA, 5 mM MgCl2, 10% glycerol, 0.02% NP40, 1 mM DTT, 0.1 mg/ml BSA, 70 mM KCl) at room temperature for 60 min. Beads were concentrated on a magnetic particle concentrator and the supernatant fraction was saved. The immobilized mono-nucleosomes were washed three times with exchange buffer. Both beads and supernatant fractions were boiled in 1× Laemmli sample buffer and subjected to western blotting with primary antibodies: anti-Flag (Invitrogen, MA1-91878, 1:1000); anti-H2A (Abcam, ab242365, 1:1000); anti-H2B (Abcam, ab1790, 1:2000); anti-His (Invitrogen, MA1-21315, 1:1000).
In vitro histone exchange assay
Histone exchange assay was conducted as described with modifications (20,21). The immobilized mono-nucleosomes were reconstituted onto a 3’ biotinylated sea urchin 5S rDNA (208 bp) by salt dialysis. Histone exchange was performed with 15 nM nucleosomes, 50 nM purified LSH (WT or K254A), 30 nM free macroH2A1_Flag–H2B dimer or macroH2A2_Flag–H2B dimer and 1 mM ATP in exchange buffer (25 mM HEPES–KOH pH 7.6, 0.37 mM EDTA, 0.35 mM EGTA, 10% glycerol, 0.017% NP40, 1 mM DTT, 70 mM KCl, 3.6 mM MgCl2, 100 μg/ml BSA and protease inhibitors). The reaction was incubated at room temperature for different time points followed by adding Dynabeads M-280 to immobilize nucleosomes. After washing three times with buffer O (25 mM HEPES–KOH pH 7.6, 70 mM KCl, 10% glycerol, 0.017% NP-40, 0.37 mM EDTA, 0.35 mM EGTA), beads were treated with 0.2 U/μl MNase (Thermo Scientific) in digestion buffer (buffer O + 2 mM CaCl2) to release nucleosomes. After 5 min of incubation the reaction was stopped by adding 10 mM EDTA. The products were analyzed on 6% native gels and visualized by SYBR Green staining (Invitrogen). The gels were washed with deionized water and incubated in tris glycine transfer buffer (25 mM Tris, 192 mM glycine and 0.01% SDS) for 15 min before Western blotting onto PVDF membranes, and developed with Flag antibody (Invitrogen, MA1-91878, 1:1000).
Statistical analysis and reproducibility
All in vitro Co-IP assay, EMSA assay, in vitro histone transfer assay and in vitro histone exchange assay were performed three times with independent enzyme preparations and led to similar results, and representative results are shown. Co-IP assay and immunoblots were repeated at least three times with similar results. Each sample was from independent biological replicate. All in vitro ATPase activity assay and histone exchange time course experiment were repeated three times with independent enzyme preparations, and results were represented as mean ± SD. Statistical analyses were performed with GraphPad Prism v. 8 software by using unpaired two-tailed Student's t test between two samples or one-way ANOVA with Tukey's analysis for multiple comparisons unless otherwise stated. P < 0.05 was considered a statistically significant difference. In all cases, n.s. P > 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001.
RESULTS
LSH physically interacts with macroH2A but not with H2A.Z in vitro
Our previous study had shown that LSH induces macroH2A1 and macroH2A2 enrichment after recruitment of LSH to a specific genomic location and that LSH deficient cells show reduced genome-wide deposition of macroH2A1/2 (40). However, it remained unclear whether this activity was directly mediated by LSH or mediated through another factor recruited by LSH.
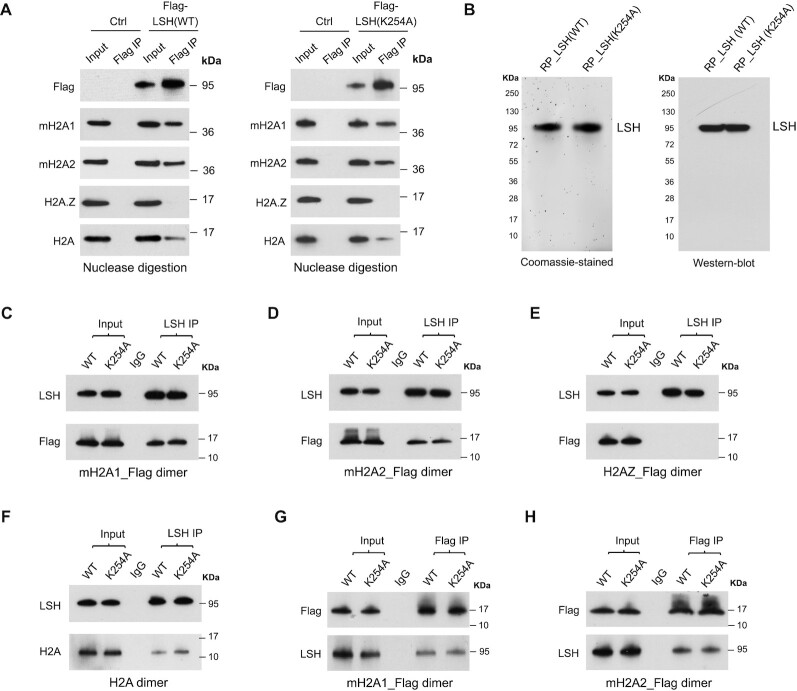
To determine a direct function of LSH in macroH2A deposition, we first examined whether LSH was capable to interact with each macroH2A variant in vivo. We have previously observed that LSH can co-precipitate macroH2A, and vice versa (40). To exclude the possibility that chromatin was responsible for this interaction, we used U2OS cells (40) that stably expressed Flag-tagged LSH_WT or Flag-tagged mutant LSH with a single amino acid substitution (K254A) within its ATP binding site (Supplementary Figure S1A), and treated U2OS cell lysates with universal nuclease and successfully removed genomic DNA (Supplementary Figure S1B). Using U2OS cells that stably expressed Flag-tagged LSH, we noted that Flag antibodies co-immunoprecipitated macroH2A1 as well as macroH2A2 but showed reduced effectivity toward canonical H2A (Figure 1A). The reaction was histone variant specific, since H2A.Z was not co-immunoprecipitated under these conditions (Figure 1A). A similar result was obtained with the ATP mutant LSH (K254A), indicating that the ATP binding site was not required for this association (Figure 1A). The persistent binding of LSH or ATP mutant of LSH to macroH2A upon nuclease digestion suggested that the interaction was not mediated through nonspecific binding to chromatin. Likewise, it has been reported that Htz1 (yeast H2A.Z) is associated with SWR1, a histone exchange complex for H2A.Z (21).
Figure 1.
Characterization of LSH and mH2A interaction. (A) Flag-IP of mH2A1, mH2A2, H2A.Z and H2A confirmed by Western-blot analysis in U2OS cells with stable expression of Flag-LSH (WT) or Flag-LSH (K254A) fusion protein. Cells without fusion protein expression were used as controls (Ctrl). Cell lysates were collected and treated with universal nuclease to remove genomic DNA for subsequent Co-IP. (B) Coomassie-stained gel of purified recombinant WT and K254A mutant LSH proteins (left panel), and Western-blot analysis with LSH antibody (right panel). RP, recombinant protein. (C–F) Western-blot analysis of purified LSH (WT) or LSH (K254A) recombinant protein Co-IP with mH2A1_Flag–H2B dimer (C), mH2A2_Flag–H2B dimer (D), H2AZ_Flag-H2B dimer (E) or H2A–H2B dimer (F) in vitro. IP was performed using beads coupled with LSH antibody or control IgG. (G-H) Western-blot analysis of mH2A1_Flag-H2B dimer (G) or mH2A2_Flag-H2B dimer (H) Co-IP with recombinant WT or K254A mutant LSH protein in vitro. IP was performed using beads coupled with Flag antibody or control IgG.
To confirm direct physical interaction between LSH and macroH2A, we generated recombinant proteins. We expressed the histone like domains of macroH2A1 and macroH2A2 as Flag-tagged proteins, and, in addition, expressed human LSH and a catalytically inactive version of LSH with a mutation (K254A) within its ATP binding site. This site is critical for ATP hydrolysis and chromatin remodeling activity of other SNF2 like factors and had been shown to be critical for LSH function in cells (37,40,47). Recombinant LSH and LSH ATP mutant showed high purity (Figure 1B) and there was no evidence of DNA contamination (Supplementary Figure S2). We found that LSH co-immunoprecipitated Flag-tagged macroH2A1 (Figure 1C) as well as Flag-tagged macroH2A2 (Figure 1D). The interaction was histone variant specific since we did not observe any interaction with H2A.Z (Figure 1E). The interaction with H2A appeared to be less strong compared to macroH2A (Figure 1F). We also detected interaction using the reverse co-immunoprecipitation approach and found that Flag-tagged macroH2A1 or macroH2A2 pulled down LSH (Figure 1G, H). Notably, the ATP mutant form of LSH behaved indistinguishable from wild type LSH, suggesting that the interaction between LSH and macroH2A was not influenced by the ATP binding site. Altogether, the data shows that LSH directly interacts with macroH2A1 or macroH2A2 in vitro.
LSH catalyzes histone transfer of macroH2A1 or macroH2A2 in vitro
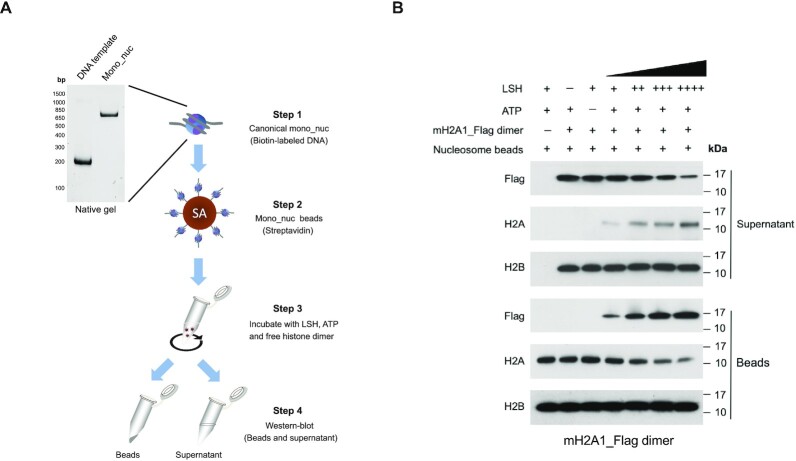
To determine whether ATP driven nucleosome disruption by LSH can lead to displacement of histone H2A and replacement with macroH2A, we conducted experiments with nucleosomes (21). We prepared mono-nucleosomes reconstituted with recombinant canonical histones and with biotinylated DNA template (Figure 2A) which showed an approximate stoichiometry of H2A:H4:H2B/H3 of 1:1:2 (Supplementary Figure S3A). At first, we tested the ability of LSH to interact with the nucleosome using an electrophoretic mobility shift assay. We found that wild type LSH as well as the ATP mutant (K254A) bound equally well to the nucleosome in a dose dependent manner (without any ATP addition), suggesting that neither ATP nor the ATP binding sites is required for interaction with the nucleosome (Supplementary Figure S4).
Figure 2.
LSH mediated transfer of mH2A-H2B dimer into the canonical nucleosomes. (A) Schematic graph to illustrate histone transfer assay in vitro. Canonical mono-nucleosomes (Mono_nuc) were immobilized on streptavidin (SA) beads, which were incubated with recombinant LSH, ATP and free reconstituted histone dimers in the transfer buffer. Incorporation of histone dimers in the nucleosome bound beads and free histone dimers in the supernatant fraction were evaluated by Western-blot analysis. (B) Immobilized reconstituted H2A containing mono-nucleosomes were incubated with recombinant LSH, ATP and free mH2A1_Flag–H2B dimer in the transfer buffer for 60 min at room temperature. Both beads and supernatant fractions were collected to detect the transfer of mH2A1–H2B dimer by LSH using western blotting. Histone H2B served as a control.
Next, we conducted a histone transfer assay (21). Nucleosomes were immobilized on streptavidin beads and these substrates were incubated with recombinant LSH, ATP and a source of free Flag-tagged macroH2A1–H2B dimers (Figure 2A). All components including the canonical nucleosome before and after immobilization, as well as the variant H2A–H2B dimers were examined on protein gels to validate their proper stoichiometry (Supplementary Figure S3A, B and S5A–C). After incubation, the components of the immobilized nucleosomes (‘beads’) and the factors released in the reaction buffer (‘supernatant’) were evaluated by western blotting. We detected an increase of Flag-tagged macroH2A1 in the nucleosomes and a concomitant loss of free macroH2A1 in the supernatant, while H2A decreased in the nucleosome fraction and was released into the reaction buffer (Figure 2B). The LSH dependent incorporation of Flag-tagged macroH2A1 was LSH concentration dependent and increased with time of incubation. H2B served as a control and amounts did not change in dependence of LSH, as had been reported for other H2A–H2B dimer exchange reactions (21). The same type of LSH mediated histone dimer exchange reaction was observed for the histone variant macroH2A2 (Supplementary Figure S6).
Specificity of the histone transfer reaction
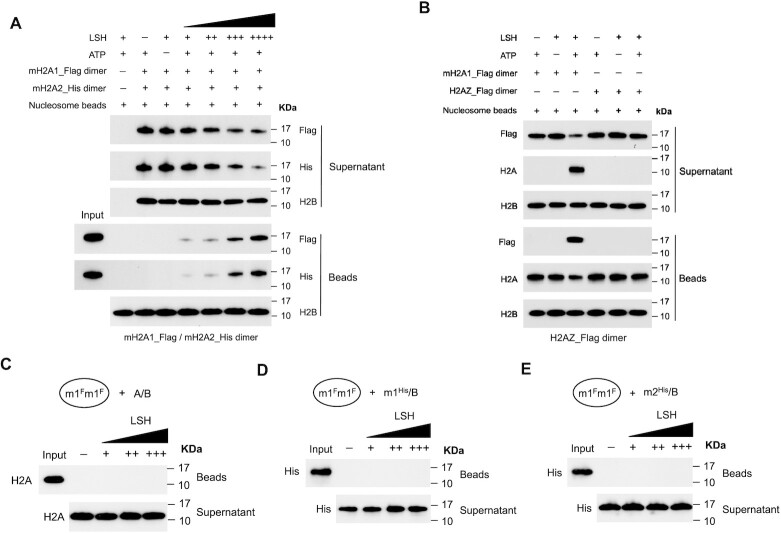
To determine a potential preference for one of the two macroH2A variant types by LSH, we used Flag-tagged macroH2A1–H2B dimer mixed with equimolar concentrations of His-tagged macroH2A2–H2B dimers in the transfer reaction. There was no overt difference in the displacement reaction under these competitive conditions and a similar efficient transfer to nucleosomes was observed for either variant macroH2A1 and macroH2A2 (Figure 3A).
Figure 3.
Preference and specificity evaluation of the histone transfer reaction by LSH. (A) Immobilized canonical nucleosomes were incubated with purified LSH, ATP and a mixture of mH2A1_Flag–H2B and mH2A2_His–H2B dimers at equimolar concentrations in order to determine the potential preference of histone transfer activity by LSH. Western blotting analysis showing the histone transfer competition results of mH2A1_Flag–H2B and mH2A2_His–H2B dimer using standard histone transfer assay. Histone H2B served as a control. (B) To determine histone variant specificity of LSH’s transfer activity, immobilized mono-nucleosomes were mixed with recombinant LSH, ATP and H2AZ_Flag–H2B dimer, and analyzed by Western blots for beads and supernatant fractions. The mH2A1_Flag-H2B dimer served as a positive control group. (C–E) To determine the unidirectional histone transfer activity of LSH, immobilized mH2A1_Flag nucleosomes (m1Fm1F) were incubated with LSH, ATP and free H2A–H2B dimer (A/B, C), mH2A1_His-H2B dimer (m1His/B, D) or mH2A2_His-H2B (m2His/B, E) dimer in the transfer buffer. After standard histone transfer reactions, nucleosome bound beads and supernatant fraction were subjected to western blotting analysis.
To determine histone variant specificity, we used the histone variant H2A.Z for comparison. While LSH readily induced a transfer of macroH2A1 into a canonical nucleosome, LSH showed no capacity to promote an exchange reaction with H2A.Z-H2B dimer (Figure 3B). This suggested selectively of LSH mediated exchange reactions and was, in part, explained by the failure of LSH to interact with the histone variant H2A.Z (Figure 1E).
Next, we tested the direction of the transfer reaction. Mono-nucleosomes were assembled with macroH2A1–H2B dimers and then incubated with LSH, ATP and free H2A–H2B dimers. Notably, LSH was inactive on this substrate (Figure 3C). This indicated that the reverse reaction of incorporating H2A-H2B dimer into a macroH2A containing nucleosome was not catalyzed. There was also no evidence that LSH promoted the exchange of a His-tagged macroH2A1–H2B or macroH2A2–H2B dimer into a nucleosome reconstituted with Flag-tagged macroH2A1 (Figure 3D, E). The same observations hold true for nucleosomes reconstituted with macroH2A2 (Supplementary Figure S7A–C) suggesting that the displacement reaction showed a strong substrate specificity for canonical nucleosomes and that the exchange reaction was unidirectional. This characteristic of LSH is similar to SWR1 transfer activity, which catalyzes histone replacement of the variant H2A.Z only in one direction (20,21).
Dependence of ATP and the ATP binding site
Chromatin remodelers of the SNF2 family require ATP hydrolysis for their in vitro and in vivo activity (2,4). SNF2 ATPases share highly conserved helicase ATP-binding domain in association with helicase C-terminal domain, and compared to ISWI, SWI/SNF, INO80/SWR1 and CHD subfamily members LSH shows 39 - 51% identity at ATP-binding domain and 46–55% identity at C-terminal domain, respectively (Supplementary Figure S8). We have previously shown that wild type LSH but not the ATP mutant (K254A) of LSH was able to induce macroH2A deposition at a targeted genomic location indicating a dependence on the ATP binding sites for in vivo function of macroH2A deposition (40).
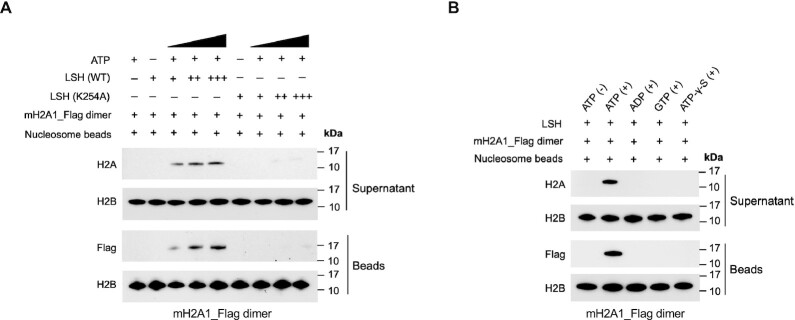
To characterize ATP dependence in vitro, we tested the LSH ATP mutant K254A for its ability to induce histone transfer. We found that ATP mutant LSH failed to transfer either macroH2A1 or macroH2A2 into canonical nucleosomes, while wild type LSH was able to do so (Figure 4A and Supplementary Figure S9A) implying that the ATP binding sites of LSH is critical for histone transfer function. Similarly, it had been shown that a point mutation in the ATP binding site of Swr1, the SNF2 like component of SWR1, is critical for the histone exchange reaction (21).
Figure 4.
ATP-dependent catalysis of histone transfer activity by LSH. (A) Histone transfer reactions were conducted using recombinant LSH (WT) or LSH (K254A, mutation at the ATP binding site) with addition of immobilized canonical nucleosomes, ATP and free mH2A1_Flag–H2B dimer. Western blots showing the replacement of mH2A1_Flag–H2B dimer with H2A–H2B dimer using beads and supernatant fractions. Histone H2B served as control. (B) Western blots showing the histone transfer activity of LSH using mH2A1_Flag–H2B dimer with addition of different ATP analogs. Reactions were incubated with or without ATP, or ATP was substituted with adenosine diphosphate (ADP), guanosine triphosphate (GTP) or nonhydrolyzable ATP analog (ATP-γ-S). Histone H2B served as a control.
Next, we determined the dependence of the transfer reaction on ATP hydrolysis and substituted ATP with adenosine diphosphate (ADP), guanosine triphosphate (GTP), and nonhydrolyzable source of ATP (ATP-γ-S). We noted that the transfer reaction of either macroH2A1 or macroH2A2 was prevented with any of these nucleotides, except ATP, suggesting that ATP hydrolysis was critical for the transfer reaction (Figure 4B and Supplementary Figure S9B).
Stimulation of ATPase activity
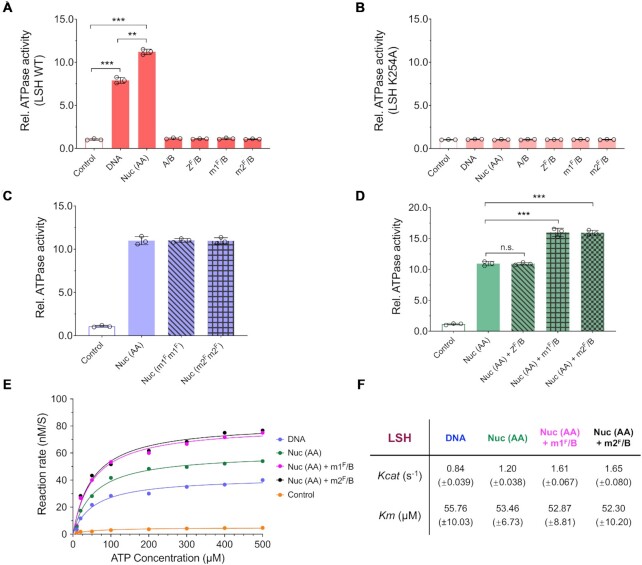
To understand more about the mechanism of macroH2A incorporation, we investigated the requirement for LSH ATPase stimulation in response to substrate binding. Since LSH can bind to nucleosome and DNA (Supplementary Figure S4 and Figure S10A, B), we tested the effect of both stimuli. ATPase function was indirectly measured by monitoring the generation of a free phosphate ion, which is released together with ADP during ATP hydrolysis. Wild type LSH was stimulated by DNA and increasingly stimulated by canonical nucleosomes (Figure 5A). The ATP mutant (K254A) was severely impaired and did not raise above background activity (Figure 5B). This result is consistent with previous reports which have also shown that LSH or the Arabidopsis homologue DDM1 has DNA and nucleosome-stimulated ATPase activity (28,38,39). Moreover, ATP analogs including ADP, GTP and ATP-γ-S failed to stimulate ATPase activity induced by DNA or nucleosomes (Supplementary Figure S11A, B). Canonical nucleosome, as well as nucleosomes reconstituted with macroH2A1 or macroH2A2 stimulated ATPase function (Figure 5C). Free H2A–H2B dimer or macroH2A1/2–H2B dimers failed to stimulate ATPase activity (Figure 5A), neither did the free dimers increase the ATPase activity beyond that of DNA alone (Supplementary Figure S12). Importantly, macroH2A1–H2B dimers or macroH2A2–H2B dimers hyper-stimulated ATPase activity in conjunction with the canonical nucleosome (Figure 5D). This hyper-stimulation was histone variant specific and was not induced by H2A.Z-H2B dimers (Figure 5D). This suggested that the recognition of H2A may occur in the context of the nucleosome and only the presence of the correct histone variant can ensure optimal ATPase activity for macroH2A–H2A exchange. Similarly, addition of H2A.Z-H2B dimers to canonical nucleosomes further increases ATPase activity of the SWR1 complex (20).
Figure 5.
Stimulation of ATPase activity by nucleosome and mH2A–H2B dimer. (A, B) ATPase activity assay of LSH (WT, A) or LSH mutant (K254A, B) recombinant protein with addition of DNA, H2A containing mono-nucleosome (Nuc (AA)), H2A–H2B dimer (A/B), H2AZ_Flag–H2B dimer (ZF/B), mH2A1_Flag–H2B dimer (m1F/B) or mH2A2_Flag–H2B dimer (m2F/B). Reactions without cofactors served as controls. ** P < 0.01 and *** P < 0.001. (C) Comparisons of ATPase activity of LSH with addition of Nuc (AA), or nucleosomes reconstituted with mH2A1_Flag (Nuc (m1Fm1F)) or with mH2A2_Flag (Nuc (m2Fm2F)). Samples without cofactors served as controls. (D) Comparisons of ATPase activity of LSH by addition of Nuc (AA) alone, or in combination with free histone dimers ZF/B, m1F/B or m2F/B. *** P < 0.001 and n.s. means not significant. (E, F) Kinetic analysis of LSH’s ATPase activity with addition of DNA or Nuc (AA) alone, or combination of Nuc (AA) with m1F/B or m2F/B (E). The kinetic parameters were determined by nonlinear fitting of the Michaelis-Menten curve over plotted values. Turnover number of Kcat (obtained from dividing maximal velocity by total enzyme concentration, s–1) and Km (ATP concentration at half maximal velocity, μM) were shown as mean ± SD from three independent experiments with individual enzyme preparations (F). Samples were incubated with different ATP concentration ranging from 10 to 500 μM for 30 min. Samples without cofactors served as controls.
When we determined kinetic parameters for LSH mediated ATP hydrolysis, we found that the turnover rate (Kcat, obtained from dividing Vmax by total enzyme concentration) was about of 0.84 s−1 in the presence of DNA and 1.20 s−1 in the presence of nucleosome (Figure 5E, F). The rate was further increased (1.3-fold) in the presence of either macroH2A1–H2B or macroH2A2–H2B with saturating nucleosome concentrations. The Michaelis constant (Km), which represents the ATP concentration at half maximal velocity (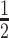 Vmax) remained somewhat unchanged under different stimulation conditions (Figure 5F), an observation shared with results obtained of the SWR1 complex (20). The value of Km were similar with reported DDM1 ATPase activity (39).
Vmax) remained somewhat unchanged under different stimulation conditions (Figure 5F), an observation shared with results obtained of the SWR1 complex (20). The value of Km were similar with reported DDM1 ATPase activity (39).
LSH exchanges nucleosomal H2A with macroH2A
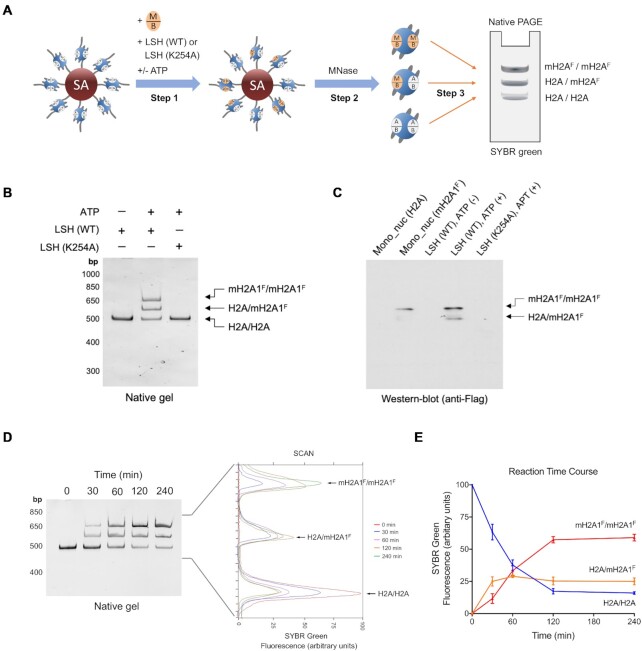
To examine the fate of the nucleosome after the histone transfer reaction we used a native Polyacrylamide gel electrophoresis (PAGE) (Figure 6A). We reasoned that nucleosomes that had incorporated macroH2A instead of H2A would show a different mobility, since the migration of Flag-tagged macroH2A1 or macroH2A2 was distinguishable from H2A due to their different molecular weight (Supplementary Figure S13A, B). Furthermore, homotypic macroH2A1 or macroH2A2 containing nucleosomes showed a mobility that was distinct from canonical nucleosomes suggesting that the mobility assay would reveal the copy number of macroH2A per nucleosome (Supplementary Figure S13C). Using free macroH2A1-H2B dimer and canonical nucleosomes, we observed after incubation with ATP and LSH that two bands appeared that differed in mobility from the canonical nucleosome (Figure 6B). This suggested the formation of heterotypic nucleosomes (containing one copy of macroH2A1 and one copy of H2A) and homotypic (two copies of macroH2A1) nucleosomes which was confirmed by immunoblot analysis of the native PAGE (Figure 6C). Likewise, the generation of heterotypic and homotypic macroH2A2 containing nucleosomes could be confirmed by native PAGE and immunoblot analysis (Supplementary Figure S14A, B). The incorporation of macroH2A variants took only place after addition of ATP and was dependent on the ATP binding site of LSH (Figure 6B, C and Supplementary Figure S14A, B).
Figure 6.
Histone exchange assay showing the stepwise assembly of mH2A containing nucleosomes by LSH. (A) Schematic graph to illustrate the in vitro histone exchange experiment. Immobilized canonical mono-nucleosomes containing H2A–H2B dimer (A/B) were incubated with free mH2A–H2B (M/B) dimer, LSH (WT) or LSH (K254A), with or without ATP (Step 1). After washing, MNase was used to liberate mono-nucleosomes from streptavidin (SA) beads in Step 2. The products were subsequently loaded onto a native PAGE (Step 3). Homotypic H2A nucleosomes (H2A/H2A, bottom), heterotypic mH2A_Flag nucleosomes (H2A/mH2AF, middle) and homotypic mH2A_Flag nucleosomes (mH2AF/mH2AF, top) were stained with SYBR green and could be distinguished by their difference in mobility. (B, C) After the histone exchange experiment, native PAGE and SYBR green staining showing the mono-nucleosomes (B), and western-blot analysis to confirm the components of heterotypic (H2A/mH2A1F) and homotypic (mH2A1F/mH2A1F) nucleosomes using Flag antibody (C). Reactions were incubated with immobilized nucleosomes, free mH2A1_Flag-H2B dimer, purified LSH (WT) or LSH (K254A), and with or without ATP for 60 min at room temperature. Homotypic H2A and mH2A1F nucleosomes served as comparisons for Western-blot analysis. (D, E) In vitro histone exchange time course experiment using mH2A1_Flag-H2B dimer was shown in Native PAGE and stained by SYBR green with densitometric measurement (D). The line chart showing the kinetic histone replacement for the stepwise assembly of mH2A1_Flag containing nucleosomes (E). LSH-mediated histone exchange reactions were stopped at various time points (0, 30, 60, 120 and 240 min).
Lastly, we studied the kinetics and found increased formation first of heterotypic and then of homotypic macroH2A1 nucleosomes over time which reached its plateau within 120 min (Figure 6D, E). By this time >85% of the canonical nucleosome had incorporated macroH2A1. A majority (about 60%) of the nucleosomal product consisted of two macroH2A1 molecules and about 25% was a heterotypic nucleosome composed of macroH2A1 and H2A. A similar result was obtained for macroH2A2 (Supplementary Figure S14C, D). A stepwise dimer replacement has been reported for SWR1 resulting in heterotypic nucleosomes as intermediates followed by the generation of homotypic H2A.Z nucleosomes (20).
DISCUSSION
A few factors, including the chaperone FACT have been implicated in the removal of macroH2A from chromatin in vivo, but no deposition machinery had been identified and in vitro data on macroH2A exchange was lacking (5,8). Using a cellular system, we have recently demonstrated that LSH promotes macroH2A incorporation into chromatin in vivo. However, it remained unknown whether LSH mediated its effect directly or whether LSH could conduct the exchange on its own and without the help of co-factors. Here we report a robust macroH2A exchange activity of recombinant LSH which depends on ATP, LSH concentration and time of incubation. This is to our knowledge the first report that identifies a depositing mechanism and demonstrates a macroH2A histone exchange activity involving a chromatin remodeler in vitro.
Complexes that promote exchange of histone variants, such as H2A.Z or H3.3, involve a SNF2 ATPase homologue of the INO80/SWR1 subfamily as key factor of catalytic activity (4,48). For example, EP400 is the SNF2 ATPase of the EP400/TIP60 complex which promotes deposition of H2A.Z into nucleosomes in vitro (49,50). This reaction is not exclusive for H2A.Z since EP400 also stimulates the transfer of H3.3 into nucleosomes. Furthermore, EP400 performs the reverse exchange of H2A and H3.1 into variant chromatin, albeit at lower efficiency for H2A (50). TIP48 and TIP49, components of a TIP60 HAT complex, catalyze the incorporation of H2A.Z into acetylated nucleosomes, this catalytic activity enfolds solely when they act as a complex (51). Other chromatin remodelers perform histone exchange only as part of a multi-subunit complexes. For example, the 14-subunit SWR1 complex is responsible for loading H2A.Z (Htz1 yeast) in vivo into chromatin and transferring H2A.Z in vitro into nucleosomes (21,52,53). The SRCAP (SNF2-related CREBBP activator protein) complex, a mammalian paralog of the SWR1 complex, supports ATP-dependent exchange of histone dimers containing H2A.Z and H2B into canonical mono-nucleosomes (54). The yeast INO80 complex can stimulate the reverse H2A.Z histone variant-exchange activity such that the enzyme can replace nucleosomal H2A.Z–H2B with H2A–H2B dimer in vitro (55).
The original classification of SNF2 ATPases identified six subgroups (17) and placed LSH among the SNF2-like subfamily with ISWI (human SNF2H and SNF2L) as closest homologue. ISWI subfamily members are known for their ability to induce nucleosome sliding, as has been previously shown for LSH in conjunction with CDCA7 (38) and for the Arabidopsis LSH homologue DDM1 (39). It is perhaps unexpected that LSH has the ability of histone variant exchange which has been so far ascribed to EP400, SRCAP, SWR1 and INO80, all of which are SNF2 ATPases of the INO80/SWR1 subfamily. However, as mentioned above neither LSH nor its Arabidopsis homologue DDM1 comprise any additional domain that distinguishes these subfamilies such as the HAND-SANT-SLIDE domain for ISWI, or the HSA domain for INO80/SWR1 subfamily. Perhaps for this reason LSH and DDM1 have been also placed in their own subgroup distinct from ISWI or INO80/SWR1 (56,57). A recent report linked the Arabidopsis homologue of LSH, DDM1, to the deposition of the histone variant H2A.W associated with heterochromatin (58). It is also noteworthy to mention that LSH is not the only SNF2 ATPase family member that conducts histone variant exchange and nucleosome sliding. INO80 is also capable of repositioning nucleosomes and histone variant exchange, although the molecular mechanisms determining these different outcomes requires further investigation (18,59).
Structural studies have shed some light on the mechanism of H2A.Z histone variant exchange. The SWR1 complex associates with the nucleosome through histone and DNA contacts and the interaction with the nucleosome results in distortion of the DNA (52,53,60). SWR1 partially unwraps DNA from the histone core, which is dynamically altered by ATP consumption, and a 1 bp translocation occurs (60). A stepwise mechanism has been observed for SWR1 mediated histone variant exchange with one H2A.Z–H2B dimer at a time leading first to heterotypic and subsequently to homotypic nucleosomes (20). Our observation that in the first 30 min more heterotypic than homotypic macroH2A nucleosomes are produced and the heterotypic form declines whereas homotypic nucleosomes still increases between 120 and 240 min, is consistent with a model of stepwise dimer exchange. However, it remains unknown how LSH modulates the conformational dynamics of the nucleosome during the exchange reaction and structural data will be important for future research. The interaction regions between LSH and macroH2A are currently unknown. For yeast SWR1 complex a critical region for interaction has been identified in the alpha2 helix of H2A, which differs by one or three residues comparing macroH2A1 and macroH2A2 with H2A, respectively (61). Additional regions in alpha1 helix and in L2 have been identified for SWR1 complex interactions that may contribute to interaction or may act by allostery (61). The alpha1 helix and the L2 region differ by four or three residues, respectively comparing macroH2A1/2 and H2A. It will be interesting to characterize the interaction sites with macroH2A in the future, and to determine if and how it differs from other chromatin remodelers.
Recently, it has been shown that mutations in the helicase SRCAP the catalytic component of the SRCAP complex which mediates the transfer of histone H2A.Z-H2B dimers into nucleosomes, is associated with the Floating–Harbor Syndrome, a rare but severe developmental disorder which shows an abnormal deposition of H2A.Z variants at enhancers (62). Since LSH mutation causes the human ICF4 syndrome, and patient derived cells show abnormal incorporation of macroH2A into chromatin (40), our study here implies a link between the histone variant macroH2A and a human inherited genetic disease. A hallmark of the ICF4 syndrome is genomic instability (23). The role of LSH in DNA repair and genomic instability has been shown to depend on the ATP binding site implying that LSH chromatin remodeling function plays a role in these processes (28–30). It is currently not known whether some of the previous observations are mediated by macroH2A deposition or DNA methylation or whether LSH has distinct molecular functions depending on co-factors, the type of DNA damage or repair process and the genomic location. However, LSH protects nascent DNA at stalled forks against nucleolytic degradation and this effect is attributed in part through macroH2A deposition (63).
Taken together, our results indicate that the human LSH protein catalyzes ATP-dependent incorporation of macroH2A-H2B dimers into nucleosomes making LSH the first example of an macroH2A exchange factor of the SNF2 family of chromatin remodeling enzymes.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Jane Jones and Carissa Grose in the NCI-Frederick Protein and Nucleic Acid Production Core (Center for Cancer Research) for excellent technical performance and assistance in the generation of the recombinant proteins in this project.
Contributor Information
Kai Ni, Epigenetics Section, Mouse Cancer Genetics Program, National Cancer Institute, Frederick, MD 21702, USA.
Kathrin Muegge, Epigenetics Section, Frederick National Laboratory for Cancer Research in the Mouse Cancer Genetics Program, National Cancer Institute, Frederick, MD 21702, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
This Research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. Funding for open access charge: National Cancer Institute institutional charges.
Conflict of interest statement. None declared.
REFERENCES
- 1.Ho L., Crabtree G.R.. Chromatin remodelling during development. Nature. 2010; 463:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swygert S.G., Peterson C.L.. Chromatin dynamics: interplay between remodeling enzymes and histone modifications. Biochim. Biophys. Acta. 2014; 1839:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talbert P.B., Henikoff S.. Histone variants on the move: substrates for chromatin dynamics. Nat. Rev. Mol. Cell. Biol. 2017; 18:115–126. [DOI] [PubMed] [Google Scholar]
- 4.Clapier C.R., Cairns B.R.. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009; 78:273–304. [DOI] [PubMed] [Google Scholar]
- 5.Martire S., Banaszynski L.A.. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell. Biol. 2020; 21:522–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesh S., Workman J.L.. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell. Biol. 2015; 16:178–189. [DOI] [PubMed] [Google Scholar]
- 7.Pehrson J.R., Fried V.A.. MacroH2A, a core histone containing a large nonhistone region. Science. 1992; 257:1398–1400. [DOI] [PubMed] [Google Scholar]
- 8.Sun Z., Bernstein E.. Histone variant macroH2A: from chromatin deposition to molecular function. Essays Biochem. 2019; 63:59–74. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy S., Gundimella S.K., Caron C., Perche P.Y., Pehrson J.R., Khochbin S., Luger K.. Structural characterization of the histone variant macroH2A. Mol. Cell. Biol. 2005; 25:7616–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Changolkar L.N., Pehrson J.R.. Reconstitution of nucleosomes with histone macroH2A1.2. Biochemistry. 2002; 41:179–184. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthy S., Luger K.. The histone variant macro-H2A preferentially forms “hybrid nucleosomes”. J. Biol. Chem. 2006; 281:25522–25531. [DOI] [PubMed] [Google Scholar]
- 12.Changolkar L.N., Singh G., Cui K., Berletch J.B., Zhao K., Disteche C.M., Pehrson J.R.. Genome-wide distribution of macroH2A1 histone variants in mouse liver chromatin. Mol. Cell. Biol. 2010; 30:5473–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douet J., Corujo D., Malinverni R., Renauld J., Sansoni V., Posavec Marjanović M., Cantariño N., Valero V., Mongelard F., Bouvet P.et al.. MacroH2A histone variants maintain nuclear organization and heterochromatin architecture. J. Cell. Sci. 2017; 130:1570–1582. [DOI] [PubMed] [Google Scholar]
- 14.Chakravarthy S., Patel A., Bowman G.D.. The basic linker of macroH2A stabilizes DNA at the entry/exit site of the nucleosome. Nucleic Acids Res. 2012; 40:8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willhoft O., Wigley D.B.. INO80 and SWR1 complexes: the non-identical twins of chromatin remodelling. Curr. Opin. Struct. Biol. 2020; 61:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dürr H., Flaus A., Owen-Hughes T., Hopfner K.P.. Snf2 family ATPases and DExx box helicases: differences and unifying concepts from high-resolution crystal structures. Nucleic Acids Res. 2006; 34:4160–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaus A., Martin D.M., Barton G.J., Owen-Hughes T.. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006; 34:2887–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clapier C.R., Iwasa J., Cairns B.R., Peterson C.L.. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell. Biol. 2017; 18:407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan A., LeGresley S., Fischer C.. Remodeler catalyzed nucleosome repositioning: influence of structure and stability. Int. J. Mol. Sci. 2020; 22:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luk E., Ranjan A., Fitzgerald P.C., Mizuguchi G., Huang Y., Wei D., Wu C.. Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell. 2010; 143:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuguchi G., Shen X., Landry J., Wu W.H., Sen S., Wu C.. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004; 303:343–348. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis C.D., Geiman T., Vila-Storm M.P., Osipovich O., Akella U., Candeias S., Nathan I., Durum S.K., Muegge K.. A novel putative helicase produced in early murine lymphocytes. Gene. 1996; 169:203–207. [DOI] [PubMed] [Google Scholar]
- 23.Thijssen P.E., Ito Y., Grillo G., Wang J., Velasco G., Nitta H., Unoki M., Yoshihara M., Suyama M., Sun Y.et al.. Mutations in CDCA7 and HELLS cause immunodeficiency-centromeric instability-facial anomalies syndrome. Nat. Commun. 2015; 6:7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De La Fuente R., Baumann C., Fan T., Schmidtmann A., Dobrinski I., Muegge K.. Lsh is required for meiotic chromosome synapsis and retrotransposon silencing in female germ cells. Nat. Cell. Biol. 2006; 8:1448–1454. [DOI] [PubMed] [Google Scholar]
- 25.Han Y., Ren J., Lee E., Xu X., Yu W., Muegge K.. Lsh/HELLS regulates self-renewal/proliferation of neural stem/progenitor cells. Sci. Rep. 2017; 7:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y., Ren J., Xu X., Ni K., Schwader A., Finney R., Wang C., Sun L., Klarmann K., Keller J.et al.. Lsh/HELLS is required for B lymphocyte development and immunoglobulin class switch recombination. Proc. Natl. Acad. Sci. U.S.A. 2020; 117:20100–20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiman T.M., Muegge K.. Lsh, an SNF2/helicase family member, is required for proliferation of mature T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 2000; 97:4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burrage J., Termanis A., Geissner A., Myant K., Gordon K., Stancheva I.. The SNF2 family ATPase LSH promotes phosphorylation of H2AX and efficient repair of DNA double-strand breaks in mammalian cells. J. Cell. Sci. 2012; 125:5524–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kollárovič G., Topping C.E., Shaw E.P., Chambers A.L.. The human HELLS chromatin remodelling protein promotes end resection to facilitate homologous recombination and contributes to DSB repair within heterochromatin. Nucleic Acids Res. 2020; 48:1872–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unoki M., Funabiki H., Velasco G., Francastel C., Sasaki H.. CDCA7 and HELLS mutations undermine nonhomologous end joining in centromeric instability syndrome. J. Clin. Invest. 2019; 129:78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litwin I., Bakowski T., Szakal B., Pilarczyk E., Maciaszczyk-Dziubinska E., Branzei D., Wysocki R.. Error-free DNA damage tolerance pathway is facilitated by the Irc5 translocase through cohesin. Embo j. 2018; 37:e98732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muegge K.Lsh, a guardian of heterochromatin at repeat elements. Biochem. Cell Biol. 2005; 83:548–554. [DOI] [PubMed] [Google Scholar]
- 33.Yu W., Briones V., Lister R., McIntosh C., Han Y., Lee E.Y., Ren J., Terashima M., Leighty R.M., Ecker J.R.et al.. CG hypomethylation in Lsh-/- mouse embryonic fibroblasts is associated with de novo H3K4me1 formation and altered cellular plasticity. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:5890–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu W., McIntosh C., Lister R., Zhu I., Han Y., Ren J., Landsman D., Lee E., Briones V., Terashima M.et al.. Genome-wide DNA methylation patterns in LSH mutant reveals de-repression of repeat elements and redundant epigenetic silencing pathways. Genome Res. 2014; 24:1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xi S., Zhu H., Xu H., Schmidtmann A., Geiman T.M., Muegge K.. Lsh controls Hox gene silencing during development. Proc. Natl. Acad. Sci. U.S.A. 2007; 104:14366–14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren J., Finney R., Ni K., Cam M., Muegge K.. The chromatin remodeling protein Lsh alters nucleosome occupancy at putative enhancers and modulates binding of lineage specific transcription factors. Epigenetics-US. 2019; 14:277–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J., Briones V., Barbour S., Yu W., Han Y., Terashima M., Muegge K.. The ATP binding site of the chromatin remodeling homolog Lsh is required for nucleosome density and de novo DNA methylation at repeat sequences. Nucleic Acids Res. 2015; 43:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenness C., Giunta S., Muller M.M., Kimura H., Muir T.W., Funabiki H.. HELLS and CDCA7 comprise a bipartite nucleosome remodeling complex defective in ICF syndrome. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E876–E885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brzeski J., Jerzmanowski A.. Deficient in DNA methylation 1 (DDM1) defines a novel family of chromatin-remodeling factors. J. Biol. Chem. 2003; 278:823–828. [DOI] [PubMed] [Google Scholar]
- 40.Ni K., Ren J., Xu X., He Y., Finney R., Braun S.M.G., Hathaway N.A., Crabtree G.R., Muegge K.. LSH mediates gene repression through macroH2A deposition. Nat. Commun. 2020; 11:5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehalko J.L., Esposito D. Engineering the transposition-based baculovirus expression vector system for higher efficiency protein production from insect cells. J. Biotechnol. 2016; 238:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dyer P.N., Edayathumangalam R.S., White C.L., Bao Y., Chakravarthy S., Muthurajan U.M., Luger K.. Reconstitution of nucleosome core particles from recombinant histones and DNA. Methods Enzymol. 2004; 375:23–44. [DOI] [PubMed] [Google Scholar]
- 43.Luger K., Rechsteiner T.J., Richmond T.J.. Preparation of nucleosome core particle from recombinant histones. Methods Enzymol. 1999; 304:3–19. [DOI] [PubMed] [Google Scholar]
- 44.Meersseman G., Pennings S., Bradbury E. Mobile nucleosomes–a general behavior. Embo j. 1992; 11:2951–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennings S., Meersseman G., Bradbury E.M.. Mobility of positioned nucleosomes on 5 S rDNA. J. Mol. Biol. 1991; 220:101–110. [DOI] [PubMed] [Google Scholar]
- 46.Bertin A., Durand D., Renouard M., Livolant F., Mangenot S.. H2A and H2B tails are essential to properly reconstitute nucleosome core particles. Eur. Biophys. J. 2007; 36:1083–1094. [DOI] [PubMed] [Google Scholar]
- 47.Corona D.F., Längst G., Clapier C.R., Bonte E.J., Ferrari S., Tamkun J.W., Becker P.B.. ISWI is an ATP-dependent nucleosome remodeling factor. Mol. Cell. 1999; 3:239–245. [DOI] [PubMed] [Google Scholar]
- 48.Ryan D.P., Owen-Hughes T.. Snf2-family proteins: chromatin remodellers for any occasion. Curr. Opin. Chem. Biol. 2011; 15:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gévry N., Chan H.M., Laflamme L., Livingston D.M., Gaudreau L.. p21 transcription is regulated by differential localization of histone H2A.Z. Genes Dev. 2007; 21:1869–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pradhan S.K., Su T., Yen L., Jacquet K., Huang C., Côté J., Kurdistani S.K., Carey M.F.. EP400 deposits H3.3 into promoters and enhancers during gene activation. Mol. Cell. 2016; 61:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi J., Heo K., An W.. Cooperative action of TIP48 and TIP49 in H2A.Z exchange catalyzed by acetylation of nucleosomal H2A. Nucleic Acids Res. 2009; 37:5993–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giaimo B.D., Ferrante F., Herchenröther A., Hake S.B., Borggrefe T.. The histone variant H2A.Z in gene regulation. Epigenet. Chromatin. 2019; 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scacchetti A., Becker P.B.. Variation on a theme: Evolutionary strategies for H2A.Z exchange by SWR1-type remodelers. Curr. Opin. Cell. Biol. 2020; 70:1–9. [DOI] [PubMed] [Google Scholar]
- 54.Ruhl D.D., Jin J., Cai Y., Swanson S., Florens L., Washburn M.P., Conaway R.C., Conaway J.W., Chrivia J.C.. Purification of a human SRCAP complex that remodels chromatin by incorporating the histone variant H2A.Z into nucleosomes. Biochemistry. 2006; 45:5671–5677. [DOI] [PubMed] [Google Scholar]
- 55.Papamichos-Chronakis M., Watanabe S., Rando O.J., Peterson C.L.. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell. 2011; 144:200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lusser A., Kadonaga J.T.. Chromatin remodeling by ATP-dependent molecular machines. Bioessays. 2003; 25:1192–1200. [DOI] [PubMed] [Google Scholar]
- 57.Verbsky M.L., Richards E.J.. Chromatin remodeling in plants. Curr. Opin. Plant Biol. 2001; 4:494–500. [DOI] [PubMed] [Google Scholar]
- 58.Osakabe A., Jamge B., Axelsson E., Montgomery S.A., Akimcheva S., Kuehn A.L., Pisupati R., Lorković Z.J., Yelagandula R., Kakutani T.et al.. The chromatin remodeler DDM1 prevents transposon mobility through deposition of histone variant H2A.W. Nat. Cell Biol. 2021; 23:391–400. [DOI] [PubMed] [Google Scholar]
- 59.Sundaramoorthy R., Owen-Hughes T.. Chromatin remodelling comes into focus. F1000Res. 2020; 9:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willhoft O., Ghoneim M., Lin C.L., Chua E.Y.D., Wilkinson M., Chaban Y., Ayala R., McCormack E.A., Ocloo L., Rueda D.S.et al.. Structure and dynamics of the yeast SWR1-nucleosome complex. Science. 2018; 362:eaat7716. [DOI] [PubMed] [Google Scholar]
- 61.Ranjan A., Wang F., Mizuguchi G., Wei D., Huang Y., Wu C.. H2A histone-fold and DNA elements in nucleosome activate SWR1-mediated H2A.Z replacement in budding yeast. Elife. 2015; 4:e06845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenberg R.S., Long H.K., Swigut T., Wysocka J.. Single amino acid change underlies distinct roles of H2A.Z subtypes in human syndrome. Cell. 2019; 178:1421–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X., Ni K., He Y., Ren J., Sun C., Liu Y., Aladjem M.I., Burkett S., Finney R., Ding X.et al.. The epigenetic regulator LSH maintains fork protection and genomic stability via MacroH2A deposition and RAD51 filament formation. Nat. Commun. 2021; 12:3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.