Abstract
Obesity has been associated with an increased risk of breast cancer recurrence and death. Some readily available biomarkers associated with systemic inflammation have been receiving attention as potential prognostic indicators in cancer, including neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). This study aimed to explore the correlation between body mass index (BMI) and invasive breast cancer and the association of NLR, PLR, and BMI with breast cancer outcomes. We undertook a retrospective study to evaluate patients treated for breast cancer over 14 years. Clinicopathological data was obtained before receiving any treatment. Of the 1664 patients included with stage I-III, 567 (34%) were obese (BMI≥30 kg/m2). Obese patients had larger tumors compared to non-obese patients. Higher BMI was associated with recurrence and worse survival only in patients with stage I disease. NLR and PLR were classified into high and low level groups. The NLRhigh (NLR>4) was found to be an independent prognostic factor for recurrence and mortality, while the PLRhigh (PLR>150) group had no impact on survival. A subgroup of patients with NLRhigh and BMIhigh had the worst disease-free survival (P=0.046), breast cancer-specific survival (P<0.001), and overall survival (P=0.006), compared to the other groups. Patients with early-stage breast cancer bearing NLRhigh and BMIhigh had worse outcomes, and this might be explained by the dysfunctional milieu of obesity in adipose tissue and its effects on the immune system. This study highlights the importance of lifestyle measures and the immune system interference with clinical outcomes in the early breast cancer setting.
Keywords: Breast cancer, Obesity, Neutrophil-to-lymphocyte ratio, Prognosis, Survival
Introduction
Breast cancer is the most common malignant neoplasm among women, accounting for approximately 25% of new cancer cases and 15% of cancer deaths worldwide (1). In Brazil alone, an estimated 66,280 new cases are expected to be diagnosed each year between 2020 and 2022 (2). Breast cancer is known to be a heterogeneous disease, with different clinical presentations and molecular subtypes (3).
Clinical and pathological staging, molecular profiling, and environmental factors can influence breast cancer outcomes (4). Among environmental factors, obesity has been reported as one of the most prevalent modifiable risk factors associated with chronic disease, raising awareness of its relation to cancer (5). Despite numerous studies evaluating obesity and breast cancer mortality (6), the mechanisms through which obesity exerts its effects on breast cancer survival have not been fully elucidated. Obesity seems to be particularly relevant in postmenopausal women and those with hormone receptor positive tumors (7).
Obesity and carcinogenesis have an important feature in common, which is the involvement of the inflammatory pathways (8). Dysregulated metabolism and a state of chronic subclinical inflammation, along with elevated levels of proinflammatory and immune mediators in the peripheral blood and local breast tissues, play important roles (9). There is evidence that chronic inflammation could influence tumor initiation, promotion, invasion, and metastasis (10).
Two inflammatory biomarkers recently described as prognostic factors for cancer are the peripheral blood neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) (11,12). Although their significance has not been fully elucidated, there is evidence that an inflammatory stimulus may increase the production and release of neutrophils from the bone marrow, mediated by the granulocyte colony-stimulating factor (G-CSF), interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) (13). IL-6 also stimulates the differentiation of megakaryocytes to platelets and thrombopoietin production, thus increasing the blood platelet count (14). In contrast, neutrophilia, particularly due to TNF-α and IL-1β, has an inverse relationship with the lymphocyte count, while lymphopenia is also correlated with poor prognosis (12). NLR and PLR as biomarkers are cheap, easy to measure and monitor over time, and have been previously shown to predict worse outcomes and decreased response to treatment in some breast cancer patients (15,16).
Here, we explored the association between obesity, assessed by body mass index (BMI), and early-stage breast cancer at presentation and survival. We further investigated the prognostic significance of NLR and PLR for breast cancer recurrence and survival and the influence of BMI on these biomarkers.
Material and Methods
Study participants
This was a retrospective study with all patients diagnosed and treated for malignant breast neoplasms at the Breast Disease Division of Clinics Hospital of Ribeirão Preto School of Medicine, University of São Paulo (USP), between 1999 and 2013. The clinical and pathological data was obtained from the patients’ files (including paper charts and electronic medical records). The variables obtained were: age, histological type and grade, TNM stage, tumor size, positive lymph nodes, immunohistochemistry (IHC), menopausal status, weight, height, and total blood count. Treatment characteristics were also collected including surgical treatments, hormone therapy, chemotherapy, and radiotherapy. The study was approved by the local ethical committee (approval number 2.638.453/2018).
Of the 1967 patients selected, 136 did not have available information about the height or weight and were not included in the study. We further restricted the cohort to those with stage I to III cancers. Other patients with missing variables were also excluded from the analyses; however, none of them had more than 10% of missing data. Data were collected from the date of diagnosis until death or until July 1, 2018, which was chosen as the final date of observation. In case of loss to follow-up, the time was censored at the date of the last information in the medical records. Disease-free survival (DFS) was defined as the time from the diagnosis to the development of locoregional or contralateral recurrence or evidence of distant metastasis. Breast cancer-specific survival (BCSS) was defined as the duration from the date of diagnosis until death due to breast cancer and overall survival (OS) was defined as the duration from the date of diagnosis to death, with no restriction on the cause of death. Patients with unknown menopausal status (n=129) were considered to be postmenopausal if they were ≥51 years old. This assumption was based on existing studies conducted in the same region (17) (Figure 1).
Figure 1. Flow diagram showing enrollment of women in the study.
Tumor subtypes and BMI
The amplification/overexpression of human epidermal growth factor receptor 2 (HER2) and the expression of hormonal receptors were determined by IHC in accordance with specific guidelines (18,19). HER2 positivity was established in accordance with the pathology report and the protocols followed at the time of diagnosis, as recorded in the clinical chart. Fluorescent in situ hybridization (FISH) was used in patients with HER2 2+ IHC results. The subtype was considered to be luminal-like if the estrogen receptor (ER) and/or the progesterone receptor (PR) were positive and HER2 was negative; luminal/HER2-like if ER and/or PR were positive and HER2 was positive; HER2-like if ER and PR were negative and HER2 was positive; and triple negative (TN) when ER, PR, and HER2 were negative. For staging, we used the American Joint Committee on Cancer Staging Manual, 7th edition (20).
Height and weight data were collected prior to the first cancer treatment. BMI was calculated as weight (in kilograms) divided by height (in meters) squared, and obesity was defined as BMI≥30 kg/m2 (21). BMI was initially explored as a continuous variable, but as there was no difference in the results, we chose to analyze it as a categorical variable, consistent with the majority of published studies.
Laboratory investigations
Hematologic samples collected prior to the first cancer treatment were considered in the analyses. Absolute blood counts of different cell types were determined. The ratios of NLR, which is the absolute number of neutrophils divided by the absolute number of lymphocytes, and PLR, which is the absolute value of platelets divided by the absolute value of lymphocytes, were calculated (11). We used receiver operating characteristic (ROC) curve analyses to estimate the best cut-off values for both parameters.
Statistical analysis
To evaluate the differences between the groups, we used the chi-squared test for categorical variables and the Mann-Whitney or Kuskall-Wallis tests for continuous variables. Normality was tested with the Shapiro-Wilk test. We used Cox multivariate regression model for analyzing variables considered significant (P<0.05) with univariate analyses and BMI.
Kaplan-Meier curves were used to estimate cancer recurrence, BCSS, and OS at 10 years, and the survival differences between the groups were tested by the log-rank test. We set the level of significance at 0.05 and conducted all analyses using the R software version 3.6.1 (R Core Team, Austria).
Results
Population characteristics
Overall, 1664 early breast cancer patients were included in the study, of which 567 (34%) had a BMI≥30 kg/m2 at the time of diagnosis. Obese patients were older (P=0.03) and had a larger median tumor size (median 22 vs 20 mm, P=0.01) compared to non-obese patients. There were no significant differences between obese and non-obese women in terms of the histologic type and grade, cancer staging, hormone receptors, and HER2. With regard to the treatment variables, obese patients were slightly more likely to receive radiotherapy during the course of the treatment (P=0.03), but with no difference in type of surgery or stage. Table 1 describes the cohort characteristics according to the BMI.
Table 1. Baseline characteristics of all patients with breast cancer (n=1664), stratified by the body mass index (BMI).
| Characteristics | BMI≥30 kg/m2 (n=567) | BMI<30 kg/m2 (n=1097) | P value |
|---|---|---|---|
| BMI, average (SD) | 35.0 (4.5) | 25.1 (3.1) | 0.01 |
| Age (years), median (range) | 56 (25.0-89.0) | 54.2 (23.6-93.8) | 0.03 |
| Tumor size (mm), median (range) | 22 (0-154) | 20 (0-140) | 0.01 |
| n (%) | n (%) | ||
| Postmenopausal status | 370 (65) | 662 (60) | 0.05 |
| Histology | |||
| Ductal | 506 (89) | 967 (88) | 0.46 |
| Lobular | 25 (4) | 45 (4) | |
| Other | 31 (6) | 77 (7) | |
| Unknown | 5 (1) | 8 (1) | |
| Gradea | |||
| 1 | 118 (21) | 224 (20) | 0.96 |
| 2 | 304 (54) | 585 (53) | |
| 3 | 132 (23) | 246 (22) | |
| Unknown | 13 (2) | 42 (4) | |
| Stage (TNM) | |||
| I (A+B) | 110 (20) | 215 (20) | 0.25 |
| II (A+B) | 228 (40) | 482 (44) | |
| III (A+B+C) | 229 (40) | 400 (36) | |
| Subtype | |||
| Luminal-like | 351 (62) | 640 (59) | 0.32 |
| Luminal/HER2-like | 77 (14) | 157 (15) | |
| HER2-like | 45 (8) | 115 (10) | |
| Triple negative | 85 (15) | 169 (15) | |
| Unknown | 9 (1) | 16 (1) | |
| Estrogen receptor status | |||
| Positive | 423 (75) | 789 (73) | 0.25 |
| Negative | 138 (24) | 297 (26) | |
| Unknown | 6 (1) | 11 (1) | |
| Progesterone receptor status | |||
| Positive | 360 (64) | 645 (59) | 0.07 |
| Negative | 201 (35) | 440 (40) | |
| Unknown | 6 (1) | 12 (1) | |
| HER2 status | |||
| Positive | 122 (22) | 272 (25) | 0.15 |
| Negative | 437 (77) | 809 (74) | |
| Unknown | 8 (1) | 16 (1) | |
| NLR median (range) | 1.93 (0.23-15.17) | 1.99 (0.18-31.67) | 0.67 |
| PLR median (range) | 122.61 (26.35-875) | 129.36 (15.29-655) | 0.40 |
| First treatment | |||
| Upfront surgery | 352 (56) | 697 (58) | 0.58 |
| Neoadjuvant chemotherapy | 243 (39) | 459 (38) | |
| NEO endocrine therapy | 25 (4) | 46 (4) | |
| NEO radiotherapy | 0 (0) | 5 (0) | |
| Treatment | |||
| Surgery approach | |||
| Conservative surgery | 312 (56) | 577 (54) | 0.34 |
| Mastectomy | 240 (44) | 493 (46) | |
| Axillary approach | |||
| Sentinel node biopsy | 181 (34) | 325 (32) | 0.36 |
| Axillary node dissection | 369 (66) | 738 (68) | |
| Chemotherapy | 401 (68) | 792 (68) | 0.93 |
| Endocrine therapy | 411 (73) | 751 (68) | 0.08 |
| Radiotherapy | 433 (78) | 783 (73) | 0.03 |
| Trastuzumab | 57 (10) | 121 (11) | 0.56 |
Nottingham histologic score system; BMI: body mass index; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; NEO: neoadjuvant. Bold type indicates statistical significance (chi-squared test for categorical variables and Kuskall-Wallis test for continuous variables).
Association between BMI, NLR, and PLR
The median pre-treatment NLR and PLR were 1.93 and 122.6 for obese patients and 1.99 and 129.3 for non-obese patients, respectively. ROC curves did not identify an ideal cut-off point for NLR and PLR (AUC=0.53 and 0.51, respectively). Hence, we decided to use NLR>4 (NLRhigh) and PLR >150 (PLRhigh) as cut-off, since these values demonstrated statistical significance and were chosen in previously published studies (22,23).
On univariate analysis, NLR greater than 4 had an impact on DFS, BCSS, and OS only in obese patients, while a higher PLR showed an influence on DFS and BCSS, but not on OS in obese. Non-obese patients were not impacted by high NLR or PLR (Supplementary Table S1).
Recurrence and survival analysis
The median follow-up time was 6.7 years in obese patients and 6.9 years in non-obese patients (P=0.91). During the study period, 482 patients experienced a locoregional or distant recurrences; the proportion was 26.3% in non-obese patients and 26.5% in obese patients (P=0.93). In the total population, there were 409 deaths due to breast cancer, and 531 deaths due to all causes, with no differences between the two groups (P=0.75) (Supplementary Table S2).
On performing multivariate analysis, including BMI and all variables with statistical significance on univariate analysis, BMI was not found to be a significant predictor of cancer recurrence, BCSS, and OS. The main independent predictors of worse outcomes were grade, stage III, HER2, TN subtypes, and NLRhigh. The PLR was not statistically significant on multivariate analysis and was not considered an independent predictor of worse outcomes (Supplementary Table S3). When patients were stratified by menopausal status, we found that BMI was not a predictor of more aggressive tumors or worse outcomes in the population of postmenopausal women on multivariate analysis (Supplementary Table S4).
Kaplan-Meier curves with log-rank tests for recurrence and overall survival at ten years showed that BMI did not influence outcomes in the total population (Figure 2A and B). However, when stratified by stage, we observed a negative impact of obesity only in patients with stage I disease, where it was associated with worse OS (log-rank test P=0.0048) and slightly shorter, but not significant, DFS (log-rank test P=0.076) (Figure 2C and D). In more advanced breast cancer stages, we did not find any significant correlation. Additionally, Figure 3A and B showed recurrence and survival curves of NLRhigh compared to NLRlow in total population (log-rank P<0.001).
Figure 2. Survival analysis of stage I to III breast cancer patients, according to the body mass index (BMI). A, Disease-free survival in all patients; B, overall survival in all patients; C, disease-free survival in stage I patients at diagnosis; D, overall survival in stage I patients at diagnosis.
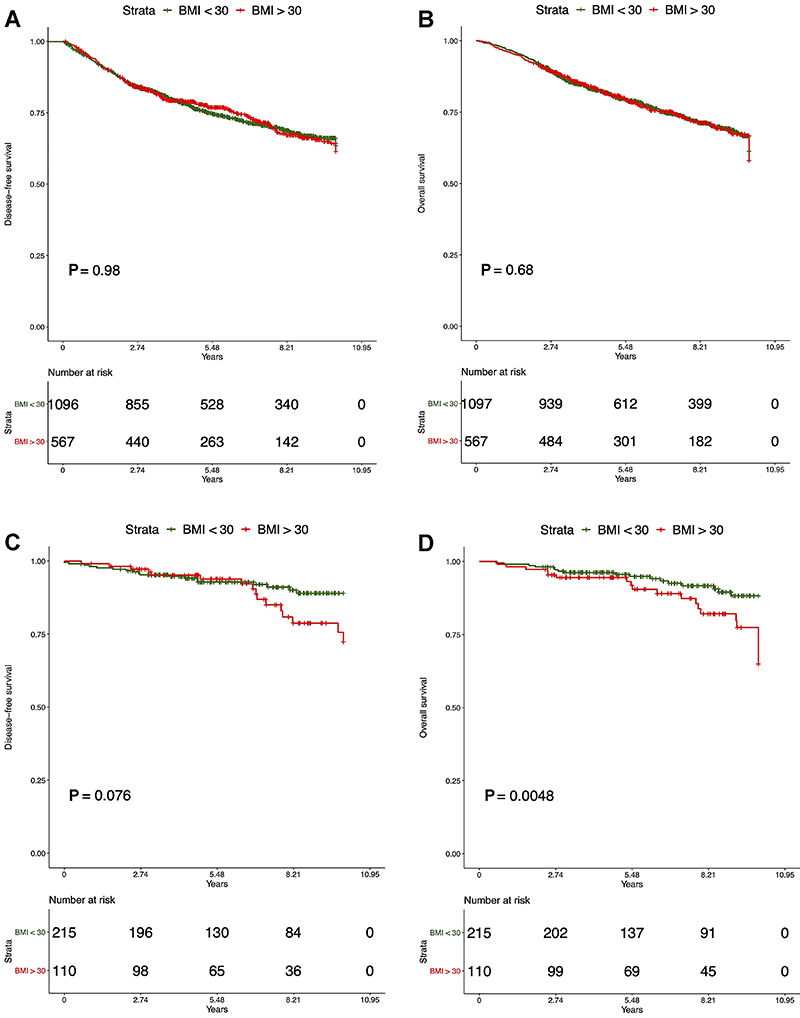
Figure 3. Survival analysis of stage I to III breast cancer patients, according to the neutrophil-to-lymphocyte ratio (NLR). A, Disease-free survival; B, overall survival. Curves (C and D) show disease-free survival and overall-survival for combined groups of NLR and body mass index (BMI), respectively. The black line indicates the patients with both NLRhigh (>4) and BMIhigh (≥30 kg/m2), who had the worst outcomes.

On examining the correlation of NLR and BMI with the outcomes, we observed that a subgroup of breast cancer patients with NLRhigh and BMI ≥30 kg/m2 (BMIhigh) had a significantly lower DFS (P=0.046, log-rank) and OS (P=0.006, log-rank) compared to the other groups (Figure 3C and D).
Discussion
In this study, we explored the association between BMI and inflammatory biomarkers in a large cohort of patients with invasive breast cancer. Here we showed that patients with non-metastatic breast cancer bearing NLRhigh and BMIhigh had worse outcomes. NLRhigh status at diagnosis was observed to be an independent prognostic factor associated with a shorter DFS and worse survival, particularly in the subset of patients with a NLRhigh and obesity.
Some recent meta-analyses have evaluated the association between obesity and breast cancer. Chen et al. (24) analyzed 31 studies with more than three million subjects and described an increased risk of postmenopausal breast cancer among obese women (RR: 1.33 (95%CI: 1.20-1.48). Chan et al. (6) evaluated four BMI categories among 213,075 patients and concluded that the relative risk in obese versus non-obese patients was 1.41 (95%CI: 1.29-1.53) for all-cause mortality and 1.35 (95%CI: 1.24-1.47) for BCSS. Protani et al. (25) pooled 43 studies and showed that obese patients had worse outcomes than non-obese patients, with a hazard ratio (HR) of 1.33 (95%CI: 1.21-1.47) for OS and 1.33 (95%CI: 1.19-1.50) for BCSS.
These meta-analyses were based mostly on studies conducted in high-income countries with established and structured screening programs and widespread access to healthcare services, as demonstrated by the fact that up to 80% of these patients were diagnosed in early-stages of breast cancer (26).
To our knowledge, our study is the first to explore the association between BMI and inflammatory biomarkers in the prognosis of Brazilian breast cancer patients. In Brazil, where more than 20% of the women are considered to be obese (27), approximately 40% of breast cancer cases are diagnosed at locally advanced or metastatic stages (28). This seems to be related to the absence of a well-structured population-based screening program as well as difficulties in accessing healthcare services (29). Our findings were also consistent with a previous study by Moore et al. (30) that found that obesity had a negative impact only in early-stage breast cancer patients. This suggests that obesity has a negative impact on a group of women with favorable prognostic features, since the modest effect of obesity may be mitigated by the worse prognosis and the amount of therapies used in the more advanced stages (7,30).
We did find larger tumors among the obese patients, which may be due to the fact that obese women often have larger breasts with less palpable masses (31). These women may also be less likely to participate in breast cancer screening programs, due to low self-esteem and poor body image (32). In terms of age and menopause, it is well known that women tend to gain weight after menopause, and that the breast cancer risk increases with age (33).
In this study, it was observed that PLR, despite having a negative association with recurrence and BCSS, was not an independent prognostic factor in multivariate analysis. This finding was in agreement with a previous study by Azab et al. (34) that demonstrated that NLR was superior to PLR as a prognostic factor for worse outcomes in breast cancer. We did not find an association between these biomarkers and breast cancer subtypes, although three recent meta-analyses have demonstrated that these biomarkers are more commonly associated with HER2 and TN breast cancers (11,12,35). Although there is increasing evidence that these ratios, when obtained prior to treatment, can act as prognostic biomarkers of breast cancer, the cut-off values have not yet been established. In a recent meta-analysis with 8,563 breast cancer patients, it was demonstrated that a higher NLR was associated with worse OS (HR: 2.56; 95%CI: 1.96-3.35; P<0.001) and DFS (HR: 1.74; 95%CI: 1.47-2.07; P<0.001). The cut-off values for a high NLR ranged from 1.9 to 5.0 in the 15 studies included (36). In line with our study, some previous studies have used a cut-off value of 4.0 for NLR (23,36). Similarly, several cut-off values have been used for PLR, but no value has been established (12,15).
Some previous studies have suggested that despite the increase in the number of both neutrophils and lymphocytes with weight gain, the NLR remains stable regardless of the BMI category, and could be less influenced by other physiological and pathological factors (34,37,38).
In our study, we found that a subset of patients with a high NLR and high BMI had the shortest DFS and worse survival. These findings may be explained by the obesity-associated inflammation and its effects on the immune response. A recent review described the mechanisms whereby chronic adipose tissue inflammation, with altered levels of adipokines and upregulation of cytokines (IL-1β, IL-6, and TNF-α), plays an important role in breast cancer prognosis (10). In addition, obese women are known to have more frequent surgical complications, chemotherapy under-dosing, and more baseline comorbidities (9).
This study has some limitations. Although BMI is typically used in retrospective studies, it may not be ideal to measure obesity in elderly women, who are the primary age group at risk of breast cancer. This is because with menopause and advancing age, women lose height, bone mass, and muscle mass, as well as accumulate visceral fat (39), all factors that are not measured by BMI. Moreover, studies have shown that sarcopenia and muscle mass can be underestimated by BMI and can be important predictors of breast cancer mortality, even in the setting of a normal BMI (40). Another limitation is that we could not evaluate the lifetime use of hormonal therapy because these data were not available. In addition, due to the inherent limitations of observational studies, we were unable to explore potentially interesting factors such as lifestyle, abdominal and hip circumference, physical activity, smoking status, and diet. Further studies are required to explore the role of obesity in breast cancer with more accurate measures of body fat and body mass. Thus, prospective studies are needed to validate standard cut-off values defining high PLR and NLR.
In conclusion, patients with non-metastatic breast cancer bearing NLRhigh and BMIhigh had worse outcomes, and this might be explained by the obesity-associated inflammation in adipose tissue and its effects on the immune system. NLRhigh, but not PLRhigh, was an independent prognostic factor for worse breast cancer recurrence and survival, particularly, in a subgroup of patients with high NLR and obesity. This study highlights the importance of lifestyle measures and the immune system interference with outcomes in the early breast cancer setting.
Acknowledgments
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)-Programa de Excelência Acadêmica (PROEX), Brasil, for financial support and Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA) for funding the publication charges.
Supplementary Material.
Click here to view [pdf].
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piãeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Estimate/2020-Cancer Incidence in Brazil/Instituto Nacional de Câncer José Alencar Gomes da Silva . Rio de Janeiro: INCA; 2019. [Google Scholar]
- 3.De Mattos-Arruda L, Shen R, Reis-Filho JS, Cortés J. Translating neoadjuvant therapy into survival benefits: one size does not fit all. Nat Rev Clin Oncol. 2016;13:566–579. doi: 10.1038/nrclinonc.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray JM, Rasanayagam S, Engel C, Rizzo J. State of the evidence 2017: an update on the connection between breast cancer and the environment. Environ Health. 2017;16:94. doi: 10.1186/s12940-017-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34:4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 8.Simpson ER, Brown KA. Minireview: obesity and breast cancer: a tale of inflammation and dysregulated metabolism. Mol Endocrinol. 2013;27:715–725. doi: 10.1210/me.2013-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argolo DF, Hudis CA, Iyengar NM. The impact of obesity on breast cancer. Curr Oncol Rep. 2018;20:47. doi: 10.1007/s11912-018-0688-8. [DOI] [PubMed] [Google Scholar]
- 10.Kolb R, Zhang W. Obesity and breast cancer: a case of inflamed adipose tissue. Cancers. 2020;12:1686. doi: 10.3390/cancers12061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019;8:4135–4148. doi: 10.1002/cam4.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Si W, Sun Q, Qin B, Zhao W, Yang J. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget. 2017;8:1023–1030. doi: 10.18632/oncotarget.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinger MHF, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 15.Koh CH, Bhoo-Pathy N, Ng KL, Jabir RS, Tan GH, See MH, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150–158. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbeau I, Jacot W, Guiu S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: a systematic review. Cancers. 2020;12:958. doi: 10.3390/cancers12040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hachul H, Polesel DN, Nozoe KT, Sanchez ZM, Prado MCO, Andersen ML, et al. The age of menopause and their associated factors: a cross-sectional population-based study. J Women's Heal Care. 2016;5:5. [Google Scholar]
- 18.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 19.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene FL. In: AJCC cancer staging manual. Edge SB, editor. New York: Springer; 2010. [Google Scholar]
- 21.Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, et al. Platelet-lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PLoS One. 2016;11:e0153459. doi: 10.1371/journal.pone.0153459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dirican A, Kucukzeybek BB, Alacacioglu A, Kucukzeybek Y, Erten C, Varol U, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol. 2015;20:70–81. doi: 10.1007/s10147-014-0672-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Liu L, Zhou Q, Imam MU, Cai J, Wang Y, et al. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: a dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health. 2017;17:936. doi: 10.1186/s12889-017-4953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313:165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 27.Vigitel Brazil 2018: surveillance of risk and protective factors for chronic diseases by telephone survey: estimates of frequency and sociodemographic distribution of risk and protective factors for chronic diseases in the capitals of the 26 Brazilian sta . Brasília Ministério da Saúde. 2019. [Google Scholar]
- 28.Abrahão KDS, Bergmann A, Aguiar SS De, Thuler LCS. Determinants of advanced stage presentation of breast cancer in 87,969 Brazilian women. Maturitas. 2015;82:365–370. doi: 10.1016/j.maturitas.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Dos-Santos-Silva I, De Stavola BL, Renna NL, Nogueira MC, Aquino EML, Bustamante-Teixeira MT, et al. Ethnoracial and social trends in breast cancer staging at diagnosis in Brazil, 2001-14: a case only analysis. Lancet Glob Heal. 2019;7:e784–e797. doi: 10.1016/S2214-109X(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore AH, Trentham-Dietz A, Burns M, Gangnon RE, Greenberg CC, Vanness DJ, et al. Obesity and mortality after locoregional breast cancer diagnosis. Breast Cancer Res Treat. 2018;172:647–657. doi: 10.1007/s10549-018-4932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui Y, Whiteman MK, Flaws JA, Langenberg P, Tkaczuk KH, Bush TL. Body mass and stage of breast cancer at diagnosis. Int J Cancer. 2002;98:279–283. doi: 10.1002/ijc.10209. [DOI] [PubMed] [Google Scholar]
- 32.Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21:41. doi: 10.1007/s11912-019-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P. Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric. 2012;15:419–429. doi: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- 34.Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30:432. doi: 10.1007/s12032-012-0432-4. [DOI] [PubMed] [Google Scholar]
- 35.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 37.Furuncuoǧlu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016;20:1300–1306. [PubMed] [Google Scholar]
- 38.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 39.Banack HR, Wactawski-Wende J, Hovey KM, Stokes A. Is BMI a valid measure of obesity in postmenopausal women? Menopause. 2018;25:307–313. doi: 10.1097/GME.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]


