ABSTRACT
Diagnosis of latent tuberculosis infection (LTBI) is considered key in the control of tuberculosis. Interferon gamma (IFN-γ) release assays, such as the QuantiFERON-TB Gold Plus test (QFT-Plus), are now widely implemented for the in vitro diagnosis of LTBI. To date, the detection and quantification of IFN-γ has been mostly performed with semiautomated enzyme-linked immunosorbent assays (ELISAs), but several limitations currently exist. The study aims to evaluate the chemiluminescence immunoassay (CLIA) analyzer Liaison XL compared to ELISA for the performance of the QFT-Plus test. Between February and April 2020, 333 heparin blood samples from 323 adult patients were collected at a tertiary teaching hospital in Barcelona, Spain. Overall, the CLIA analyzer Liaison XL performed well for the detection of IFN-γ compared to the ELISA method, demonstrating substantial agreement (κ, 0.872) and great correlation between assays (r, >0.950). CLIA produced significantly higher values of IFN-γ IU per milliliter than the ELISA (P = 0.004 for the TB1 tube and P = 0.010 for the TB2 tube). Many discrepant cases (8/15, 53.3%) corresponded to indeterminate results with ELISA (NIL-corrected mitogen value of <0.5 IU/ml), which, when analyzed with the CLIA analyzer Liaison XL, reverted to interpretable results. In conclusion, this analysis suggests that CLIA presents a greater sensitivity for the identification of LTBI, especially among immunocompromised patients. Furthermore, the analytical variability reported between both ELISA and CLIA methods, especially around the standardized 0.35-IU/ml positivity threshold, suggests the need to refine the interpretative algorithm.
KEYWORDS: tuberculosis, QuantiFERON-TB, chemiluminescence, Liaison
INTRODUCTION
The identification and treatment of latent tuberculosis infection (LTBI) in individuals at increased risk for the development of active disease is considered key in the control of tuberculosis in low-incidence tuberculosis settings (1, 2). The tuberculin skin test (TST) traditionally has been used for the detection of LTBI cases, but several important limitations currently exist (1). For that reason, interferon gamma (IFN-γ) release assays (IGRAs), such as the QuantiFERON-TB Gold Plus test (QFT-Plus; Qiagen, Hilden, Germany), are now widely implemented in clinical laboratories for the in vitro diagnosis of LTBI (3).
The QFT-Plus is based on the detection of IFN-γ released by T-cell-mediated immune response following in vitro stimulation of human whole blood by antigens specific to the Mycobacterium tuberculosis complex (4). These antigenic peptides contained in TB1 and TB2 QFT-Plus tubes are designed to preferably elicit a CD4 response and CD4 and CD8 T-cell responses, respectively, which presumably improve decision-making in LTBI treatment (5, 6). To date, the detection and quantification of IFN-γ has been performed mostly with semiautomated enzyme-linked immunosorbent assays (ELISAs). However, ELISAs require well-trained laboratory technicians, and they are not fully optimized regarding the use of controls and calibrators. Recently, new chemiluminescence immunoassays (CLIAs) have been developed for the implementation of QFT-Plus test in laboratory routines (7–10), such as the novel CLIA system adapted on the fully automated Liaison XL analyzer (DiaSorin, Saluggia, Italy) (8–10).
In this study, we evaluate the clinical and analytical performance of the CLIA analyzer Liaison XL compared to ELISA for the detection of IFN-γ in the diagnosis of LTBI in a low-incidence tuberculosis setting. Furthermore, a comprehensive analysis of discrepant results between both methods was conducted to clinically assess the relevance of these cases in the management of LTBI.
MATERIALS AND METHODS
Sample selection and laboratory procedures.
Between February and April 2020, 333 heparin blood samples from 323 adult patients were collected for the routine performance of the QFT-Plus test at the Bellvitge University Hospital, a tertiary teaching hospital in L´Hospitalet de Llobregat, Barcelona, Spain. The study period corresponded to the onset of the COVID-19 pandemic in Spain, and 61 study patients (18.9%) were infected with SARS-CoV-2. Since lymphopenia and other immunological deviations are a key feature in COVID-19 disease (11), the study population may have represented a particular challenge for the performance of the QFT-Plus test.
During the study period, specimens were prospectively and simultaneously analyzed using the QFT-Plus ELISA (Qiagen, Hilden, Germany) on the DS2 automated platform (Dynex, Chantilly, VA) and the Liaison XL instrument. Results were interpreted according to the manufacturer´s criteria, with a positivity threshold established at 0.35 IU/ml. Furthermore, borderline results were those with IFN-γ values within the equivocal range established between 0.20 and 0.70 IU/ml, in line with reports in other low-incidence tuberculosis settings (12, 13).
Ethical approval for the study was obtained from the Bellvitge University Hospital-IDIBELL Ethics Committee (approval number PR083/20).
Statistical analyses.
Statistical analyses were performed using GraphPad Prism version 8.0.1 (GraphPad Software Inc., USA). The Cohen’s kappa statistic (κ) was used to evaluate the agreement between both assays, and 95% confidence intervals (CI) were calculated by exact methods. The Mann-Whitney test was used to assess differences in IFN-γ quantification between methodologies, and differences with a P value of <0.05 were considered statistically significant. Level of correlation was expressed with the Pearson correlation coefficient (r). Since both IFN-γ quantification procedures, ELISA and CLIA, cannot accurately determine IFN-γ values of >10 IU/ml, 10 IU/ml was attributed in these cases by convention as already adopted in other investigations (10, 14).
RESULTS
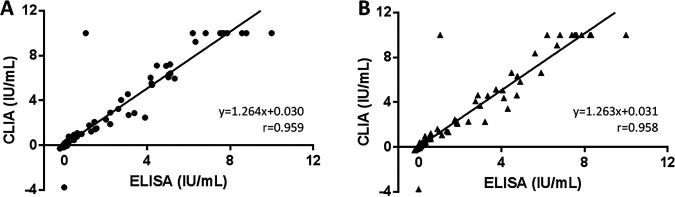
Qualitative results of the 333 study samples are displayed in Table 1. Overall, the level of concordance between both assays was 95.5% (95% CI, 92.7% to 97.5%), with a κ value of 0.872 (0.809 to 0.935). The analytical comparison of IFN-γ levels, related to TB1 and TB2 QFT-Plus tubes, is presented in Fig. 1. Overall, there was substantial correlation between ELISA and CLIA methods for both TB1 and TB2 antigens (r, 0.959 and 0.958, respectively). Furthermore, CLIA yielded significantly higher values of IFN-γ than ELISA in both TB1 (P = 0.004) and TB2 (P = 0.010) tubes, consistent with regression slopes in both cases (1.264 and 1.263, respectively). When cases with IFN-γ levels within the equivocal interval (defined as between 0.20 and 0.70 IU/ml) were analyzed, the correlation obtained between methods was 0.906 for TB1 and 0.890 for TB2 (Fig. 2). CLIA also provided significantly higher values of IFN-γ than ELISA in both TB1 (P = 0.003) and TB2 (P = 0.006) QFT-Plus tubes, again consistent with regression slopes in both cases (1.335 and 1.484, respectively).
TABLE 1.
Qualitative evaluation of the Liaison XL for the performance of the QuantiFERON-TB Gold Plus assay compared to ELISA resultsa
| QFT CLIA result | QFT ELISA result |
Level of concordance [% (95% CI)] | Kappa value (95% CI) | ||
|---|---|---|---|---|---|
| Positive | Indeterminate | Negative | |||
| Positive | 49 | 0 | 6 | 95.5 (92.7–97.5) | 0.872 (0.809–0.935) |
| Indeterminate | 0 | 14 | 1 | ||
| Negative | 0 | 8 | 255 | ||
Abbreviations: CI, confidence interval; CLIA, chemiluminescence immunoassay; ELISA, enzyme-linked immunosorbent assay.
FIG 1.
Quantitative comparison of TB1 (A) and TB2 (B) IFN-γ levels in QuantiFERON-TB Gold Plus tests. Regression lines for the NIL-subtracted antigen tubes are plotted. Abbreviations: IU, international units; CLIA, chemiluminescence immunoassay; ELISA, enzyme-linked immunosorbent assay.
FIG 2.
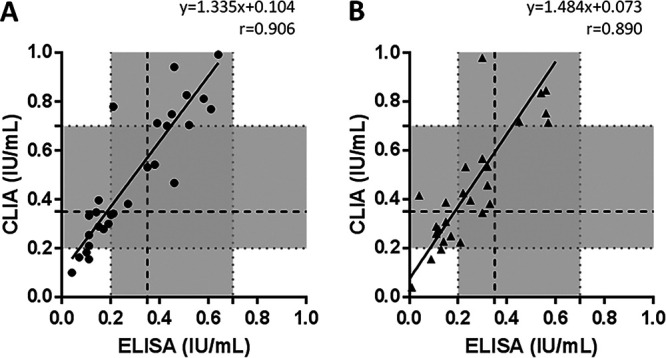
Quantitative comparison of TB1 (A) and TB2 (B) IFN-γ levels in QuantiFERON-TB Gold Plus borderline tests. The area colored in gray corresponds to the equivocal range between 0.20 and 0.70 IU/ml. The dashed line indicates the standardized 0.35-IU/ml positivity cutoff. Regression lines for the NIL-subtracted antigen tubes are plotted. Abbreviations: IU, international units; CLIA, chemiluminescence immunoassay; ELISA, enzyme-linked immunosorbent assay.
A comprehensive analysis of discrepant results is shown in Table 2. Eight of the 15 (53.3%) discrepant results corresponded to indeterminate results with ELISA (NIL-corrected mitogen value, <0.5 IU/ml), which, when analyzed with the CLIA analyzer Liaison XL, reverted to interpretable analyses. Six of these 8 discrepancies (75.0%) occurred in patients with COVID-19 disease. An exhaustive analysis of concordant indeterminate results with both IFN-γ detection assays is also displayed in Table S1 in the supplemental material.
TABLE 2.
Comprehensive analysis of QuantiFERON-TB Gold Plus discrepant casesh
| Case | Age (yr) | Gender | Setting | QFT CLIA (IU/ml) |
QFT ELISA (IU/ml) |
No. of lymphocytes (×109/liter)a | Relevant clinical historyg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resultf | NIL | TB1-NIL | TB2-NIL | MIT-NIL | Resultf | NIL | TB1-NIL | TB2-NIL | MIT-NIL | ||||||
| 1 | 49 | M | Outpatient | P* | 0.03 | 0.10 | 0.42 | 10.00 | N | 0.03 | 0.04 | 0.04 | 8.78 | 2.33 | Tuberculosis contactb |
| 2 | 47 | F | Outpatient | P* | 0.15 | 0.25 | 0.57 | 10.00 | N* | 0.10 | 0.11 | 0.30 | 7.86 | 1.97 | Multiple sclerosis |
| 3 | 56 | F | HCW | I | 9.07 | 10.00 | 10.00 | 10.00 | N | 7.58 | 1.03 | 1.03 | 1.03 | ND | Nonec |
| 4 | 54 | M | Hospitalized | N | 0.01 | 0.10 | 0.16 | 0.55 | I | 0.03 | 0.00 | 0.00 | 0.36 | 1.17 | Severe COVID-19** |
| 5 | 70 | M | Hospitalized | N | 0.02 | −0.01 | −0.01 | 0.63 | I | 0.06 | −0.04 | −0.04 | 0.31 | 0.32 | Severe COVID-19** |
| 6 | 81 | F | Hospitalized | N | 0.05 | −0.00 | −0.00 | 1.09 | I | 0.03 | 0.00 | 0.00 | 0.43 | 0.53 | Severe COVID-19** |
| 7 | 47 | F | Hospitalized | N | 0.00 | 0.00 | 0.11 | 0.56 | I | 0.03 | −0.01 | 0.00 | 0.28 | 0.38 | Severe COVID-19** and history of kidney transplant |
| 8 | 23 | F | Hospitalized | P | 0.24 | 0.78 | 0.98 | 10.00 | N* | 0.08 | 0.21 | 0.30 | 6.22 | 1.13 | Tuberculous pleurisyd |
| 9 | 76 | M | Hospitalized | P* | 0.01 | 0.30 | 0.39 | 1.24 | N | 0.02 | 0.19 | 0.15 | 0.68 | 2.06 | Severe COVID-19** |
| 10 | 73 | M | Hospitalized | N | 0.03 | 0.00 | 0.01 | 0.56 | I | 0.03 | −0.01 | −0.01 | 0.49 | ND | Severe COVID-19** |
| 11 | 64 | F | Outpatient | N | 0.02 | 0.06 | −0.00 | 0.64 | I | 0.01 | 0.00 | 0.00 | 0.37 | 2.46 | None |
| 12 | 65 | F | Outpatient | P* | 0.10 | 0.40 | 0.53 | 10.00 | N* | 0.03 | 0.15 | 0.23 | 7.57 | ND | Psoriasis, tuberculosis contact |
| 13 | 42 | M | Hospitalized | N | 0.00 | 0.01 | 0.01 | 0.78 | I | 0.01 | 0.00 | 0.00 | 0.47 | 0.84 | Severe COVID-19** |
| 14 | 56 | F | Hospitalized | N | 0.02 | 0.02 | 0.02 | 0.50 | I | 0.03 | 0.00 | 0.00 | 0.13 | ND | Non-COVID-19-related pneumonia, SLEe |
| 15 | 53 | F | HCW | P* | 0.04 | 0.38 | 0.20 | 10.00 | N* | 0.04 | 0.27 | 0.13 | 10.00 | 1.66 | None |
Lymphocyte count was determined at the time of the QuantiFERON-TB Gold Plus test.
Individual under treatment with Rifinah, following prior diagnosis of latent tuberculous infection.
Individual with history of diagnosis of latent tuberculous infection.
Patient under a 7-day treatment with Rimstar, following prior diagnosis of active tuberculosis.
Chronic disease patient under treatment with immunosuppressive drugs. Additionally, the individual had a history of conversion (defined as having a positive test that followed a negative one) by QuantiFERON-TB Gold Plus test.
*, borderline results (equivocal range between 0.20 and 0.70 IU/ml).
**, most severe COVID-19 patients were under treatment with immunosuppressive drugs (corticosteroids, tocilizumab, anakinra, etc.) at the time of the QuantiFERON-TB Gold Plus test.
Abbreviations: IU, international units; CLIA, chemiluminescence immunoassay; ELISA, enzyme-linked immunosorbent assay; MIT, mitogen; M, male; F, female; HCW, health care worker; P, positive; N, negative; I, indeterminate; ND, not determined; SLE, systemic lupus erythematosus.
On the other hand, one case (number 8) revealed a relevant disagreement between both IGRA methods in a female patient with tuberculous pleurisy, being positive with CLIA and negative, but borderline, with ELISA. Additionally, case number 3 presented an important discrepancy, with an indeterminate result with CLIA caused by IFN-γ values of >8.00 IU/ml in the NIL tube (9.07 IU/ml) but interpretable (negative) with ELISA (NIL value of 7.58 IU/ml).
DISCUSSION
The CLIA analyzer Liaison XL performed well for the detection of IFN-γ in QFT-Plus test compared to the ELISA method, with substantial agreement between assays (κ, 0.872). Furthermore, the quantitative analytical comparison indicated substantial correlation between both assays. Nevertheless, as was already been reported (9, 10), CLIA produced significantly higher values of IFN-γ in IU per milliliter than the ELISA. Previously suggested by Bisognin et al. (10), our study confirms that these differences are not a matter of preanalytical variability but rather the intrinsic chemistry beyond the chemiluminescence technology. Overall, this evaluation provides further evidence regarding the comparable performance of chemiluminescence and ELISA for the detection of IFN-γ in the QFT-Plus test (7–10) and, specifically, with the Liaison XL analyzer (9, 10).
To our knowledge, this is the first study in which a detailed clinical and analytical assessment of discrepant results has been conducted. With a low number of discrepancies, most interestingly corresponded to indeterminate results with ELISA that, analyzed with the CLIA analyzer Liaison XL, reverted to interpretable analyses. This indicates that CLIA is a more powerful tool in the diagnosis of LTBI among immunocompromised patients and in individuals with COVID-19 disease. As observed in this series, COVID-19-driven immune disturbances have been suggested to significantly alter IGRA testing into indeterminate results (15). Furthermore, a relevant disagreement between both IGRA methods occurred in a female patient with tuberculous pleurisy, being positive with CLIA and negative, but borderline, with ELISA. In accordance with the analytical results, this finding may represent a greater sensitivity of the Liaison XL for the detection of IFN-γ and the identification of tuberculosis infection. In this regard, in one case an indeterminate result with CLIA reverted to an interpretable negative result with ELISA. This indeterminate CLIA result was caused by an IFN-γ value of 9.07 IU/ml in the NIL tube (of note, results of >8.00 IU/ml should not be interpreted according to the manufacturer´s criteria), which decreased up to 7.58 IU/ml when utilizing the ELISA. This was a matter of preanalytical perturbances, excessive levels of circulating IFN-γ, or the presence of heterophile antibodies. The high IFN-γ levels present in the NIL tube makes “indeterminate” a more conservative and, likely, the most appropriate result in this specific case. Nevertheless, the clinical relevance of these discrepancies must be further elucidated.
The analytical variability of both ELISA and CLIA methods, especially around the standardized 0.35-IU/ml positivity threshold that may lead to important qualitative discrepancies, suggests the need to refine the interpretative algorithm (9, 10). This means adjusting the positivity cutoff, currently established at 0.35 IU/ml according to the manufacturer´s criteria, and utilizing a standardized equivocal range for QFT-Plus results, perhaps also depending on the clinical characteristics of the study population. In the present study, borderline results were considered those with IFN-γ values between 0.20 and 0.70 IU/ml, in line with other authors (12, 13). Whether this range needs to be revised remains controversial (9, 10).
On the other hand, apart from the analytical differences described, the Liaison XL has some technical characteristics that require further discussion. First, the CLIA instrument is fully automated, limiting variability, ensuring reproducibility, and significantly reducing laboratory work. Moreover, since the analyzer does not work in batches (it is based on a continuous-load system), it can be easily and efficiently implemented in routine disregarding of variations in sample flow, which are frequent in clinical laboratories. In addition, the Liaison XL result turnaround time is less than 1 h. Compared with ELISA-based instruments, this CLIA analyzer provides prompt results that are key in the clinical management of tuberculosis-infected individuals. Finally, the optimization in the use of controls and calibrators makes the Liaison XL potentially less expensive than ELISA instruments for the performance of the QFT-Plus assay.
In conclusion, the CLIA analyzer Liaison XL performs well for the detection of IFN-γ in the clinical diagnosis of latent tuberculosis in our low-incidence tuberculosis settings. Furthermore, the greater sensitivity of the Liaison XL for the detection of IFN-γ may decrease the rate of indeterminate results in QFT-Plus tests and, presumably, optimize the diagnosis of LTBI in immunocompromised patients. Nevertheless, this analytical variability of both ELISA and CLIA methods, especially around the standardized 0.35-IU/ml positivity threshold, suggests the need to refine the interpretative algorithm.
ACKNOWLEDGMENTS
DiaSorin, the developer and manufacturer of the analyzer and the chemiluminescence kits, supplied the reagents utilized for this study. DiaSorin did not have any role in the clinical evaluation, including data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no competing interests to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Fernando Alcaide, Email: falcaide@bellvitgehospital.cat.
Christine Y. Turenne, University of Manitoba
REFERENCES
- 1.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. 2014. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2020. Global tuberculosis report 2020. World Health Organization, Geneva, Switzerland. https://www.who.int/teams/global-tuberculosis-programme/tb-reports. [Google Scholar]
- 3.Sotgiu G, Saderi L, Petruccioli E, Aliberti S, Piana A, Petrone L, Goletti D. 2019. QuantiFERON TB Gold Plus for the diagnosis of tuberculosis: a systematic review and meta-analysis. J Infect 79:444–453. doi: 10.1016/j.jinf.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Qiagen. 2014. QuantiFERON-TB Gold Plus ELISA package insert. https://www.quantiferon.com/products/quantiferon-tb-gold-plus-qft-plus/package-inserts/.
- 5.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, Dal Monte P, Di Serio C, Goletti D, Lombardi G, Lipman M, Rancoita PM, Tadolini M, Cirillo DM. 2016. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 47:1587–1590. doi: 10.1183/13993003.02033-2015. [DOI] [PubMed] [Google Scholar]
- 6.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2016. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 73:588–597. doi: 10.1016/j.jinf.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Kim JJ, Park Y, Choi D, Kim HS. 2020. Performance evaluation of a new automated chemiluminescent immunoanalyzer-based interferon-gamma releasing assay AdvanSure I3 in comparison with the QuantiFERON-TB-Gold in-tube assay. Ann Lab Med 40:33–39. doi: 10.3343/alm.2020.40.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brantestig S, Kinnunen A, Almeflo S, Restorp K, Ahlqvist J, Dyrdak R. 2020. Comparative evaluation of CLIA and EIA for Quantiferon-TB Gold Plus. APMIS 128:343–349. doi: 10.1111/apm.13025. [DOI] [PubMed] [Google Scholar]
- 9.De Maertelaere E, Vandendriessche S, Verhasselt B, Coorevits L, André E, Padalko E, Boelens J. 2020. Evaluation of QuantiFERON-TB Gold Plus on Liaison XL in a low-tuberculosis-incidence setting. J Clin Microbiol 58:e00159-20. doi: 10.1128/JCM.00159-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisognin F, Lombardi G, Re MC, Dal Monte P. 2020. QuantiFERON-TB Gold Plus with chemiluminescence immunoassay: do we need a higher cutoff? J Clin Microbiol 58:e00780-20. doi: 10.1128/JCM.00780-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. 2020. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres Costa J, Silva R, Sá R, Cardoso MJ, Nienhaus A. 2011. Serial testing with the interferon-γ release assay in Portuguese healthcare workers. Int Arch Occup Environ Health 84:461–469. doi: 10.1007/s00420-010-0571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J, Kumar K, Reading J, Harvey J, Murthy S, Capocci S, Hopkins S, Seneviratne S, Cropley I, Lipman M. 2017. Frequency and significance of indeterminate and borderline Quantiferon Gold TB IGRA results. Eur Respir J 50:1701267. doi: 10.1183/13993003.01267-2017. [DOI] [PubMed] [Google Scholar]
- 14.Lombardi G, Petrucci R, Corsini I, Bacchi Reggiani ML, Visciotti F, Bernardi F, Landini MP, Cazzato S, Dal Monte P. 2017. Quantitative analysis of interferon-γ release assay response in children with latent and active tuberculosis. J Clin Microbiol 56:e01360-17. doi: 10.1128/JCM.01360-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torre A, Aliberti S, Castellotti PF, Cirillo DM, Grisolia A, Mangioni D, Marchetti G, Rossotti R, Santus P, Besozzi G, Villa S, Codecasa LR, Bandera A, Blasi F, Campisi D, Ferrarese M, Gramegna A, Lombardi A, Mancon A, Mantero M, Muscatello A, Passerini M, Piscaglia M, Saporiti M, Schiuma M, Milan TB-COVID-19 Study Group. 2020. Preliminary observations on IGRA testing for TB infection in patients with severe COVID-19 eligible for immunosuppressive therapy. Respir Med 175:106204. doi: 10.1016/j.rmed.2020.106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download JCM.00603-21-s0001.pdf, PDF file, 214 KB (213.6KB, pdf)