LETTER
Enzyme-mediated antibiotic inactivation is a common mechanism of drug resistance in bacteria (1). The genes encoding these resistance proteins are frequently associated with mobile elements, making them particularly concerning due to their propensity for horizontal transfer through bacterial populations. Given the specificity of enzymes for their substrates, which has often coevolved with antibiotic production over millennia (2), it is not surprising that natural-product antibiotics and their semisynthetic derivatives are particularly vulnerable to enzymatic inactivation. Various hydrolases (β-lactams), kinases (aminoglycosides and macrolides), nucleotidyltransferases (lincosamides and aminoglycosides), acyltransferases (chloramphenicol and aminoglycosides), oxygenases (tetracyclines and rifamycins), and many others are known. In contrast, enzyme-mediated inactivation of synthetic antibiotics is rare. Examples include monooxygenase-mediated inactivation of sulfonamides by soil bacteria (3) and coopting of an aminoglycoside acetyltransferase, AAC(6′)-Ib-cr, for the modification of fluoroquinolone antibiotics containing a piperazine moiety with a free secondary amine, such as ciprofloxacin (CIP) and norfloxacin (4, 5).
The 2018 report of a novel ciprofloxacin-inactivating enzyme, CrpP, encoded by a gene located on plasmid pUM505 in Pseudomonas aeruginosa (6), is therefore highly unusual. The crpP gene is predicted to encode a small, 65-amino-acid protein sharing sequence similarity with a short region of an aminoglycoside phosphotransferase (APH) from Mycobacterium smegmatis and an APH(3′)-IIb from P. aeruginosa M18. CrpP possesses Gly7 and Ile26, conserved residues in APH enzymes with roles in catalysis and ATP binding, respectively. This similarity prompted the hypothesis that CrpP was a fluoroquinolone phosphotransferase. Purification of the protein followed by enzyme assays using the canonical coupled pyruvate kinase/lactate dehydrogenase assay for ADP release showed concentration-dependent saturable activity with ciprofloxacin and ATP, consistent with enzyme activity. Mass spectrometry of CrpP-inactivated ciprofloxacin identified many signals interpreted as being the initial formation of a ciprofloxacin-ATP adduct (covalently linked through the carboxyl of ciprofloxacin and the γ-phosphate of ATP). The plasmid pUM505 harboring crpP confers a 2-fold (levofloxacin) to 8-fold (moxifloxacin) increase in the fluoroquinolone MIC in P. aeruginosa PAO1 compared to P. aeruginosa ATCC 27853. The MICs for ciprofloxacin and norfloxacin were increased 4-fold (0.5 to 2.0 μg/ml). Paradoxically, the expression of crpP on the high-copy-number plasmid pUCP20 in P. aeruginosa PAO1 had either no effect (ciprofloxacin and norfloxacin) or a 2-fold increase (levofloxacin and moxifloxacin) in the MIC compared to P. aeruginosa ATCC 27853. The expression of pUC:crpP in Escherichia coli did not affect the MICs of some fluoroquinolones (norfloxacin, levofloxacin, and moxifloxacin) and had a modest 2-fold effect (0.004 μg/ml increased to 0.008 μg/ml) for ciprofloxacin.
We were intrigued by this discovery given the rarity of enzymes inactivating synthetic antibiotics, especially in clinical strains. We did, however, have questions about the initial hypothesis. The region of protein similarity between CrpP and the M. smegmatis kinase shown by those authors is in a part of APH enzymes that has some minor overlap with the Mg2+ binding site but is not directly involved in catalysis or ATP binding. Those authors also noted that CrpP shows similarity to a 79-amino-acid APH(3)-IIb from P. aeruginosa M18 (7). However, this protein (GenBank accession no. AEO73307.1) lacks the requisite amino acid residues for phosphotransfer (8) and is likely a pseudogene. Indeed, the authors of the P. aeruginosa M18 genome sequence report noted that the isolate was more susceptible to kanamycin than other strains (7). A second APH in this genome (GenBank accession no. AEO75667.1) shows homology to the APH(3′) streptomycin/spectinomycin kinase family, has the necessary active-site residues for kinase activity, and is likely responsible for the spectinomycin resistance phenotype of P. aeruginosa M18. The similarity of CrpP with APHs is therefore tenuous.
Similarly, the mass spectrometry results in Fig. 4 of the original CrpP publication (6) are challenging to interpret. The transfer of a phosphate from ATP to antibiotics or other substrates such as proteins generally occurs through nucleophilic attack of the phosphate acceptor on the γ-phosphate (or occasionally on the β-phosphate via the transfer of pyrophosphate followed by hydrolysis to generate the monophosphorylated product [8]). The mass spectrum interpretation reported a chemically unprecedented ciprofloxacin-ATP adduct linked by the carboxyl of the antibiotic and the γ-phosphate of ATP, which is highly unlikely. Those authors suggest that this adduct decomposes to the ciprofloxacin acyl-phosphate. Again, this compound is predicted to be highly chemically unstable and may not be detectable in the mass spectrum. Indeed, we have attempted to synthesize this molecule, even under anhydrous conditions, without success.
Since the discovery of CrpP, Chavez-Jacobo et al. have delved deeper into understanding this novel resistance element. In 2018, they began by searching for homologous crpP genes in extended-spectrum-β-lactamase (ESBL)-producing transconjugants and clinical isolates of Enterobacteriaceae in Mexican hospitals (12). Sequence analysis of the homologs revealed that crpP-like genes contain the conserved residues Gly7, Thr8, Asp9, Lys33, Gly34, and Cys40. Mutagenesis experiments later confirmed that Gly7, Asp9, and Lys33 are involved in ATP binding in the N-terminal region and that Cys40 is a CrpP-specific conserved C-terminal residue essential for activity (6). Comparatively, Ortiz de la Rosa et al. (9) identified four CrpP homologs (CrpP2, CrpP3, CrpP4, and CrpP5) across P. aeruginosa clinical isolates from Europe, and sequence analysis revealed that each of these proteins has a missense mutation in the previously reported “essential catalytic residue” Gly7. To measure the activity of these homologs compared to CrpP, each gene was cloned into the shuttle vector pUCp24 and transformed into Escherichia coli TOP10. All homologs were found to modestly decrease ciprofloxacin susceptibility except for CrpP1 (the original CrpP), which had no activity against the drug (9). No such effect was detected in P. aeruginosa PAO1. These conflicting results suggest that Gly7 is, in fact, nonessential for enzymatic activity and that CrpP may utilize a different mechanism of action than previously reported. To further complicate the CrpP literature, Hernandez-Garcia et al. (10) recently reported two additional homologs (CrpP6 and CrpP7) in P. aeruginosa clinical isolates from Spain and Portugal. Susceptibility assays against a number of clinical isolates expressing the various crpP genes determined that CrpP7 is the only homolog associated with a decrease in ciprofloxacin susceptibility. It was hypothesized that this result reflected the presence of a missense mutation in the catalytic residue Gly7 in CrpP2 to CrpP6 that is absent in CrpP and CrpP7. Those authors concluded that the presence of crpP genes is not always associated with ciprofloxacin resistance, but the central hypothesis that CrpP is a ciprofloxacin kinase remains unchallenged.
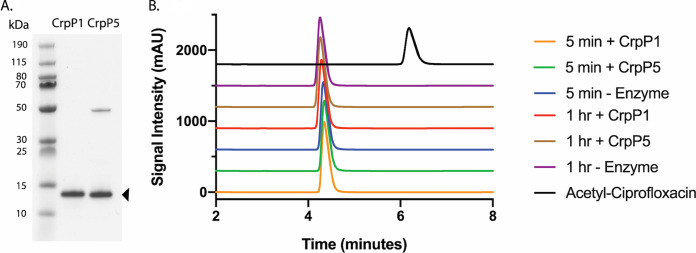
Given the conflicting results and questions associated with these reports, we revisited the analysis of CrpP. We synthesized the original gene for expression in E. coli. Several cloning attempts into our standard pGDP vectors (constitutive lac and bla promoters) used to express resistance genes (11) resulted in the selection for crpP alleles with various mutations, suggesting that the gene product may be toxic. We successfully overexpressed the protein in E. coli BL21(DE3)/pET28a:crpP to suppress expression in the absence of isopropyl-β-d-thiogalactopyranoside (IPTG). We measured the susceptibility to ciprofloxacin, levofloxacin, norfloxacin, and moxifloxacin in the presence of 0.5 mM IPTG by broth microdilution studies but could not detect resistance (Table 1). We also measured ciprofloxacin resistance by an Etest, with the same result (not shown). We next overexpressed the protein for purification using E. coli BL21(DE3)/pET28a:crpP. This construct generated a soluble His6-tagged protein (Fig. 1A). Using the purified sample, we attempted to measure kinase activity using the coupled pyruvate kinase/lactate dehydrogenase ADP release assay. However, we could not detect ADP release activity (the highest concentrations tested were 2 mM ATP, 0.1 mM ciprofloxacin, and 5 μg/ml CrpP). We then established a high-performance liquid chromatography (HPLC) assay to measure any phosphorylation of ciprofloxacin catalyzed by CrpP (2 mM ATP, 1 mM ciprofloxacin, and 5 μg/ml CrpP) (the HPLC limit of detection for CIP is 1.6 ng, with ∼1,655 ng injected per sample). Incubations over 5 to 60 min showed no change in the ciprofloxacin peak (Fig. 1B). High-resolution mass spectrometry analysis of the reaction mixture showed no evidence of ciprofloxacin modification (the limit of detection for CIP is 1.5 ng, with ∼165.5 ng injected per sample) (Fig. 2). Due to the unavailability of standard phosphorylated ciprofloxacin, acetyl-ciprofloxacin, the product of AAC(6′)-Ib-cr, was used as a positive control to demonstrate the ability of our HPLC method to detect inactive modified fluoroquinolones. To address the activity of CrpP homologs, we overexpressed and purified CrpP5 using E. coli BL21(DE3)/pET28a:crpP5 (Fig. 1A). The activity of CrpP5 was measured by HPLC under the same conditions as the ones described above for CrpP, and the results showed that CrpP5 does not modify ciprofloxacin (Fig. 1B).
TABLE 1.
MICs for E. coli BL21(DE3)/pET28a:crpP and E. coli BL21(DE3)/pET28a in the presence and absence of 0.5 mM IPTG
| Drug and strain | Presence or absence of 0.5 mM IPTG | MIC (μg/ml) |
|---|---|---|
| Moxifloxacin | ||
| E. coli BL21(DE3)/pET28a:crpP | + | 0.0156 |
| E. coli BL21(DE3)/pET28a:crpP | − | 0.0156–0.0312 |
| E. coli BL21(DE3)/pET28a | + | 0.0156 |
| E. coli BL21(DE3)/pET28a | − | 0.0156 |
| Levofloxacin | ||
| E. coli BL21(DE3)/pET28a:crpP | + | 0.0156 |
| E. coli BL21(DE3)/pET28a:crpP | − | 0.0156 |
| E. coli BL21(DE3)/pET28a | + | 0.0156 |
| E. coli BL21(DE3)/pET28a | − | 0.0078 |
| Norfloxacin | ||
| E. coli BL21(DE3)/pET28a:crpP | + | 0.0312 |
| E. coli BL21(DE3)/pET28a:crpP | − | 0.0312 |
| E. coli BL21(DE3)/pET28a | + | 0.0312 |
| E. coli BL21(DE3)/pET28a | − | 0.0312 |
| Ciprofloxacin | ||
| E. coli BL21(DE3)/pET28a:crpP | + | 0.0039 |
| E. coli BL21(DE3)/pET28a:crpP | − | 0.0039–0.0078 |
| E. coli BL21(DE3)/pET28a | + | 0.0039 |
| E. coli BL21(DE3)/pET28a | − | 0.0039–0.0078 |
FIG 1.
Purification and characterization of recombinant CrpP and CrpP5. (A) SDS-polyacrylamide gel of His6-tagged CrpP and CrpP5 purified from E. coli BL21(DE3)/pET28a:crpP and E. coli BL21(DE3)/pET28a:crpP5, respectively, demonstrating the purity of the protein used in these studies. A minor immobilized metal affinity chromatography contaminant is present at ∼50 kDa in the CrpP5 sample. The gel was stained with Coomassie brilliant blue. (B) HPLC trace of ciprofloxacin (1 mM) following incubation with ATP (2 mM) in the presence and absence of purified CrpP or CrpP5 (5 μg/ml). Reaction mixtures were incubated for both 5 min and 1 h. Acetyl-ciprofloxacin was used as a positive control to demonstrate that the HPLC method can detect modified drugs. No modified ciprofloxacin was detected in the enzyme reactions. Signal intensity was measured in milliabsorbance units (mAU).
FIG 2.
High-resolution mass spectrometry analysis of ciprofloxacin (1 mM) following incubation with ATP (2 mM) in the presence of purified CrpP (5 μg/ml) after reacting for 1 h. (A) Positive electrospray ionization (+ESI) spectra at a retention time (Rt) of 2.873 min (the Rt for ciprofloxacin). Ciprofloxacin can be detected as [M + H]+ at m/z 332.1420 and [M + Na]+ at m/z 354.1223. Note that the signal at m/z 288.1506 is a result of losing the carboxyl group during the mass spectrometry analysis and is representative of [M − CO2H + H]+. The protonated form of HEPES buffer is detected at m/z 239.1065. No phosphorylated ciprofloxacin is present (expected m/z 412.10), nor is there evidence of the previously reported ATP-ciprofloxacin adduct (m/z 820.12). * indicates that the signal for [M + H]+ is oversaturated. The reference compound used is HP-0921 (m/z 922.0099). (B) +ESI extracted-ion chromatogram (EIC) at m/z 332.15 for [M + H]+. Note that due to the oversaturated signal, the EIC was obtained for m/z 332.15 rather than m/z 332.14 as seen in panel A. A high-intensity signal with the same Rt as [M + H]+ in panel A is detected (Rt = 2.8 min). (C and D) +ESI EIC at m/z 412.10 and m/z 821.12 for ciprofloxacin acyl-phosphate (calculated exact mass, m/z 412.1068) (C) and the proposed ATP-ciprofloxacin adduct (calculated exact mass, m/z 812.1257) (D). There is no signal for either compound.
Given these rigorous experimental results together with the protein sequence analysis, we show that CrpP alone is not a ciprofloxacin kinase, nor is it responsible for ciprofloxacin resistance in E. coli. We cannot rule out that CrpP, perhaps combined with other gene products, may be involved in the low-level fluoroquinolone resistance detected by others. However, the cumulative data are consistent with the position that CrpP is not an enzyme that confers clinically relevant antibiotic resistance.
ACKNOWLEDGMENTS
We thank Laurent Poirel and José Manuel Ortiz de la Rosa for valuable discussions.
This research was funded by a Canadian Institutes of Health Research grant (FRN-148463), an Ontario graduate scholarship (to H.L.Z.), an Alexander Graham Bell Canada graduate scholarship (to H.L.Z.), and a Canada research chair in antibiotic biochemistry (to G.D.W.).
REFERENCES
- 1.De Pascale G, Wright GD. 2010. Antibiotic resistance by enzyme inactivation: from mechanisms to solutions. Chembiochem 11:1325–1334. 10.1002/cbic.201000067. [DOI] [PubMed] [Google Scholar]
- 2.Waglechner N, McArthur AG, Wright GD. 2019. Phylogenetic reconciliation reveals the natural history of glycopeptide antibiotic biosynthesis and resistance. Nat Microbiol 4:1862–1871. 10.1038/s41564-019-0531-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim DW, Thawng CN, Lee K, Wellington EMH, Cha CJ. 2019. A novel sulfonamide resistance mechanism by two-component flavin-dependent monooxygenase system in sulfonamide-degrading actinobacteria. Environ Int 127:206–215. 10.1016/j.envint.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88. 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 5.Vetting MW, Park CH, Hegde SS, Jacoby GA, Hooper DC, Blanchard JS. 2008. Mechanistic and structural analysis of aminoglycoside N-acetyltransferase AAC(6′)-Ib and its bifunctional, fluoroquinolone-active AAC(6′)-Ib-cr variant. Biochemistry 47:9825–9835. 10.1021/bi800664x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez-Jacobo VM, Hernandez-Ramirez KC, Romo-Rodriguez P, Perez-Gallardo RV, Campos-Garcia J, Gutierrez-Corona JF, Garcia-Merinos JP, Meza-Carmen V, Silva-Sanchez J, Ramirez-Diaz MI. 2018. CrpP is a novel ciprofloxacin-modifying enzyme encoded by the Pseudomonas aeruginosa pUM505 plasmid. Antimicrob Agents Chemother 62:e02629-17. 10.1128/AAC.02629-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu DQ, Ye J, Ou HY, Wei X, Huang X, He YW, Xu Y. 2011. Genomic analysis and temperature-dependent transcriptome profiles of the rhizosphere originating strain Pseudomonas aeruginosa M18. BMC Genomics 12:438. 10.1186/1471-2164-12-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright GD, Thompson PR. 1999. Aminoglycoside phosphotransferases: proteins, structure, and mechanism. Front Biosci 4:D9–D21. 10.2741/wright. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz de la Rosa JM, Nordmann P, Poirel L. 2020. Pathogenicity genomic island-associated CrpP-like fluoroquinolone-modifying enzymes among Pseudomonas aeruginosa clinical isolates in Europe. Antimicrob Agents Chemother 64:e00489-20. 10.1128/AAC.00489-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Garcia M, Garcia-Castillo M, Garcia-Fernandez S, Lopez-Mendoza D, Diaz-Reganon J, Romano J, Passaro L, Paixao L, Canton R. 2021. Presence of chromosomal crpP-like genes is not always associated with ciprofloxacin resistance in Pseudomonas aeruginosa clinical isolates recovered in ICU patients from Portugal and Spain. Microorganisms 9:388. 10.3390/microorganisms9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox G, Sieron A, King AM, De Pascale G, Pawlowski AC, Koteva K, Wright GD. 2017. A common platform for antibiotic dereplication and adjuvant discovery. Cell Chem Biol 24:98–109. 10.1016/j.chembiol.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Ramírez KC, Reyes-Gallegos RI, Chávez-Jacobo VM, Díaz-MagaÞa A, Meza-Carmen V, Ramírez-Díaz MI. 2018. A plasmid-encoded mobile genetic element from Pseudomonas aeruginosa that confers heavy metal resistance and virulence. Plasmid 98:15–21. 10.1016/j.plasmid.2018.07.003. [DOI] [PubMed] [Google Scholar]