ABSTRACT
Stenotrophomonas maltophilia bloodstream infections (BSI) are associated with considerable mortality in the hematologic malignancy population. Trimethoprim-sulfamethoxazole (TMP-SMX) is the treatment of choice; however, it is not routinely included in empirical treatment regimens, both because of its adverse event profile and the relative rarity of S. maltophilia infections. We developed a risk score to predict hematologic malignancy patients at increased risk for S. maltophilia BSI to guide early (TMP-SMX) therapy. Patients ≥12 years of age admitted to five hospitals between July 2016 and December 2019 were included. Two separate risk scores were developed, (i) a “knowledge-driven” risk score based upon previously identified risk factors in the literature in addition to variables identified by regression analysis using the current cohort, and (ii) a risk score based upon automatic variable selection. For both scores, discrimination (receiver operator characteristic [ROC] curves and C statistics) and calibration (Hosmer-Lemeshow goodness-of-fit test and graphical calibration plots) were assessed. Internal validation was assessed using leave-one-out cross-validation. In total, 337 unique patients were included; 21 (6.2%) had S. maltophilia BSI. The knowledge-driven risk score (acute leukemia, absolute neutrophil count category, mucositis, central line, and ≥3 days of carbapenem therapy) had superior performance (C statistic = 0.75; 0.71 after cross-validation) compared to that of the risk score utilizing automatic variable selection (C statistic = 0.63; 0.38 after cross-validation). A user-friendly risk score incorporating five variables easily accessible to clinicians performed moderately well to predict hematologic malignancy patients at increased risk for S. maltophilia BSI. External validation using a larger cohort is necessary to create a refined risk score before broad clinical application.
KEYWORDS: Stenotrophomonas maltophilia, bloodstream infection, predictor, risk score
INTRODUCTION
Stenotrophomonas maltophilia is a nonfermenting Gram-negative bacillus that causes a variety of infections—most notably, pulmonary infections and bloodstream infections (BSI). S. maltophilia infections are associated with mortality rates upwards of 40% in vulnerable populations (1–3). Timely treatment of S. maltophilia BSI with effective antibiotic agents is critical to reducing mortality (2, 4, 5). Owing to extensive health care exposures, high rates of broad-spectrum antibiotic use, and impaired immune systems, patients with hematologic malignancies are at particularly high risk for S. maltophilia BSI (4, 6, 7).
S. maltophilia is intrinsically resistant to most beta-lactam agents (including carbapenems) and aminoglycosides (4, 8, 9). Consequently, standard empirical antibiotic regimens for Gram-negative infections usually do not include coverage for S. maltophilia. Trimethoprim-sulfamethoxazole (TMP-SMX) is generally regarded as the treatment of choice for S. maltophilia infections (4, 10). However, the adverse event profile of TMP-SMX, which includes bone marrow suppression, hyperkalemia, and hypersensitivity syndromes, limits its routine use as empirical therapy (11). Prompt treatment of Gram-negative BSIs (GN-BSIs) in hematologic malignancy patients therefore poses a clinical challenge. On the one hand, with the increased risk and severity of S. maltophilia infections in this population, empirical initiation of TMP-SMX may be reasonable. On the other hand, S. maltophilia BSIs remain relatively rare compared to other GN-BSIs, and indiscriminate TMP-SMX therapy is associated with important side effects. Ideally, clinicians could determine, at the time a Gram stain identifies a GN-BSI in patients with a hematologic malignancy, for which patients the benefits of empirical TMP-SMX therapy for potential S. maltophilia BSI outweigh the risks.
To address this challenge, we sought to identify the subgroup of hematologic malignancy patients at greatest risk for a S. maltophilia BSI. We sought to develop and validate a user-friendly clinical risk score to predict, among the subgroup of patients with GN-BSIs, when empirical TMP-SMX therapy for S. maltophilia may be warranted.
RESULTS
Study population.
In total, 337 unique patients were identified as having a hematologic malignancy or hematopoietic stem cell transplant (HSCT) and GN-BSI, of whom 21 (6.2%) had S. maltophilia bacteremia (Table 1). Demographics, types of underlying malignancies, severity of illness, and median number of days of hospital exposure in the past 3 months were similar between the S. maltophilia and other GN-BSI groups. The median absolute neutrophil counts (ANC) on day 1 of bacteremia were not statistically different between the two groups, nor were the proportions of those with prolonged neutropenia (≥7 days). There were, however, some significant differences between the two groups. Mucositis was present in 5 (23.8%) of the S. maltophilia patients compared to 24 (7.6%) of other GN-BSI patients (odds ratio [OR] = 3.80; 95% confidence interval [CI], 1.28 to 11.27; P = 0.02) (Table 2). Patients with S. maltophilia BSI were more likely to have received at least 3 days of carbapenem therapy in the past 3 months compared to patients with other GN-BSIs (8 [38.1%] versus 40 [12.7%]; OR = 4.25; 95% CI 1.66 to 10.88; P < 0.01).
TABLE 1.
Baseline characteristics of hematologic malignancy patients with Stenotrophomonas maltophilia bacteremia and with other GN-BSIs
| Patient characteristicf | Total (N = 337) | S. maltophilia BSI (N = 21) | Other GN-BSI (N = 316) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age in yrs (median [IQR]) | 62 (49–70) | 62 (44–70) | 62 (49–70) | 0.56 |
| Sex, female (no. [%]) | 140 (41.54) | 13 (61.90) | 127 (40.19) | 0.07 |
| Type of malignancy or receipt of HSCT (no. [%]) | ||||
| Acute myelogenous leukemia | 109 (32.34) | 8 (38.10) | 101 (31.96) | 0.52 |
| Acute lymphocytic leukemia | 35 (10.39) | 3 (14.29) | 32 (10.13) | 0.52 |
| T-cell lymphoma | 7 (2.08) | 1 (4.76) | 6 (1.90) | 0.52 |
| Chronic lymphocytic lymphoma | 12 (3.56) | 0 | 12 (3.80) | 0.52 |
| Chronic myelomonocytic leukemia | 2 (0.59) | 0 | 2 (0.63) | 0.52 |
| Chronic myelogenous leukemia | 3 (0.89) | 0 | 3 (0.95) | 0.52 |
| Hodgkin lymphoma | 7 (2.08) | 1 (4.76) | 6 (1.90) | 0.52 |
| Non-Hodgkin lymphoma | 25 (7.42) | 1 (4.76) | 24 (7.59) | 0.52 |
| Multiple myeloma | 42 (12.46) | 0 | 42 (13.29) | 0.52 |
| Myelodysplastic syndrome | 25 (7.72) | 1 (4.76) | 24 (7.59) | 0.52 |
| Diffuse large B-cell lymphoma | 40 (11.87) | 3 (14.29) | 37 (11.71) | 0.52 |
| Othera | 29 (8.61) | 3 (14.29) | 26 (8.23) | 0.52 |
| HSCT within preceding 12 mo | 92 (27.30) | 8 (38.10) | 84 (26.58) | 0.31 |
| Degree of immunosuppression, potential risk factors | ||||
| ANC (cells/mm3) (median [IQR]) | 49 (0–1,880) | 180 (0–1,010) | 30 (0–1,895) | 0.87 |
| Neutropeniab for ≥7 days (no. [%]) | 170 (51.36) | 14 (66.67) | 156 (50.32) | 0.18 |
| Mucositis present (no. [%]) | 29 (8.61) | 5 (23.81) | 24 (7.59) | 0.03 |
| Central venous catheter (no. [%]) | 260 (77.15) | 20 (95.24) | 240 (75.95) | 0.06 |
| Severity of illness | ||||
| Pitt bacteremia score (median [IQR]) | 1 (0–3) | 1 (0–2) | 1 (0–3) | 0.48 |
| ICU on day 1 of bacteremia (no. [%]) | 68 (20.18) | 3 (14.29) | 65 (20.57) | 0.78 |
| Prior colonization or infection in preceding 6 mo (no. [%]) | ||||
| S. maltophilia | 8 (2.37) | 1 (4.76) | 7 (2.22) | 0.41 |
| Other multidrug-resistant Gram-negative organismsc | 48 (14.24) | 2 (9.52) | 46 (14.56) | 0.75 |
| Antibiotic history in preceding 3 mo (no. [%]) | ||||
| Carbapenemd for ≥3 days | 48 (14.24) | 8 (38.1) | 40 (12.7) | <0.01 |
| Cefepime for ≥3 days | 113 (33.53) | 11 (52.38) | 102 (32.28) | 0.09 |
| Piperacillin-tazobactam for ≥3 days | 98 (29.08) | 8 (38.10) | 90 (28.48) | 0.33 |
| Prophylactic antibiotice | 230 (68.25) | 17 (80.95) | 213 (67.41) | 0.23 |
| Prior hospitalization in preceding 3 mo (median [IQR]) | ||||
| No. of days hospitalized | 11 (1–22) | 15 (1–24) | 11 (1–22) | 0.83 |
| No. of days in ICU | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.07 |
Includes aplastic anemia (n = 4), myelofibrosis (2), central nervous system (CNS) lymphoma and B-cell lymphoma (1), mixed-phenotype (B-cell and myeloid/monocytic lineages) acute leukemia (1), chronic neutrophilic leukemia and myelofibrosis (1), bilineage acute leukemia (1), large granular lymphocytic leukemia (1), mucosa-associated lymphoid tissue (MALT) lymphoma (1), CNS lymphoma (1), multiple myeloma and myelodysplastic syndrome (1), hairy cell leukemia (1), Waldenstrom’s macroglobulinemia versus lymphoproliferative disorder (1), acute promyelocytic leukemia (1), mantle cell lymphoma (1), monoclonal gammopathy and cardiac amyloid (1), follicular lymphoma (1), blastic plasmacytoid dendritic cell neoplasm (1), T-cell lymphoma (1), myeloproliferative neoplasm (1), high-grade B-cell lymphoma (1), primary plasma cell leukemia (1), prolymphocytic leukemia (1), cutaneous T-cell lymphoma (1), immunodeficiency (1), and sickle cell disease (1).
Neutropenia with ANC of <1,500 cells/mm3.
Other multidrug-resistant Gram-negative organisms include presence of an extended-spectrum beta-lactamase, carbapenem resistant Enterobacterales, and multidrug-resistant Enterobacterales, Pseudomonas, Acinetobacter, or Achromobacter species defined as resistant to at least 1 drug in 3 drug categories (piperacillin-tazobactam, extended-spectrum cephalosporins, fluoroquinolones, aminoglycosides, or carbapenems (or ampicillin-sulbactam specifically for Acinetobacter spp.).
Prior carbapenem use includes prior meropenem and/or ertapenem; no patients received imipenem-cilastatin.
Any antibiotic received as prophylaxis for ongoing neutropenia, as determined by chart review.
IQR, interquartile range; HSCT, hematopoietic stem cell transplant; ANC, absolute neutrophil count; ICU, intensive care unit.
TABLE 2.
Logistic regression analyses for Stenotrophomonas maltophilia bloodstream infection (BSI) compared to other GN-BSIs among patients with hematologic malignancy or a hematopoietic stem cell transplant
| Variable | Odds ratio (95% CI) | P | Adjusted odds ratio (95% CI) | P |
|---|---|---|---|---|
| Acute leukemia (AML or ALL) | 1.51 (0.62–3.67) | 0.36 | 0.78 (0.30–2.04) | 0.61 |
| ANC (no. of cells/mm3) | 0.87 (0.70–1.08) | 0.22 | 0.94 (0.77–1.15) | 0.53 |
| Mucositis present | 3.80 (1.28–11.27) | 0.02 | 4.05 (1.25–13.15) | 0.02 |
| Central venous catheter | 6.33 (0.84–47.98) | 0.07 | 4.89 (0.62–38.78) | 0.13 |
| Carbapenem receipta for ≥3 days | 4.25 (1.66–10.88) | <0.01 | 4.06 (1.49–11.02) | <0.01 |
Prior carbapenem use includes prior meropenem and/or ertapenem as treatment in the preceding 3 months; no patients received imipenem-cilastatin.
Knowledge-driven risk score.
Five variables were identified for inclusion in the multivariable logistic regression model that generated regression coefficients for the knowledge-driven risk score (Table 2). These included two variables with a P value of <0.05 in univariable and multivariable analysis (i.e., mucositis [adjusted odds ratio (aOR) = 4.05; 95% CI, 1.25 to 13.15; P = 0.02] and ≥3 days of carbapenem therapy in the previous 3 months [aOR = 4.06; 95% CI, 1.49 to 11.02; P < 0.01]) and three variables previously identified as risk factors for S. maltophilia BSI in the published literature (i.e., ANC, acute leukemia, and presence of a central venous catheter). Points were assigned as follows: acute myelogenous leukemia or acute lymphocytic leukemia (1 point); central venous catheter present (22 points); ANC level (3 points per each increasing level, up to a maximum of 12 points, with 0 to 99 as reference level with no points); mucositis (23 points); and ≥3 days of carbapenem use in the preceding 3 months (19 points).
Patient point scores ranged from −1 to 67, with a median score of 22 (interquartile range [IQR], 21 to 34) (Table 3). The C statistic was 0.75 for the multivariable logistic model and remained 0.75 after transformation to integer points. Following cross-validation, the C statistic for the risk score was 0.71. There was acceptable calibration (Hosmer-Lemeshow goodness-of-fit test P = 0.22) of the multivariable logistic model. Following transformation to a point scale, the knowledge-driven risk score underestimated the probability of S. maltophilia BSI along several points of the risk continuum (Hosmer-Lemeshow goodness-of-fit P < 0.001) (Fig. 1A).
TABLE 3.
Regression models and corresponding point scoring system to predict S. maltophilia bloodstream infection among patients with hematologic malignancy or a hematopoietic stem cell transplant
| Variable | β coefficient | Odds ratio (95% CI) | No. of points assigneda |
|---|---|---|---|
| Risk score 1 (“knowledge-driven” model risk score) | |||
| Intercept | −5.14 | ||
| Acute leukemia | −0.08 | 0.92 (0.35–2.44) | 1 |
| Absolute neutrophil count levelb | 0.22 | 1.24 (0.93–1.67) | 3/increasing group (max 12 points) |
| Mucositis | 1.80 | 6.07 (1.70–21.71) | 23 |
| Central venous catheter present | 1.77 | 5.84 (0.74–46.33) | 22 |
| Carbapenem receiptc for ≥3 days | 1.48 | 4.41 (1.61–12.06) | 19 |
| Risk score 2 (“automatic variable selection” risk score) | |||
| Intercept | −3.27 | ||
| Mucositis | 1.41 | 4.10 (1.33–12.63) | 1 |
| Carbapenem receiptc for ≥3 days | 1.50 | 4.46 (1.71–11.67) | 1 |
Points were created based upon the smallest model coefficient (1.41 for weeks of carbapenem in backwards selection model, 0.08 for acute leukemia in full selection model). Points were then calculated based upon scaling of these. For example, in the full model, acute leukemia received 1 point, by dividing by 0.08. All other coefficients were then also divided by 0.08 and rounded to the nearest integer.
ANC level defined by groups of 0 to 99, 100 to 499, 500 to 999, 1,000 to 1,499, and ≥1,500 cells/mm3.
Prior carbapenem use includes prior meropenem and/or ertapenem as treatment in the preceding 3 months; no patients received imipenem-cilastatin.
FIG 1.
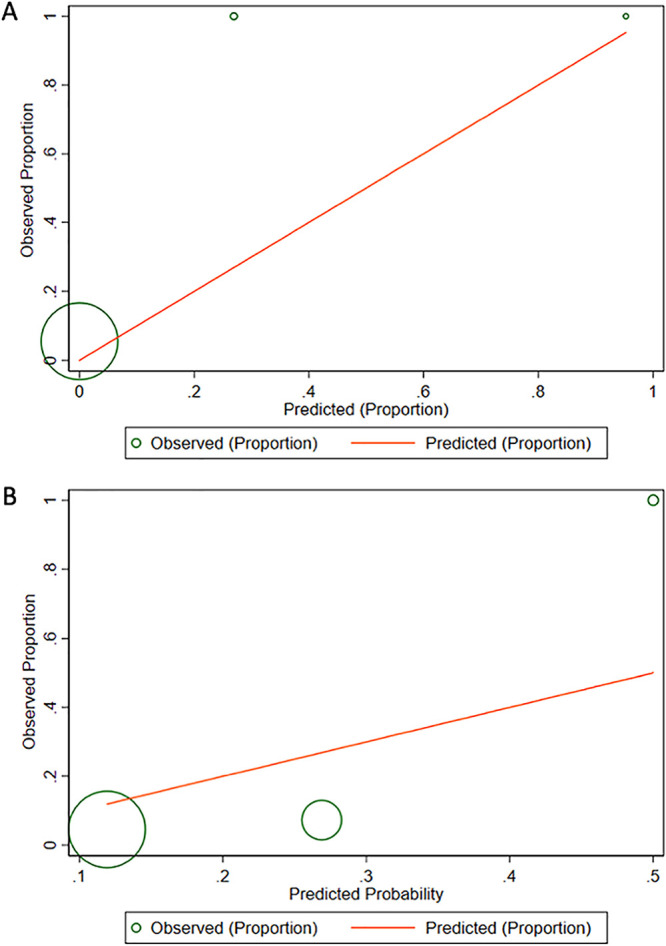
Calibration plots for the risk score models, after conversion to points. (A) Calibration plot of observed proportion versus Stenotrophomonas maltophilia BSI predicted by risk score 1 (“knowledge-driven” risk score model), by decile groups. (B) Calibration plot of observed proportion versus S. maltophilia BSI predicted by risk score 2 (“automatic variable selection” risk score model), by decile groups.
Automatic variable selection risk score.
Risk score 2, using the “automatic variable selection” approach, yielded two variables, mucositis and ≥3 days of carbapenem therapy in the preceding 3 months (Table 3). Patient point scores ranged from 0 to 2, with a median score of 0 (IQR, 0). The C statistic for the risk score was 0.63 (0.64 prior to coefficient-to-points transformation). Following cross-validation, the C statistic for the risk score was 0.38. There was not acceptable calibration (Hosmer-Lemeshow goodness-of-fit test with P < 0.01) of the raw multivariable model or following point conversion (P < 0.001). The risk score overestimated the probability of S. maltophilia BSI at low and middle deciles and underestimated the probability at high deciles (Hosmer-Lemeshow goodness-of-fit P < 0.001) (Fig. 1B).
The sensitivity and specificity at different cutoff points for each risk score are described in Table 4. Both risk scores displayed high sensitivity at relatively low cutoff values, and higher specificity at higher values. In the risk score 1 group, >80% of observations were correctly classified at ≥41 points (maximum of 67 points), while for risk score 2, >75% of observations were correctly classified at ≥1 point (maximum of 2 points).
TABLE 4.
Sensitivity, specificity, and classification accuracy for each risk score at various cutoff points for predicting S. maltophilia bloodstream infectiona
| Risk score cutoff pointb | Risk scorec |
|||||
|---|---|---|---|---|---|---|
| 1 |
2 |
|||||
| Sensitivity (%) | Specificity (%) | Correctly classified (%) | Sensitivity (%) | Specificity (%) | Correctly classified (%) | |
| ≥0 | 100.0 | 2.3 | 8.5 | 100.0 | 0.0 | 6.2 |
| ≥1 | 42.9 | 79.8 | 77.5 | |||
| ≥2 | 19.1 | 100.0 | 95.0 | |||
| ≥3 | 100.0 | 5.8 | 11.9 | |||
| ≥5 | 100.0 | 10.1 | 15.8 | |||
| ≥6 | 100.0 | 10.4 | 16.1 | |||
| ≥8 | 100.0 | 11.4 | 17.0 | |||
| ≥11 | 100.0 | 12.0 | 17.3 | |||
| ≥12 | 100.0 | 12.0 | 17.6 | |||
| ≥18 | 100.0 | 20.8 | 25.8 | |||
| ≥21 | 100.0 | 21.1 | 26.1 | |||
| ≥22 | 95.2 | 38.6 | 42.3 | |||
| ≥23 | 85.7 | 55.8 | 57.8 | |||
| ≥24 | 85.7 | 56.8 | 58.7 | |||
| ≥25 | 81.0 | 58.2 | 59.6 | |||
| ≥27 | 81.0 | 61.0 | 62.3 | |||
| ≥28 | 76.2 | 62.0 | 62.6 | |||
| ≥30 | 71.4 | 63.6 | 64.1 | |||
| ≥31 | 66.8 | 64.9 | 65.1 | |||
| ≥33 | 66.8 | 66.6 | 66.6 | |||
| ≥34 | 61.9 | 72.4 | 71.7 | |||
| ≥40 | 42.9 | 82.1 | 79.6 | |||
| ≥41 | 38.1 | 87.3 | 84.2 | |||
| ≥43 | 28.6 | 89.3 | 85.4 | |||
| ≥44 | 19.1 | 89.9 | 85.4 | |||
| ≥45 | 19.1 | 94.8 | 90.0 | |||
| ≥46 | 14.3 | 96.4 | 91.2 | |||
| ≥49 | 14.3 | 97.1 | 91.8 | |||
| ≥50 | 14.3 | 97.7 | 92.4 | |||
| ≥52 | 14.3 | 98.1 | 92.7 | |||
| ≥53 | 14.3 | 98.7 | 93.3 | |||
| ≥63 | 14.3 | 100.0 | 94.5 | |||
| ≥67 | 4.8 | 100.0 | 93.9 | |||
From a cohort of patients with hematologic malignancy or hematopoietic stem cell transplant and Gram-negative bloodstream infections.
Cutoff points of <0 are not shown, as sensitivity remained 100% and specificity was 0% (for the risk score based on the “full” model of variables). For simplicity, whole numbers of integers are shown.
Risk score 1, knowledge-driven; risk score 2, automatic variable selection.
DISCUSSION
We found that among patients with hematologic malignancy or HSCT in a multicenter observational cohort, a knowledge-driven risk score incorporating five variables readily available to treating clinicians performed better than a risk score purely based upon automatic variable selection to predict patients with hematologic malignancy or HSCT who are at greatest risk for S. maltophilia infection. The knowledge-driven risk score included the following variables: acute leukemia, absolute neutrophil count category, mucositis as determined by an oncologist, central venous catheter present, and ≥3 days of carbapenem therapy within the previous 3 months. A risk score is useful while awaiting organism identification in blood culture bottles, which are dependent on bacterial growth. Moreover, rapid diagnostic assays capable of identifying S. maltophilia are still not widely available in many clinical microbiology laboratories (12).
S. maltophilia BSI is associated with poor outcomes, with attributable mortality as high as 38%, making early identification of patients at highest risk for S. maltophilia critical to ensure they are placed on effective antibiotic therapy as early as possible (1). S. maltophilia can be challenging to treat with antibiotics due to its resistance to several antibiotic classes. S. maltophilia has a number of diverse mechanisms of antimicrobial resistance, including chromosomally encoded beta-lactamases (L1, a metallo-beta-lactamase, and L2, a serine beta-lactamase), multidrug efflux pumps, chromosomally encoded Smqnr resistance genes, and aminoglycoside-modifying enzymes (4, 8–10). Given the antibiotic resistance associated with S. maltophilia, even once identified in clinical cultures, determining effective treatment can be challenging, as resistance to antibiotics expected to be active against wild-type S. maltophilia is also increasing (8). In addition, robust comparative effectiveness treatment studies for S. maltophilia are lacking (13–17).
Similar to what others have shown, recent carbapenem use was associated with increased S. maltophilia BSI in our cohort, potentially because carbapenem therapy can reduce intestinal colonization of a broad range of aerobic and anaerobic Gram-positive and Gram-negative bacteria, but not that of S. maltophilia, due to its intrinsic carbapenem resistance (7, 18). Mucositis has also been described as a risk factor for S. maltophilia BSI (19, 20), although not consistently. Mucositis represents toxicity of chemotherapy regimens/immunosuppression and a decreased epithelial barrier, which may explain its role in translocation of S. maltophilia resulting in invasive infections. Aitken and colleagues demonstrated that for each 1% increase in relative abundance of S. maltophilia in the oral microbiome, there is an associated 3% increase in the hazard of S. maltophilia infection in patients with acute myelogenous leukemia receiving chemotherapy (18). Variables such as neutropenia, the presence of a central venous catheter, and leukemia, in addition to mucositis and prior carbapenem use, have been previously associated with an increased risk of S. maltophilia infections (3, 6, 7, 18).
Existing studies that have explored risk factors for S. maltophilia infections have been informative but also have limitations that include their mostly single-center nature; heterogeneous study populations (i.e., not limited to hematologic malignancy or HSCT), which make extrapolation of findings to specific high-risk patient populations more challenging; and their inclusion of diverse S. maltophilia specimen types that capture multiple sources of infection as well as potential colonization. Moreover, no previous studies investigating S. maltophilia risk factors have attempted to construct risk scores to simplify end user application and to predict which patients have S. maltophilia infections. To explain further, although traditional risk factor analyses are useful for understanding etiologic causes of disease (and thus potential prevention targets), such causal risk factors may not necessarily be effective at distinguishing between those who do or do not have the outcome of interest, depending upon their distribution in a cohort. In contrast, sufficiently discriminative risk scores can help answer prediction questions, e.g., “Who may benefit the most from empirical TMP-SMX therapy to target S. maltophilia in addition to usual empirical antimicrobial regimens?”
We built two risk scores to identify patients with S. maltophilia BSI, a knowledge-driven approach that included variables selected based upon the prior literature or significance in univariable analysis (risk score 1), and an automatic variable selection approach that included only those variables that were retained following stepwise variable selection procedures (risk score 2). Risk score 1 performed well (area under the curve [AUC] = 0.75 and 0.71 following cross-validation). In comparison, risk score 2 had inferior discrimination compared to that of risk score 1 (AUC = 0.63 and 0.38 following cross-validation). The knowledge-driven risk score model was only marginally more complex, at five variables, and had superior performance despite including some variables that did not achieve statistical significance.
While automatic variable selection is commonly employed in the literature in the risk score-building process, our results illustrate some of its potential pitfalls. As stated previously, independent causal risk factors do not necessarily make ideal predictors (21, 22). For example, the identification of an independent risk factor assists in understanding what is contributing to S. maltophilia BSI, and thus illuminates a target where one may be able to intervene (i.e., by limiting use of carbapenem therapy). However, a risk score (in our example) focuses on predicting which patients have S. maltophilia BSI while awaiting confirmatory testing; the goal is not necessarily to identify intervention targets, but rather to understand what variables best discriminate between those who do and do not have S. maltophilia BSI.
Importantly, risk scores also exhibit end user flexibility, where the cutoff point can be varied to tailor the desired sensitivity and specificity. For example, increasing the sensitivity of the cutoff value (i.e., lower point values) increases the proportion of patients receiving TMP-SMX and increases the likelihood of providing adequate empirical coverage for a patient with S. maltophilia BSI; however, this decision would come with more “false-positive patients” receiving empirical TMP-SMX. Alternatively, prioritizing specificity by raising the cutoff point value would reduce the number of patients unnecessarily exposed to TMP-SMX but could lead to “missed” cases of S. maltophilia BSI. Even with a relatively low prevalence of S. maltophilia BSI of 6% in our cohort, our opinion would be that prioritizing sensitivity may be preferred, given the high morbidity and mortality associated with delayed treatment for S. maltophilia BSIs in this vulnerable population. For example, by choosing a threshold cutoff of ≥27 points in our knowledge-driven risk score, a clinician would identify 17 S. maltophilia BSI patients for early appropriate treatment, who would otherwise have received empirical therapy ineffective against S. maltophilia, and miss 4 cases, while 123 or 39% of patients would be false positives who would unnecessarily receive empirical TMP-SMX therapy. On the other hand, by selecting a threshold of ≥40 points, a clinician would identify 9 S. maltophilia BSI patients for early appropriate therapy and miss 12 cases, with 57 (or 18%) false-positive patients receiving unnecessary TMP-SMX empirical therapy.
Our study has several limitations. First, while this is a multicenter study, it was from a single region. There may be regional differences in chemotherapy regimens and their associated degree of immunosuppression that might change observed associations in cohorts from other regions and cancer centers. Additionally, prior studies have suggested that microbiome domination may precede infection in hematologic malignancy patients. In the hospitals included in our cohort, hematologic malignancy and HSCT patients undergo weekly rectal surveillance cultures for Gram-negative organisms with resistance to third-generation cephalosporins. However, S. maltophilia growth on surveillance cultures is not routinely reported in the electronic medical record. Therefore, we were unable to rigorously investigate if antecedent colonization status would be an important predictor for S. maltophilia BSI in our study population. Given the importance of mucositis in our models, this would be an important target for future research. Additionally, our study had a small number of S. maltophilia BSI events, making the development of a risk score challenging. The small sample size likely contributed to poor calibration after conversion to point scales. Nevertheless, the discrimination of our knowledge-driven risk score remained acceptable even after cross-validation, suggesting that despite small sample sizes, this model was not overfitted to the data. External validation of our risk score in a larger, geographically diverse data set, and possibly with more events, would be essential prior to broad clinical implementation.
In conclusion, given the high mortality associated with S. maltophilia BSI and the lack of therapeutic efficacy with antibiotics traditionally prescribed for patients with hematologic malignancies/HSCT, a risk score that can be utilized easily by clinicians to predict which patients may benefit from early, effective therapy for S. maltophilia (e.g., TMP-SMX), despite the known toxicities, is of value. We developed and internally validated a risk score that performed moderately well to identify such patients. External validation in a larger cohort is necessary to create a refined risk score that can be broadly applied.
MATERIALS AND METHODS
Setting and participants.
This was an observational cohort study of unique patients 12 years of age and older admitted between 1 July 2016 and 1 December 2019 to one of five hospitals within the Johns Hopkins Health System, which serves the greater Maryland region. Patients met eligibility criteria if they had at least one blood culture with an Enterobacterales or a nonfermenting Gram-negative bacillus and were actively receiving chemotherapy for a hematologic malignancy and/or received a hematopoietic stem cell transplant (HSCT) within the preceding 12 months. Only the first episode of GN-BSI (including polymicrobial episodes) during the study period for each eligible patient was included. This study was approved by the institutional review board of the Johns Hopkins University School of Medicine, with a waiver of informed consent. Informed consent was not obtained from patients because this work was retrospective and did not modify care received.
Data extraction.
Demographics, type of malignancy and degree of immunosuppression, severity of illness, prior antibiotic use within the previous 3 months, health care exposure within the previous 3 months, and previous microbiologic results within the previous 6 months (including both S. maltophilia and other multidrug-resistant Gram-negative organisms [MDRGN]), were collected by manual review of electronic medical records and entered into a secure REDCap database. Degree of immunosuppression was determined by the absolute neutrophil count (ANC), neutropenia (<1,500 neutrophils/mm3) for 7 or more preceding days, or the presence of mucositis (based on significant oral pain or ulcers as documented by the oncology team), all on day 1 (defined as the day of blood culture collection). Severity of illness was evaluated by admission to an intensive care unit (ICU) and by the Pitt bacteremia score, both on day 1 (23). Prior antibiotic use consisted of inpatient and outpatient days of antibiotic therapy with Gram-negative activity, including antibiotic prophylaxis, within the 3 months prior to day 1. Other MDRGNs that were included were as follows: extended-spectrum beta-lactamase-producers (confirmed by phenotypic or molecular testing); carbapenem-resistant Enterobacterales; or Pseudomonas aeruginosa, Acinetobacter baumannii, Achromobacter species, or Enterobacterales that were resistant to at least 1 drug in 3 or more of the following antibiotic categories (piperacillin-tazobactam, extended-spectrum cephalosporins, fluoroquinolones, aminoglycosides, or carbapenems, or specifically for Acinetobacter baumannii, ampicillin-sulbactam) (5).
Knowledge-driven risk score.
Two risk scores were developed. The first was a knowledge-driven risk score (risk score 1). The knowledge-driven risk score includes both (i) risk factors for S. maltophilia infections in hematologic malignancy patients described in the limited published literature (18, 19, 24–27); and (ii) any additional variables that were statistically significant in our cohort based on univariable logistic regression analysis at a significance level of 0.05. The reference group was patients with GN-BSIs other than S. maltophilia, including both Enterobacterales and glucose-nonfermenting Gram-negative bacilli. In order to develop a risk score that is user friendly for manual bedside calculation, continuous variables were converted to ordinal categories. As an example, ANC values were recategorized into the following ordinal levels: 0 to 99, 100 to 499, 500 to 999, 1,000 to 1,499, and ≥1,500 cells/mm3. Fisher’s exact test compared categorical variables, and the Wilcoxon rank sum test was used for continuous variables. Univariable regression analysis evaluated the association between each variable and the outcome of S. maltophilia BSI. Variables that were statistically significant in univariable regression at a significance level of 0.05 were included in a multivariable model. Additionally, risk factors described in the published literature (18, 19, 24–27) were “forced” into the model. The regression coefficients derived from the multivariable model were rescaled by dividing by the smallest final model coefficient and rounding to the nearest integer to create risk score “points.” Patient scores were calculated by summing their respective points.
Automatic variable selection risk score.
Additionally, we constructed an automatic variable selection risk score (risk score 2). Automatic variable selection is a common, although debated approach, in the literature for building risk scores (21, 28, 29). The same procedures were used as for risk score 1, but the multivariable logistic regression model was fitted using stepwise backward variable selection at an alpha value of 0.05 across all variables (28, 29). A priori identified risk factors in the literature were not included in risk score 2. Using the same procedures as for risk score 1, risk score “points” and patient scores were calculated.
Risk score discrimination, calibration, and validation.
Discrimination evaluates the ability of a model to distinguish between those who do and do not have the outcome of interest. In the present application, it represents the probability that the risk score will predict that a patient (later confirmed to have a S. maltophilia BSI) has a higher probability of being infected with S. maltophilia compared to that of a randomly selected hematologic malignancy patient with a GN-BSI caused by an organism other than S. maltophilia. For both risk scores, discrimination was assessed via receiver operator characteristic (ROC) curves and accompanying C statistics (area under the curve [AUC]). Calibration evaluates agreement between observed and predicted outcomes and was assessed using the Hosmer-Lemeshow goodness-of-fit test and graphical calibration plots. Both risk scores were internally validated using leave-one-out cross-validation. All statistical tests were two-sided, and a P value of <0.05 was considered statistically significant. Statistical analyses were performed using Stata version 16.0 (StataCorp, College Station, TX).
ACKNOWLEDGMENTS
S.M.K. is supported by a training grant from the National Institute of Allergy and Infectious Diseases (T32-A1007291).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All authors report no conflicts of interest related to this work.
REFERENCES
- 1.Falagas ME, Kastoris AC, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, Dimopoulos G. 2009. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol 4:1103–1109. 10.2217/fmb.09.84. [DOI] [PubMed] [Google Scholar]
- 2.Looney WJ, Narita M, Mühlemann K. 2009. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis 9:312–323. 10.1016/S1473-3099(09)70083-0. [DOI] [PubMed] [Google Scholar]
- 3.Senol E, DesJardin J, Stark PC, Barefoot L, Snydman DR. 2002. Attributable mortality of Stenotrophomonas maltophilia bacteremia. Clin Infect Dis 34:1653–1656. 10.1086/340707. [DOI] [PubMed] [Google Scholar]
- 4.Chang YT, Lin CY, Chen YH, Hsueh P-R. 2015. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 6:893. 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chotiprasitsakul D, Han JH, Cosgrove SE, Harris AD, Lautenbach E, Conley AT, Tolomeo P, Wise J, Tamma PD, Antibacterial Resistance Leadership Group . 2018. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis 66:172–177. 10.1093/cid/cix767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metan G, Hayran M, Hascelik G, Uzun O. 2006. Which patient is a candidate for empirical therapy against Stenotrophomonas maltophilia bacteraemia? An analysis of associated risk factors in a tertiary care hospital. Scand J Infect Dis 38:527–531. 10.1080/00365540500452481. [DOI] [PubMed] [Google Scholar]
- 7.Hotta G, Matsumura Y, Kato K, Nakano S, Yunoki T, Yamamoto M, Nagao M, Ito Y, Takakura S, Ichiyama S. 2014. Risk factors and outcomes of Stenotrophomonas maltophilia bacteraemia: a comparison with bacteraemia caused by Pseudomonas aeruginosa and Acinetobacter species. PLoS One 9:e112208. 10.1371/journal.pone.0112208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gales AC, Seifert H, Gür D, Castanheira M, Jones RN, Sader HS. 2019. Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY Antimicrobial surveillance program (1997–2016). Open Forum Infect Dis 6:S34–S46. 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojica MF, Rutter JD, Taracila M, Abriata LA, Fouts DE, Papp-Wallace KM, Walsh TJ, LiPuma JJ, Vila AJ, Bonomo RA. 2019. Population structure, molecular epidemiology, and β-lactamase diversity among Stenotrophomonas maltophilia isolates in the United States. mBio 10:e00405-19. 10.1128/mBio.00405-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho JM-W, Juurlink DN. 2011. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ 183:1851–1858. 10.1503/cmaj.111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamma PD, Smith TT, Adebayo A, Karaba SM, Jacobs E, Wakefield T, Nguyen K, Whitfield NN, Simner PJ. 2021. Prevalence of blaCTX-M genes in Gram-negative bloodstream isolates across 66 hospitals in the United States. J Clin Microbiol 59 10.1128/JCM.00127-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YL, Scipione MR, Dubrovskaya Y, Papadopoulos J. 2014. Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 58:176–182. 10.1128/AAC.01324-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tekçe YT, Erbay A, Cabadak H, Sen S. 2012. Tigecycline as a therapeutic option in Stenotrophomonas maltophilia infections. J Chemother 24:150–154. 10.1179/1120009X12Z.00000000022. [DOI] [PubMed] [Google Scholar]
- 15.Hand E, Davis H, Kim T, Duhon B. 2016. Monotherapy with minocycline or trimethoprim/sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. J Antimicrob Chemother 71:1071–1075. 10.1093/jac/dkv456. [DOI] [PubMed] [Google Scholar]
- 16.Ko J-H, Kang C-I, Cornejo-Juárez P, Yeh K-M, Wang C-H, Cho SY, Gözel MG, Kim S-H, Hsueh P-R, Sekiya N, Matsumura Y, Lee D-G, Cho S-Y, Shiratori S, Kim Y-J, Chung DR, Peck KR. 2019. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: a systematic review and meta-analysis. Clin Microbiol Infect 25:546–554. 10.1016/j.cmi.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Nys C, Cherabuddi K, Venugopalan V, Klinker KP. 2019. Clinical and microbiologic outcomes in patients with monomicrobial Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 63:e00788-19. 10.1128/AAC.00788-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aitken SL, Sahasrabhojane PV, Kontoyiannis DP, Savidge TC, Arias CA, Ajami NJ, Shelburne SA, Galloway-Peña JR. 2020. Alterations of the oral microbiome and cumulative carbapenem exposure are associated with Stenotrophomonas maltophilia infection in patients with acute myeloid leukemia receiving chemotherapy. Clin Infect Dis 72:1507–1513. 10.1093/cid/ciaa778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apisarnthanarak A, Mayfield JL, Garison T, McLendon PM, DiPersio JF, Fraser VJ, Polish LB. 2003. Risk factors for Stenotrophomonas maltophilia bacteremia in oncology patients: a case-control study. Infect Control Hosp Epidemiol 24:269–274. 10.1086/502197. [DOI] [PubMed] [Google Scholar]
- 20.Labarca JA, Leber AL, Kern VL, Territo MC, Brankovic LE, Bruckner DA, Pegues DA. 2000. Outbreak of Stenotrophomonas maltophilia bacteremia in allogenic bone marrow transplant patients: role of severe neutropenia and mucositis. Clin Infect Dis 30:195–197. 10.1086/313591. [DOI] [PubMed] [Google Scholar]
- 21.van Diepen M, Ramspek CL, Jager KJ, Zoccali C, Dekker FW. 2017. Prediction versus aetiology: common pitfalls and how to avoid them. Nephrol Dial Transplant 32:ii1–ii5. 10.1093/ndt/gfw459. [DOI] [PubMed] [Google Scholar]
- 22.Goodman KE, Simner PJ, Klein EY, Kazmi AQ, Gadala A, Toerper MF, Levin S, Tamma PD, Rock C, Cosgrove SE, Maragakis LL, Milstone AM, CDC Prevention Epicenters Program . 2019. Predicting probability of perirectal colonization with carbapenem-resistant Enterobacteriaceae (CRE) and other carbapenem-resistant organisms (CROs) at hospital unit admission. Infect Control Hosp Epidemiol 40:541–550. 10.1017/ice.2019.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee J-Y, Kwon KT, Ki HK, Shin SY, Jung DS, Chung D-R, Ha B-C, Peck KR, Song J-H. 2009. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the acute physiology and chronic health evaluation II scoring systems. Shock 31:146–150. 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
- 24.Aisenberg G, Rolston KV, Dickey BF, Kontoyiannis DP, Raad II, Safdar A. 2007. Stenotrophomonas maltophilia pneumonia in cancer patients without traditional risk factors for infection, 1997–2004. Eur J Clin Microbiol Infect Dis 26:13–20. 10.1007/s10096-006-0243-7. [DOI] [PubMed] [Google Scholar]
- 25.Micozzi A, Venditti M, Monaco M, Friedrich A, Taglietti F, Santilli S, Martino P. 2000. Bacteremia due to Stenotrophomonas maltophilia in patients with hematologic malignancies. Clin Infect Dis 31:705–711. 10.1086/314043. [DOI] [PubMed] [Google Scholar]
- 26.Mori M, Tsunemine H, Imada K, Ito K, Kodaka T, Takahashi T. 2014. Life-threatening hemorrhagic pneumonia caused by Stenotrophomonas maltophilia in the treatment of hematologic diseases. Ann Hematol 93:901–911. 10.1007/s00277-014-2028-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim S-H, Cho SY, Kang C-I, Seok H, Huh K, Ha YE, Chung DR, Lee NY, Peck KR, Song J-H. 2018. Clinical predictors of Stenotrophomonas maltophilia bacteremia in adult patients with hematologic malignancy. Ann Hematol 97:343–350. 10.1007/s00277-017-3178-4. [DOI] [PubMed] [Google Scholar]
- 28.Goodman KE, Lessler J, Harris AD, Milstone AM, Tamma PD. 2019. A methodological comparison of risk scores versus decision trees for predicting drug-resistant infections: a case study using extended-spectrum beta-lactamase (ESBL) bacteremia. Infect Control Hosp Epidemiol 40:400–407. 10.1017/ice.2019.17. [DOI] [PubMed] [Google Scholar]
- 29.Tseng W-P, Chen Y-C, Yang B-J, Chen S-Y, Lin J-J, Huang Y-H, Fu C-M, Chang S-C, Chen S-Y. 2017. Predicting multidrug-resistant Gram-negative bacterial colonization and associated infection on hospital admission. Infect Control Hosp Epidemiol 38:1216–1225. 10.1017/ice.2017.178. [DOI] [PubMed] [Google Scholar]


