Abstract
Introduction
LncRNAs play important roles in multiple diseases including asthma, while there are a few reports on the role of lncRNA H19 about asthma. This study aimed to investigate the roles and mechanisms of lncRNA H19 in asthma.
Methods
We detected lncRNA H19 and Muc5ac mRNA by establishing a murine asthma model and an in vitro inflammation model. Regulatory roles of lncRNA H19 in asthma were explored by lncRNA H19 overexpression or knockdown in vitro. To study its mechanisms, we detect p-NF-κB and p-Akt expression, and treated 16-HBE cells with inhibitors of PI3K. To study regulatory effects of miR-675-3p on Muc5ac, miR-675-3p mimics and inhibitors were respectively transfected into 16-HBE cells.
Results
Firstly, we established a murine asthma model and an in vitro inflammation model. We found that lncRNA H19 expression was decreased, while Muc5ac mRNA was increased in lung tissues of murine asthma model and in the in vitro inflammation model. lncRNA H19 overexpression increased Muc5ac mRNA expression and lncRNA H19 knockdown decreased Muc5ac mRNA expression in 16-HBE cells. Moreover, lncRNA H19 overexpression further increased Muc5ac expression in TNFα-induced in vitro inflammation model. lncRNA H19 knockdown decreased p-Akt and p-NF-κB expression. Inhibitors of PI3K abolished Muc5ac induced by lncRNA H19 overexpression. Although miR-675-3p was increased by lncRNA H19 overexpression, it had no regulatory effects on Muc5ac expression.
Discussion
These results demonstrated that lncRNA H19 positively regulates Muc5ac expression through PI3K/Akt /NF-κB pathway in the in vitro inflammation model. Therefore, this study indicated that decreased lncRNA H19 in asthma might play a protective role relieving mucus overproduction, and lncRNA H19 might be a potential target for asthma treatment.
Keywords: asthma, lncRNA H19, Muc5ac, NF-κB, Akt
Background
Asthma is a chronic allergic disease affecting almost more than 300 million people around the world, which impairs life qualities of asthmatics and even threatens their lives.1 Typical asthmatic symptoms, including wheezing, dyspnea, chest tightness, and cough, are caused by airflow obstruction which is thought to result from inflammation-triggered AHR and mucus overproduction.2 Therefore, elucidating the regulatory mechanism of mucus overproduction might provide new insights into asthma treatment.
The glycoprotein components of airway mucus is primarily composed of secreted polymeric mucins, Muc5ac and Muc5b. In the airways, Muc5ac is specifically expressed in goblet cells, whereas Muc5b is expressed in submucosal glands.3 Expression of Muc5ac in airways is increased, while Muc5b is decreased in asthmatics compared with healthy controls. Muc5b has physiological functions on mucus clearance, while Muc5ac is pathologic.4 It has been evidenced that Muc5ac is a central effector of allergic inflammation that is required for AHR to methacholine.5 NF-κB, epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 6 (STAT6) signal pathways have been identified to mediate Muc5ac overproduction.6 Some medicine, such as Flavonoid 7,4ʹ-Dihydroxyflavone, Cucurbitacin E, Melatonin, Lyn kinase and fasudil, were demonstrated to inhibit Muc5ac expression through regulation of ERK1/2, MAPK, STAT6 and NF-κB signaling pathways.7–11 In the progress of asthma, numerous inflammatory factors in the airways lead to airway epithelial hyperplasia and metaplasia, thereby resulting in changes in mucus components and overproduction of Muc5ac.12 However, the detailed mechanisms of Muc5ac production are unclear.
Long noncoding RNA (lncRNA) represents a diverse class of RNA bearing more than 200 nucleotides, while without abilities of encoding protein. LncRNAs have been reported to be associated with a variety of diseases.13–15 Moreover, lncRNAs involved in airway diseases have also been widely reported.16–21 LncRNAs in peripheral blood are differentially expressed between asthmatic patients and healthy controls,22 and even between eosinophilic asthmatics and basophilic asthmatics.23 LncRNA PVT1 was correlated with glucocorticoid resistance in severe asthmatics, and impacted proliferation of airway smooth muscle cells and release of inflammatory factor IL-6, which could be a therapy target for airway remodeling.16 LncRNA GAS5 promoted proliferation of airway smooth muscle cells through regulation of miR-10a/BDNF signal transduction.24 lncRNA H19, an evolutionarily conserved and maternally expressed imprinted lncRNA, is located on chromosome 7 in mice and chromosome 11 in humans,25,26 and has been reported to regulate numerous diseases via diverse mechanisms. LncRNA H19 exon 1 encodes miR-675-3p and functions in numerous diseases.14 lncRNA H19 was decreased in airways of asthmatics compared with healthy controls,16 and lncRNA H19 overexpression was reported to inhibit proliferation and migration of airway smooth muscle cells.21 However, its roles and mechanisms in airway epithelial cells in asthma are not reported yet.
We hypothesized that lncRNA H19 might be involved in asthma and regulate the production of Muc5ac. In this study, we therefore established a murine asthma model and an in vitro inflammation model to explore the roles and mechanisms of lncRNA H19 on Muc5ac expression in asthma. We found that lncRNA H19 expression was decreased, while Muc5ac mRNA was increased in lung tissues of murine asthma model and in the in vitro inflammation model. Moreover, we found that lncRNA H19 positively regulated Muc5ac mRNA expression through PI3K/Akt/NF-κB pathway in the in vitro inflammation model.
Materials and Methods
Mice
BALB/c female mice aged 6–8 weeks under specific pathogen-free conditions were purchased from Chongqing Medical University, where the animal experiments took place. The Institutional Animal Care and Use Committee of Chongqing Medical University approved all animal experiments. The approval number is SYXK (Chongqing) 2012–0001. Animals were treated in accordance with Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press).
Establishment of Asthma Model
BALB/c female mice were divided randomly into two groups, 6–8 mice per group. Mice in asthmatic group were intraperitoneally sensitized with 100 μg OVA (Sigma-Aldrich, St. Louis, MO, USA) suspended to 1 mg of aluminum hydroxide (Thermo scientific, Waltham, MA, USA) in 200 µL of sterile phosphate-buffered saline (PBS). After sensitization on days 1 and 8, mice were challenged by aerosolization with 5% OVA for 30 minutes each day from day 14 to day 21. The control group was sensitized and challenged with PBS in the same way. On day 22, mice were anesthetized with 1.5% sodium pentobarbital by intraperitoneal injection and sacrificed for analysis. The experiments were performed for three times.
Analysis of Bronchoalveolar Lavage Fluid (BALF)
Tracheal intubation was performed using an indwelling needle after mice were anesthetized. BALF was obtained by lavaging 5 times with 1 mL of PBS. The cell free supernatant was used for detection of cytokines by ELISA kits after centrifugation at 800 g for 5 min at 4°C. After resuspension in 1 mL of PBS, the total number of inflammatory cells were counted by modified Neubauer Counter under a microscope. After cytospin and Wright’s staining, neutrophils, eosinophils, lymphocytes, and macrophages were differentially counted under a microscope.27
Cell Culture
Human bronchial epithelial cell line 16-HBE was purchased from American Type Culture Collection (ATCC) and cultured in 1640 medium (Hyclone) supplemented with 10% of fetal bovine serum (FBS) and 1% of penicillin and streptomycin at 37 °C with 5% of CO2. After confluence, the cells were plated in 6-well plates at a density of 2.5×105 per well for siRNAs transfection, or 5×105 per well for plasmids transfection. Almost after 24 hours, the cells were transfected with siRNAs (Tsingke, China) targeting lncRNA H19 (si-NC as controls) or phblv-H19 (GenScript, Nanjing) plasmids (phblv-vc as controls) using lipofectamine (lipo) 2000 (Invitrogen, USA). The sequences of siRNAs (from 5ʹ to 3ʹ), miR-675-3p mimics and inhibitors are listed in Table 1. After overexpression of lncRNA H19 for 24 hours, 16-HBE cells were pre-treated with inhibitors of PI3K or DMSO for 40 minutes and then treated with TNFα for 24 hours.
Table 1.
siRNAs, miRNA-675-3p Mimics and Inhibitors Used in This Study
| Sequences (5ʹ-3ʹ) | |
|---|---|
| siRNA3-F | CCAACAUCAAAGACACCAUTT |
| siRNA3-R | AUGGUGUCUUUGAUGUUGGGC |
| siRNA1-F | CUCUUUGUUUCUGAGCUUUCTT |
| siRNA1-R | GAAAGCUCAGAAACAAAGAGAC |
| siRNA415-F | GCGGGUCUGUUUCUUUACUTT |
| siRNA415-R | AGUAAAGAAACAGACCCGCUU |
| siRNA940-F | CAUUUGCACUGGUUGGAGUUGTT |
| siRNA940-R | CAACUCCAACCAGUGCAAAUGAC |
| siRNA NC-F | UUCUCCGAACGUGUCACGUTT |
| siRNA NC-R | ACGUGACACGUUCGGAGAATT |
| miRNA-675-3p mimics sense | CUGUAUGCCCUCACCGCUCA |
| miRNA-675-3p mimics antisense | AGCGGUGAGGGCAUACAGUU |
| mimics control | UUGUACUACACAAAAGUACUG |
| miRNA-675-3p inhibitors | UGAGCGGUGAGGGCAUACAG |
| Inhibitors control | CAGUACUUUUGUGUAGUACAA |
Abbreviation: siRNA, small interfering RNA.
Reverse Transcription-Quantitative Polymerase Chain Reaction
Total RNA from cells and murine lung tissues were harvested and prepared using isoplus (Invitrogen) according to the manufacturer’s instructions. Then the cDNA products were produced using a PrimeScriptTMRT reagent Kit (Takara, Shiga, Japan) according to the manufacturer’s protocol. Q-PCR Primers (Sangon, Shanghai, China) specific for lncRNA H19, Muc5ac and GAPDH were listed in Table 2. All RNA expressions were normalized to GAPDH gene expression. Q-PCR Primers (Sangon, Shanghai, China) specific for mir-675-3p were listed in Table 2. All microRNA expressions were normalized to U6 gene expression.
Table 2.
q-PCR Primers Used in This Study
| Primers | Sequences (5ʹ-3ʹ) |
|---|---|
| homo-H19-F | GACATGACATGGTCCGGTGT |
| homo-H19-R | ATGTTGTGGGTTCTGGGAGC |
| homo-Muc5ac-F | CCCAAACCTGCTTCTGCAAC |
| homo-Muc5ac-R | TCATCATCACAGCTTGGCGT |
| homo-GAPDH-F | TGCACCACCAACTGCTTAGC |
| homo-GAPDH-R | GGCATGGACTGTGGTCATGA |
| mmu-H19-F | GCAGGTAGAGCGAGTAGCTG |
| mmu-H19-R | TTCGAGACACCGATCACTGC |
| mmu-Muc5ac-F | AATGACTCAATCTGCGTGCCTTCC |
| mmu-Muc5ac-R | CAGGTTAGCGTGGCTTCCTTACAG |
| mmu-Muc5b-F | TCAGCATCCGCCTAGTCCTCAC |
| mmu-Muc5b-R | CCCGTTGAAGTCACCGCACAG |
| mmu-GAPDH-F | ATGTGTCCGTCGTGGATCTGA |
| mmu-GAPDH-R | TTGAAGTCGCAGGAGACAACCT |
| miR-675-3p-F | CGCGCTGTATGCCCTCAC |
| miR-675-3p-R | AGTGCAGGGTCCGAGGTATT |
| miR-675-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGAGCG |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
Western Blotting
The cells were lysed by RIPA lysis buffer (Beyotime, China) supplemented with 1% of PMSF. Subsequently, the total protein content was measured using a BCA protein assay kit (Beyotime Inc., China). After separation of total protein by 10% of SDS-PAGE, protein and prestained protein ladder (Thermo Scientific, Canada) were electrotransferred onto PVDF membranes (Millipore, United States). Then the PVDF membranes were blocked with 5% of nonfat-dried milk for 2 hours at 37°C and incubated respectively with primary antibodies to p-NF-κB, p-Akt and GAPDH (CST, United States) at 4°C overnight. After washing for 15 minutes per time and for 3 times, the membranes were incubated with HRP-conjugated secondary antibodies for 1 hour at 37°C. Finally the membranes were visualized using Image Lab and analyzed using Image J.
ELISA
IFN-γ, IL-5, IgE levels were detected by commercial ELISA kits (Biolegend, San Diego, CA, USA) according to the manufacturer’s instructions.
Histopathological Analysis
After fixing in 4% of paraformaldehyde, the lung tissues were embedded in paraffin and finally made into sections (5μm). The sections were stained with Hematoxylin and eosin (HE) to evaluate lung inflammation, or Periodic Acid-Schiff (PAS) to examine mucus secretion in airways under the light microscope.
Statistical Analysis
The data was shown as mean ± SD from repeated experiments for at least three times. Statistical evaluation was performed using Student’s t-test or Wilcoxon-Mann–Whitney test. P values less than 0.05 were considered statistically significant.
Results
Murine Asthma Model Was Successfully Established
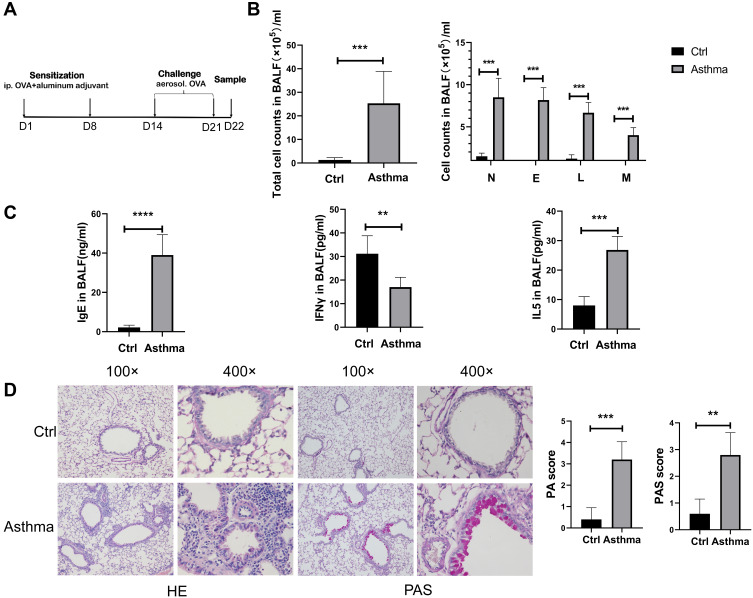
To study the role of lncRNA H19 in asthma, murine asthma model was established (Figure 1A). The total number of nucleated cells in BALF was significantly higher in asthmatic group than control group, as were the number of eosinophils, neutrophils, lymphocytes and monocytes, respectively (Figure 1B). IgE and Type 2 cytokine IL-5 in BALF were increased in asthmatic group compared with control group, while Type 1 cytokine IFN-γ was decreased (Figure 1C). Moreover, HE staining showed increased inflammation in asthmatic group than in control group. PAS staining showed that mucus secretion was much more in asthmatic group than in control group (Figure 1D). These results suggested successful establishment of murine asthma model.
Figure 1.
Successful establishment of murine asthma model. (A) Protocol of murine asthma model by using OVA. (B) The number of leukocytes, neutrophils, eosinophils, lymphocytes and monocytes in BALF of murine asthma group and control group. (C) The levels of IgE, IFNγ and IL-5 in BALF were determined by Elisa analysis. (D) HE (haematoxylin and eosin) and PAS (periodic acid-Schiff stain) analysis of lung tissues in murine asthma group and control group, and histopathology score (PA) and periodic acid-Schiff stain score (PAS). The study was repeated for three times. **P<0.01; ***P<0.001, ****P<0.0001.
LncRNA H19 Was Decreased, While Muc5ac Was Increased in Murine Asthma Model and in 16-HBE Cells Treated with TNFα
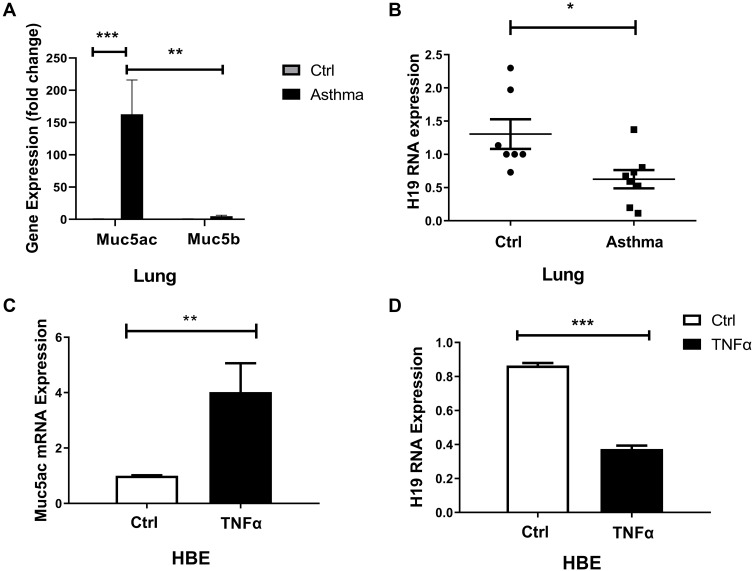
It was reported that increased mucins in airways of asthmatic were primarily composed of Muc5ac, not Muc5b, and that Muc5ac played a pathologic role.3 We found that Muc5ac was significantly increased in lung tissues of asthmatic mice compared with control group, and Muc5ac was much higher than Muc5b (Figure 2A). We hypothesized that lncRNA H19 might be involved in asthma and regulate expression of Muc5ac. Firstly, we detected lncRNA H19 expression in murine asthma model. The results showed that lncRNA H19 in lung tissues was significantly decreased in asthma group compared with control group (Figure 2B). TNFα is an important inflammatory mediator which contributes to Muc5ac overproduction in asthma. Therefore, it was used to treat 16-HBE cells to construct in vitro inflammation model of asthma.28,29 Subsequently, we detected expression of lncRNA H19 and Muc5ac in the in vitro inflammation model. Consistent with in vivo study, obviously increased Muc5ac mRNA expression (Figure 2C) and decreased lncRNA H19 expression were detected (Figure 2D) when 16-HBE cells were stimulated with TNFα. These results indicated that lncRNA H19 expression was negatively correlated with Muc5ac expression both in in vivo and in vitro model, and lncRNA H19 might be involved in the regulation of Muc5ac in asthma.
Figure 2.
Decreased lncRNA H19 and increased Muc5ac in murine asthma model and 16-HBE cells treated with TNFα. q-PCR analysis of Muc5ac, Muc5b (A) and lncRNA H19 (B), in lung tissues of murine asthma group and control group. q-PCR analysis of LncRNA Muc5ac (C) and lncRNA H19 (D) in 16-HBE cells treated with TNFα for 24 hours. The study was repeated for three times. *P<0.05; **P<0.01; ***P<0.001.
LncRNA H19 Positively Regulated Muc5ac Expression
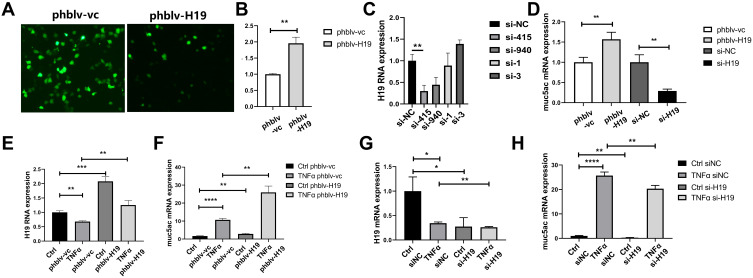
Since lncRNA H19 was decreased in asthma, we deduced that lncRNA H19 plays an important role in asthma. In order to investigate the role of lncRNA H19 in pathogenesis of asthma, plasmid vectors overexpressing lncRNA H19 were constructed and siRNAs targeting lncRNA H19 were synthesized. Plasmids phblv-vc and phblv-H19 were successfully transfected into 16-HBE cells (Figure 3A), and lncRNA H19 was obviously increased in phblv-H19 treated group compared with phblv-vc treated group (Figure 3B). siRNA-415 was the most effective in knocking down lncRNA H19 expression among the four siRNAs (Figure 3C), which was used in subsequent experiments as si-H19. Muc5ac mRNA expression was increased in phblv-H19 group compared with phblv-vc group, whereas it was decreased in si-H19 group compared with si-NC group (Figure 3D). These results suggested that lncRNA H19 positively regulated Muc5ac mRNA expression.
Figure 3.
LncRNA H19 positively regulated Muc5ac mRNA expression in 16-HBE cells. (A) Fluorescent visions of 16-HBE cells transfected with plasmids phblv-vc (left) and phblv-H19 (right), respectively. (B) Verification of lncRNA H19 overexpression by q-PCR. (C) Comparison of knockdown efficacy of four siRNAs targeting lncRNA H19 after transfection for 24 hours by q-PCR. (D) q-PCR analysis of Muc5ac in 16-HBE cells after overexpression and knockdown of lncRNA H19 for 24 hours. q-PCR analysis of lncRNA H19 (E and G) and Muc5ac (F and H) in 16-HBE cells transfected respectively with plasmids phblv-vc and phblv-H19 (E and F), or si-NC and si-H19 (G and H) for 24 hours and subsequently with TNFα treatment for 24 hours. The study was repeated for three times. *P<0.05; **P<0.01; ***P<0.001, ****P<0.0001.
TNFα treatment of 16-HBE transfected with plasmids phblv-vc or siRNAs si-NC showed a decrease in lncRNA H19 (Figure 3E and G) and an increase in Muc5ac expression (Figure 3F and H), which is consistent with the in vivo and in vitro results described earlier. lncRNA H19 was significantly increased in plasmids phblv-H19 transfected TNFα treatment group compared with plasmids phblv-vc transfected TNFα treatment group (Figure 3E), and Muc5ac expression was significantly higher in the former group than the latter group (Figure 3F). Similarly, lncRNA H19 was significantly decreased in si-H19 transfected TNFα treatment group compared with si-NC transfected TNFα treatment group (Figure 3G), and Muc5ac expression was significantly lower in the former group than the latter group (Figure 3H). These results furtherly demonstrated that lncRNA H19 positively regulated TNFα-induced Muc5ac mRNA expression (Figure 3F and H). Therefore, we speculated that decreased lncRNA H19 expression in asthma might be a protective mechanism partly relieving mucin overproduction in asthma.
LncRNA H19 Regulated Muc5ac Expression via PI3K/Akt/NF-κB Pathway in 16-HBE Cells
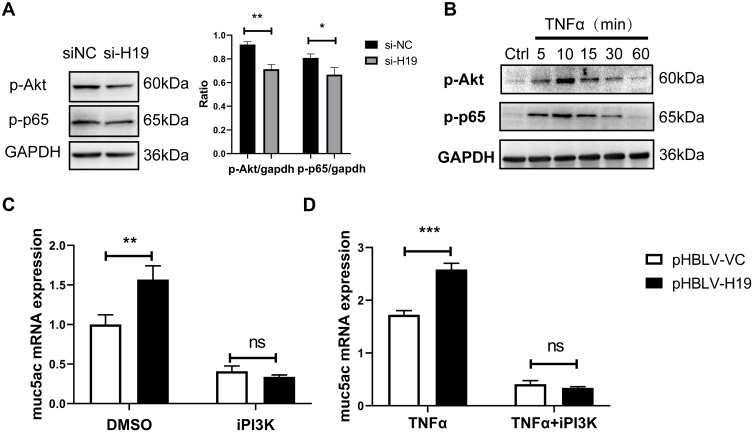
NF-κB was widely demonstrated as a downstream transcription factor of PI3K/Akt, binding to promoter region of Muc5ac gene.30 To further explore mechanisms which mediated effects of lncRNA H19 on Muc5ac expression, lncRNA H19 was knocked down in 16-HBE cells by transfection with si-H19. p-Akt and p-p65 were reduced in knockdown group compared with control group (Figure 4A). TNFα treatment rapidly activated Akt and NF-κB in 16-HBE cells (Figure 4B). Moreover, PI3K inhibitor blocked effects of lncRNA H19 on Muc5ac expression, no matter with (Figure 4D) or without TNFα treatment (Figure 4C). These results indicated that lncRNA H19 regulated Muc5ac expression via PI3K/Akt/NF-κB pathway in vitro inflammation model.
Figure 4.
LncRNA H19 regulated Muc5ac mRNA expression via PI3K/Akt/NF-κB in 16-HBE cells. (A) After knock down of lncRNA H19 in 16-HBE cells for 24 hours, p-Akt, p-p65 and GAPDH were assayed by Western Blotting. Expressions of p-Akt and p-p65 were normalized to GAPDH. (B) Western Blotting analysis of p-Akt and p-p65 in 16-HBE cells treated with TNFα for different time. After overexpression of lncRNA H19 for 24 hours (right), 16-HBE cells was advancely treated with inhibitors of PI3K for 40 minutes and then with (D) or without (C) TNFα for 24 hours. Muc5ac was assayed by q-PCR. The study was repeated for three times. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
LncRNA H19 Regulated miR-675-3p Production, Which Had No Effects on Muc5ac Expression
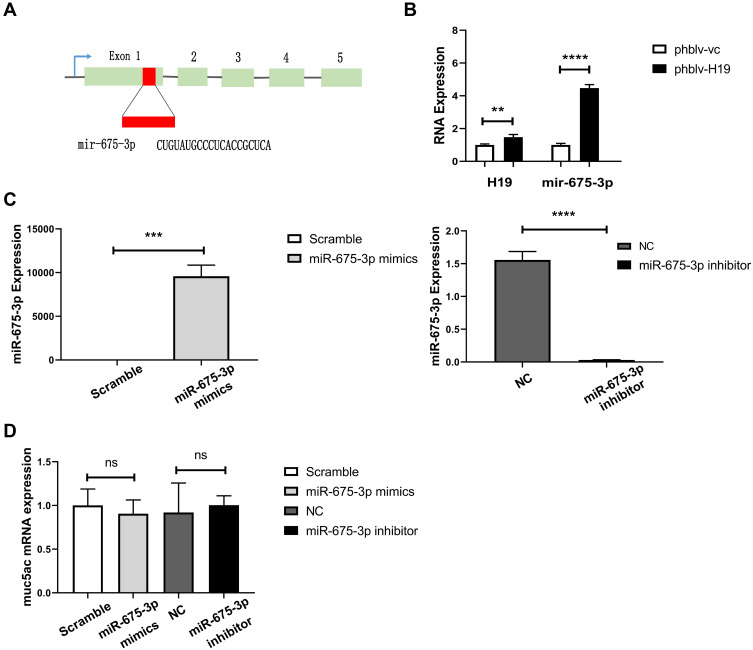
LncRNA H19 encodes miR-675-3p from its exon 1 (Figure 5A), and plays important roles in numerous diseases via regulation of mir-675 production. We found that lncRNA H19 overexpression definitely increased miR-675-3p production by transfection with plasmids phblv-H19 in 16-HBE cells (Figure 5B). To explore if miR-675-3p regulates Muc5ac expression, miR-675-3p was overexpressed or knocked down in 16-HBE cells by transfection with miR-675-3p mimics or inhibitors, respectively (Figure 5C). However, Muc5ac mRNA expression was not significantly changed (Figure 5D). These results indicated that lncRNA H19 regulated Muc5ac expression not through regulation of miR-675-3p production.
Figure 5.
miR-675 processed from lncRNA H19 had no regulatory effects on Muc5ac mRNA expression in 16-HBE cells. (A) miRNA-675 encoded by exon 1 of lncRNA H19 gene. (B) q-PCR analysis of miR-675 in 16-HBE cells after overexpression of lncRNA H19 for 24 hours. After overexpression (left) and knockdown (right) of miR-675-3p in 16-HBE cells for 24 hours, miR-675-3p (C) and Muc5ac expressions (D) were assayed by q-PCR. The study was repeated for three times. **P<0.01; ***P<0.001; ****P<0.0001, ns, not significant.
Discussion
Currently, reports on lncRNAs are increasing day by day. LncRNA H19 is a maternally expressed and conserved long noncoding RNA, widely expressed in many organs, and its roles have been largely reported in many diseases except asthma. In our study, we found that lncRNA H19 positively regulated Muc5ac mRNA expression via PI3K/Akt/NF-κB pathway in the in vitro inflammation model. Moreover, decreased lncRNA H19 levels were detected in the in vitro inflammation model and the murine asthma model. These results indicated that decreased lncRNA H19 in asthma might be a protective mechanism partly by reducing mucin overproduction in asthmatic airways.
TNFα is one of cardinal inflammatory factors in asthma, mediating persistent activation of NF-κB, which is proved to bind to the promoter region of Muc5ac gene.30 TNFα was elevated in asthmatic airways and induced EGFR expression in airway epithelium. Subsequently, EGFR-ligands increased Muc5ac production at both gene and protein levels.31 Furthermore, TNFα was reported to activate RhoA expression and thus leading to AHR.32 Anti-TNFα therapy were effective and could alleviate inflammatory cell infiltration, inflammation damages in airways and AHR.33–36 Because of crucial roles of TNFα in asthmatic airways and Muc5ac expression, we treated bronchial epithelial cells 16-HBE with TNFα and thereby induced Muc5ac production. In the in vitro inflammation model, Muc5ac was increased, while lncRNA H19 was decreased. Moreover, increased Muc5ac and decreased lncRNA H19 in lung tissues were also detected in murine asthmatic group compared with control group. Our results in the in vitro and in vivo models were consistent. These results were also consistent with asthmatics.16
In our study, lncRNA H19 was demonstrated to positively regulate Muc5ac mRNA expression by transfection with plasmids phblv-vc and phblv-H19 in 16-HBE cells. lncRNA H19 overexpression increased TNFα-induced Muc5ac expression, while lncRNA H19 knockdown reduced TNFα-induced Muc5ac expression. Interestingly, lncRNA H19 was decreased while Muc5ac was increased both in murine asthma model and in the in vitro inflammation model. These results indicate the points as follows: 1) LncRNA H19 positively regulates Muc5ac expression, and overexpression of lncRNA H19 promotes the development of asthma. 2) Decreases of lncRNA H19 are probably a protective mechanism in murine asthma model. Otherwise the expression of Muc5ac was higher. 3) It deserves further study about the mechanisms of decreases of lncRNA H19 in asthma.
Positive regulation of Muc5ac by lncRNA H19 and their opposite changes in asthma model implied that Muc5ac mRNA expression might be increased by other mechanisms such as EGFR.31 As reported, TNFα was elevated in asthmatic airways and induced EGFR expression in airway epithelium. EGFR-ligands binding to EGFR increased Muc5ac production at both gene and protein levels.31,37 H19 was reported to activate EGFR.38 Consequently, lncRNA H19 probably promotes Muc5ac production through EGFR. And thus Muc5ac expression was higher after lncRNA H19 overexpression in the in vitro inflammation model. However, Muc5ac production was partly inhibited by downregulation of lncRNA H19 through some mechanisms in murine asthma model.
LncRNAs functions in numerous diseases through various mechanisms, including regulation of transcription factors, microRNAs, regulatory proteins, chromatin-modifying complexes and mRNA targets.39 To further explore the mechanism of Muc5ac regulation by lncRNA H19, lncRNA H19 knockdown were achieved by transfection with si-H19 in 16-HBE cells, resulting in decreased p-Akt, p-NF-κB and Muc5ac. NF-κB is demonstrated as a transcription factor for Muc5ac in numerous studies, which containing a binding site at the promoter region of Muc5ac gene.28 Moreover, NF-κB was reported as a downstream transcription factor of PI3K/Akt.40–42 In the in vitro inflammation model, TNFα rapidly activated Akt and NF-κB in 16-HBE cells. In our study, transcriptional levels of Muc5ac gene were increased by lncRNA H19 overexpression when treated with or without TNFα. However, inhibitors of PI3K abolished the promotive effect of lncRNA H19 on Muc5ac expression through abrogation of phosphorylation of Akt and subsequent activation of NF-κB. Therefore, our study indicated that lncRNA H19 regulated Muc5ac expression through PI3K/AKT/NF-κB pathway.
miR-675-3p is a microRNA encoded in exon 1 of lncRNA H19 and can be processed from it. lncRNA H19 was reported to regulate intestinal epithelial barrier function via miR-675.28 Therefore, we wondered whether lncRNA H19 regulated Muc5ac expression through miR-675-3p in asthma. In our study, overexpression of lncRNA H19 increased production of miR-675-3p in 16-HBE cells. However, miR-675-3p had no effects on Muc5ac expression.
To sum up, our study demonstrated that lncRNA H19 positively regulated Muc5ac expression through PI3K/AKT/NF-κB pathway. However, there were some limitations of our study are as follows: The effect of lncRNA H19 overexpression on Muc5ac expression in murine asthma model had not been studied. Furthermore, it needs to prove that whether H19 promotes Muc5ac production through EGFR.
Many asthmatics are suffering from airway obstruction and airflow limit. Currently, the common therapy is combination treatments of various bronchodilators and corticosteroids. However, the therapeutic effects are limited and temporary. Besides, corticosteroids have side effects such as osteoporosis and pneumonia.43 Thus, new treatments for asthma are urgently needed. Given that Muc5ac is a dominant component of gels formed in airways of asthmatics, it has become a major drug target for asthma treatment.44 Therefore, our study about the regulative role of lncRNA H19 in Muc5ac expression might provide a new insight into asthma treatment from the perspective of long noncoding RNA.
Funding Statement
This work was supported by the Natural Science Foundation of China (No. 81671639 and 31270984).
Data Sharing Statement
The data generated and analysed during this study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x [DOI] [PubMed] [Google Scholar]
- 2.Fanta CH. Asthma. N Engl J Med. 2009;360(10):1002–1014. doi: 10.1056/NEJMra0804579 [DOI] [PubMed] [Google Scholar]
- 3.Welsh KG, Rousseau K, Fisher G, et al. MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest. 2017;152(4):771–779. doi: 10.1016/j.chest.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lachowicz-Scroggins ME, Yuan S, Kerr SC, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med. 2016;194(10):1296–1299. doi: 10.1164/rccm.201603-0526LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans CM, Raclawska DS, Ttofali F, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6(1):6281. doi: 10.1038/ncomms7281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samsuzzaman M, Uddin MS, Shah MA, Mathew B. Natural inhibitors on airway mucin: molecular insight into the therapeutic potential targeting MUC5AC expression and production. Life Sci. 2019;231:116485. doi: 10.1016/j.lfs.2019.05.041 [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Weir D, Busse P, et al. The flavonoid 7,4ʹ-dihydroxyflavone inhibits muc5ac gene expression, production, and secretion via regulation of NF-kappaB, STAT6, and HDAC2. Phytother Res. 2015;29(6):925–932. doi: 10.1002/ptr.5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang J, Liu W, Yin C, Chu H, Zhang M. Cucurbitacin E ameliorates lipopolysaccharide-evoked injury, inflammation and MUC5AC expression in bronchial epithelial cells by restraining the HMGB1-TLR4-NF-kappaB signaling. Mol Immunol. 2019;114:571–577. doi: 10.1016/j.molimm.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 9.Shin IS, Park JW, Shin NR, et al. Melatonin inhibits MUC5AC production via suppression of MAPK signaling in human airway epithelial cells. J Pineal Res. 2014;56(4):398–407. doi: 10.1111/jpi.12127 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Yang X, Li Y, et al. Lyn kinase represses mucus hypersecretion by regulating IL-13-induced endoplasmic reticulum stress in asthma. EBioMedicine. 2017;15:137–149. doi: 10.1016/j.ebiom.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie T, Luo G, Zhang Y, et al. Rho-kinase inhibitor fasudil reduces allergic airway inflammation and mucus hypersecretion by regulating STAT6 and NFkappaB. Clin Exp Allergy. 2015;45(12):1812–1822. doi: 10.1111/cea.12606 [DOI] [PubMed] [Google Scholar]
- 12.Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med. 2017;6(12):12. doi: 10.3390/jcm6120112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heward JA, Lindsay MA. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014;35(9):408–419. doi: 10.1016/j.it.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing R, Guo X, Yang Y, Chen W, Kang J, Zhu S. Long noncoding RNA Q associates with sox2 and is involved in the maintenance of pluripotency in mouse embryonic stem cells. Stem Cells. 2020. doi: 10.1002/stem.3180 [DOI] [PubMed] [Google Scholar]
- 15.Kotzin JJ, Spencer SP, McCright SJ, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537(7619):239–243. doi: 10.1038/nature19346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PJ, Tsitsiou E, Boardman C, et al. Transcriptional profiling identifies the long noncoding RNA plasmacytoma variant translocation (PVT1) as a novel regulator of the asthmatic phenotype in human airway smooth muscle. J Allergy Clin Immunol. 2017;139(3):780–789. doi: 10.1016/j.jaci.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swarr DT, Herriges M, Li S, et al. The long noncoding RNA falcor regulates Foxa2 expression to maintain lung epithelial homeostasis and promote regeneration. Genes Dev. 2019;33(11–12):656–668. doi: 10.1101/gad.320523.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X, Huang S, Xue P, et al. LncRNA PTPRE-AS1 modulates M2 macrophage activation and inflammatory diseases by epigenetic promotion of PTPRE. Sci Adv. 2019;5(12):eaax9230. doi: 10.1126/sciadv.aax9230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Yin Z, Fan J, Zhang S, Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;4(1):47. doi: 10.1038/s41392-019-0080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia L, Wang X, Liu L, et al. lnc-BAZ2B promotes M2 macrophage activation and inflammation in children with asthma through stabilizing BAZ2B pre-mRNA. J Allergy Clin Immunol. 2021;147(3):921–932.e929. doi: 10.1016/j.jaci.2020.06.034 [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Qi N, Zhou Q. LncRNA H19 inhibits proliferation and migration of airway smooth muscle cells induced by PDGF-BB through miR-21/PTEN/Akt axis. J Asthma Allergy. 2021;14:71–80. doi: 10.2147/JAA.S291333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu YJ, Mao D, Gao W, Hu H. Peripheral whole blood lncRNA expression analysis in patients with eosinophilic asthma. Medicine. 2018;97(8):e9817. doi: 10.1097/MD.0000000000009817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Y, Mao D, Gao W, Han G, Hu H. Analysis of lncRNA expression in patients with eosinophilic and neutrophilic asthma focusing on LNC_000127. Front Genet. 2019;10:141. doi: 10.3389/fgene.2019.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XY, Tang XY, Li N, et al. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sci. 2018;212:93–101. doi: 10.1016/j.lfs.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 25.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351(6322):153–155. doi: 10.1038/351153a0 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Tycko B. Monoallelic expression of the human H19 gene. Nat Genet. 1992;1(1):40–44. doi: 10.1038/ng0492-40 [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Zhang X, Chen X, et al. Streptococcus pneumoniae aminopeptidase N regulates dendritic cells that attenuates type-2 airway inflammation in murine allergic asthma. Br J Pharmacol. 2020;177(22):5063–5077. doi: 10.1111/bph.15216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Chen C, Gao Y, et al. Bergenin-activated SIRT1 inhibits TNF-α-induced proinflammatory response by blocking the NF-κB signaling pathway. Pulm Pharmacol Ther. 2020;62:101921. doi: 10.1016/j.pupt.2020.101921 [DOI] [PubMed] [Google Scholar]
- 29.Lea S, Li J, Plumb J, et al. P38 MAPK and glucocorticoid receptor crosstalk in bronchial epithelial cells. J Mol Med. 2020;98(3):361–374. doi: 10.1007/s00109-020-01873-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SU, Sung MH, Ryu HW, et al. Verproside inhibits TNF-alpha-induced MUC5AC expression through suppression of the TNF-alpha/NF-kappaB pathway in human airway epithelial cells. Cytokine. 2016;77:168–175. doi: 10.1016/j.cyto.2015.08.262 [DOI] [PubMed] [Google Scholar]
- 31.Takeyama K, Dabbagh K, Lee HM, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999;96(6):3081–3086. doi: 10.1073/pnas.96.6.3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiba Y, Danno S, Suto R, et al. Intranasal administration of recombinant progranulin inhibits bronchial smooth muscle hyperresponsiveness in mouse allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2018;314(1):L215–L223. doi: 10.1152/ajplung.00575.2016 [DOI] [PubMed] [Google Scholar]
- 33.Cai Y, Cao YX, Lu SM, Xu CB, Cardell LO. Infliximab alleviates inflammation and ex vivo airway hyperreactivity in asthmatic E3 rats. Int Immunol. 2011;23(7):443–451. doi: 10.1093/intimm/dxr032 [DOI] [PubMed] [Google Scholar]
- 34.Catal F, Mete E, Tayman C, Topal E, Albayrak A, Sert H. A human monoclonal anti-TNF alpha antibody (adalimumab) reduces airway inflammation and ameliorates lung histology in a murine model of acute asthma. J Clin Med. 2015;43(1):14–18. doi: 10.1016/j.aller.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 35.Kim J, McKinley L, Natarajan S, et al. Anti-tumor necrosis factor-alpha antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin Exp Allergy. 2006;36(1):122–132. doi: 10.1111/j.1365-2222.2005.02407.x [DOI] [PubMed] [Google Scholar]
- 36.Luo Y, Pang Z, Zhu Q, et al. Locally instilled tumor necrosis factor-alpha antisense oligonucleotide inhibits allergic inflammation via the induction of Tregs. J Gene Med. 2012;14(6):374–383. doi: 10.1002/jgm.2631 [DOI] [PubMed] [Google Scholar]
- 37.Jia Z, Bao K, Wei P, et al. EGFR activation-induced decreases in claudin1 promote MUC5AC expression and exacerbate asthma in mice. Mucosal Immunol. 2021;14(1):125–134. doi: 10.1038/s41385-020-0272-z [DOI] [PubMed] [Google Scholar]
- 38.Ye Y, Guo J, Xiao P, et al. Macrophages-induced long noncoding RNA H19 up-regulation triggers and activates the miR-193b/MAPK1 axis and promotes cell aggressiveness in hepatocellular carcinoma. Cancer Lett. 2020;469:310–322. doi: 10.1016/j.canlet.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 39.Hu W, Alvarez-Dominguez JR, Lodish HF. Regulation of mammalian cell differentiation by long non-coding RNAs. EMBO Rep. 2012;13(11):971–983. doi: 10.1038/embor.2012.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han W, Xiong Y, Li Y, et al. Anti-arthritic effects of clematichinenoside (AR-6) on PI3K/Akt signaling pathway and TNF-alpha associated with collagen-induced arthritis. Pharm Biol. 2013;51(1):13–22. doi: 10.3109/13880209.2012.698287 [DOI] [PubMed] [Google Scholar]
- 41.Hyam SR, Lee IA, Gu W, et al. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur J Pharmacol. 2013;708(1–3):21–29. doi: 10.1016/j.ejphar.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 42.Zhao J, Ma ST. Downregulation of lncRNA H19 inhibits migration and invasion of human osteosarcoma through the NF-kappaB pathway. Mol Med Rep. 2018;17(5):7388–7394. [DOI] [PubMed] [Google Scholar]
- 43.Hanania NA, Chapman KR, Kesten S. Adverse effects of inhaled corticosteroids. Am J Med. 1995;98(2):196–208. doi: 10.1016/S0002-9343(99)80404-5 [DOI] [PubMed] [Google Scholar]
- 44.Dey BK, Pfeifer K, Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28(5):491–501. doi: 10.1101/gad.234419.113 [DOI] [PMC free article] [PubMed] [Google Scholar]