Abstract
Background
Citrate is used as a regional anticoagulant for continuous veno-venous haemofiltration and provides 0.59 kcal/mmol. Previous studies hypothesised continuous veno-venous haemofiltration can provide 200–1300 kcal/day dependent on the anticoagulant and replacement solutions used. The aim of this study was to calculate the calorie load from citrate in our patient group.
Methods
An equation derived from a paper by Oudemans-van Straaten was used to estimate calorie provision from citrate. Citrate calorie load was defined as the difference between the citrate in the filter circuit and the removal by continuous veno-venous haemofiltration. Clinical data were recorded on 20 consecutive patients admitted to intensive care unit and commenced on citrate continuous veno-venous haemofiltration using prismacitrate 18/0 by Gambro, a tri-sodium citrate solution. Clinical data recorded included patient demographics, filter settings including blood flow, filtration factor, citrate dose and time on filtration daily.
Results
A total of 20 critically ill patients received continuous veno-venous haemofiltration for treatment of a new acute kidney injury, mean age 66 years, 65% male. Mean duration of continuous veno-venous haemofiltration was 3.7 days. Mean daily time on filtration was 20 h/day. Mean filtration fraction, citrate dose and blood flow were 30%, 3 mmol/L and 123 ml/min, respectively. Our calculation showed that a mean of 9.5 ± 1.7 cal/h were provided from citrate with a mean daily calorie load of 196 ± 69 kcal.
Conclusions
Continuous veno-venous haemofiltration with tri-sodium citrate provided an additional 196 ± 69 kcal/day. The calorie load from citrate continuous veno-venous haemofiltration should be calculated regularly as changes in filter settings, in particular citrate dose and blood flow can have a significant impact on calorie provision.
Keywords: Citrate, renal replacement therapy, critical illness, calorie
Introduction
Acute kidney injury (AKI) is a common occurrence during critical illness often occurring in the context of multiorgan failure and sepsis. AKI occurs in 35%–60% of critical care admissions1–3 with approximately two thirds of these requiring renal replacement therapy (RRT).3,4
Anticoagulation is frequently used during continuous veno-venous haemofiltration (CVVHDF) in order to prevent clotting of the extracorporeal circuit. Heparin has been the traditional anticoagulant of choice in CVVHDF; however, systemic anticoagulation with heparin in the critically ill has been associated with complications, and there have been increasing questions regarding its safety.5 Complications include increased risk of haemorrhages and heparin resistance.6 As a result, citrate anticoagulation has gained popularity due to its regional action, reducing patients’ risk of bleeding whilst maintaining a similar or improved circuit life.5 Citrate prevents the clotting cascade by chelating calcium.7 A citrate solution is infused prior to the blood entering the filter which binds to serum calcium, with a replacement infusion of calcium given after filtration to reinstate the clotting cascade6,7 as shown in Figure 1. The chelated citrate is then rapidly metabolised in the Krebs Cycle.6,7
Figure 1.
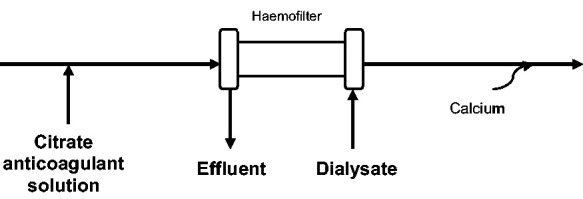
Filter set up with TSC solution.
Citrate provides a calorie value of 0.59 kcal/mmol when metabolised in the Krebs cycle7; however, the calorific gain from citrate filtration is dependent on the citrate solution used, dose infused and the amount removed by CVVHDF.6,7 Studies have hypothesised that CVVHDF using a tri-sodium citrate (TSC) solution can provide 220 to 350 kcal/day.6–8 If an anticoagulant solution containing glucose in addition to citrate is used, such as acid citrate dextrose (ACD) and replacement fluids containing lactate are used, then calorie provision will be higher with studies finding a calorie load of 350 to 600 kcal/day.6–8 Calorie load from citrate varies depending on filtration settings, blood pump rate and filtration fraction, and on citrate dose infused.5 It is important to establish the calorie provision from citrate anticoagulation and include this in nutritional calculations and feeding plans as overfeeding has been shown to have a detrimental impact on patient outcomes in the critically ill, including increased rates of hyperglycemia, respiratory insufficiency and increased risk of infectious complications.9,10
Gambro Prismacitrate 18/0 solution was introduced as the standard anticoagulation solution for CVVHDF on the general intensive care unit (ICU) at University Southampton NHS Foundation Trust (UHS) in 2017; this is TSC solution. Following this change in unit practice, this study was designed to establish the average calorie provision from citrate anticoagulation in our patient group and the variability in the calorie provision for each patient to determine if a set value could be used or the calorie provision would need to be calculated daily for individual patients. We also sought to assess the potential implications of this increased calorie load on nutritional adequacy, particularly in regard to protein and micronutrient intakes, in our patient group.
Methods
This project was registered via the UHS clinical audit system. The study was considered a service improvement project, and therefore, ethical approval was not required.
Data were collected retrospectively between April and July 2018 by the critical care dietitians. Information was collected on 20 patients consecutively commenced on CVVHDF with Gambro Prismacitrate 18/0 solution for treatment of a new AKI during this period. Information was collected from patients’ electronic medical notes and the filter on the blood flow, filtration fraction, citrate dose and time on filtration daily. If the information was not available, the last recorded data were used for that day. Patients were excluded if they were receiving CVVHDF for less than 48 h. The first and last days of CVVHDF were excluded from data collection. Information regarding demographics was also collected, and energy requirements calculated using 25 kcal/kg; for obese patients (body mass index (BMI) >30 kg/m2) calorie requirements were based on 25 kcal/kg ideal body weight (IBW) based on BMI of 25 kg/m2. Protein requirements were calculated at 1.5 g of protein/kg actual body weight or IBW if BMI >30 kg/m2.
The citrate calorie load is defined as the balance between the total citrate dose administered in the circuit and the mass removal by heamofiltration.11 An equation to estimate calorie load from citrate was derived based on the calculations found in the paper by Oudemans-van Straaten5as shown in Figure 2 and based on the assumption that in heamofiltration citrate removal is similar to the filtration fraction.7 Filtration fraction is the relationship between the replacement fluid rate and the blood flow rate calculated as: filtration fraction = replacement rate/(blood flow rate × 60). The citrate calorie load per minute calculated using the Oudemans-van Straaten equation was multiplied by 60 to provide an hourly value and then multiplied by the hours of filtration received per day to give the daily citrate calorie provision.
Results
A total of 20 patients were included in the analysis; patients were a mix of medical, surgical and oncology admissions (Figure 3). Patient baseline characteristics are shown in Table 1.
Figure 3.
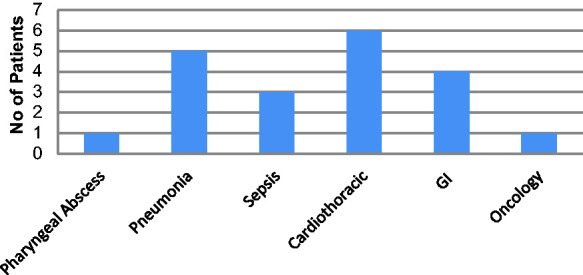
Reason for ICU admission.
Table 1.
Baseline characteristics of patients studied.
| Age (years) | 66.8 ± 12.9 |
| Gender (% male) | 70 |
| Weight (kg) | 97.2 ± 22.3 |
| Body mass index (kg/m2) | 32.6 ± 7.7 |
| Estimated calorie requirements (kcal) | 2041.7 ± 257.6 |
Data are presented as mean ± standard deviation.
Excluding the first and last day of filtration 74 days of data were captured; mean of 3.7 days per patient (range 1–18 days). Daily mean duration of CVVHDF was 20 h (range 5–24). Mean filtration fraction was 30.0 ± 2.0%; mean citrate dose was 3.0 ± 0.3 mmol/L; mean blood flow was 123.0 ± 19.6 ml/min.
Calorie provision from citrate based on our calculations ranged from 6.1 to 14.3 kcal/h (mean 9.5 ± 1.7). This resulted in a daily mean calorie provision of 196 ± 69 kcal. Citrate calories provided on average 9.7 ± 2.7% of patients estimated calorie needs.
Discussion
Our study found a mean calorie provision from citrate of approximately 200 kcal/day using a TSC solution. This is similar to other studies that found citrate provides 210–218 kcal per day.8–12
As expected, the net calorie gain in our unit was lower than that stated in some other papers, as in our protocol a TSC solution is used. The TSC solution does not contain dextrose as opposed to ACD. Previous studies have reported the net calorie gain from the glucose in anticoagulation solutions can be as high as 295 to 500 kcal/day.5,7 Further to this, our unit uses a low lactate replacement fluid which is not expected to provide a substantial gain of energy due to the small difference in glucose and lactate concentrations between the dialysate solutions and the patients’ blood.8 Papers have reported a gain of 850 to 1300 kcal/day using an ACD solution and high lactate replacement fluids.6,7,12 It should be noted that our study was a pilot study and had a small number of patients enrolled, further larger scale studies in this area are required to assess the impact of citrate calorie provision on nutritional status in patients receiving CVVDHF.
It is important to ascertain the calorie provision from citrate to reduce the risk of overfeeding critically ill patients, which in turn increases the risk of morbidity, mortality and increased length of stay.13,14 It should be noted, however, that during times of critical illness, calories from citrate may be advantageous, as it is easy for the body to utilise as no insulin is required and it can replenish the Krebs cycle if intermediates are scarce.7,11,12
Haemofiltration does not appear to increase calorie needs compared to a non-filtered patient,15,16 but protein requirements are increased due to the loss of amino acids in the filter.15 Previous studies have found that a minimum of 1.5 g of protein/kg is required to achieve positive nitrogen balance taking into account hypercatabolism and amino acid losses, with up to 1.8 g of protein/kg being found to be beneficial.11,15 This can be difficult to achieve with enteral feeds given the low protein to energy ratio of commercially available enteral feeds and reduced calorie requirement from feed due to citrate calorie provision. Citrate can also affect micronutrient needs, citrate chelates magnesium which is only partially replaced with the dialysate solution increasing the requirement for additional supplementation.5
On average, citrate provided 9.7% of individuals calorie requirements up to a maximum of 16%. To account for this, the volume of enteral feed required to meet calorie needs is reduced which has a significant impact on the provision of other nutrients, in particular protein. If fed using a standard 1 kcal/ml enteral feed to meet estimated energy requirements adjusted to account for the calorie burden from citrate, none of our patients would have adequate protein provision (1.5 g/kg) with patients only receiving an average of 60% of their protein requirements. If a high protein feed was used, protein provision was still inadequate for any patients to meet their protein needs; however, protein provision was increased to 75% of protein requirements. Addition of any other non-nutritional calorie sources, for example, propofol, will reduce the volume of feed required, and therefore, protein provision further. In our patient group, the deficit in protein needs is made up with the use of modular protein supplements and access to these products is key to meeting protein requirements in patients receiving citrate anticoagulation.
The reduction in volume of feed required to meet calorie requirements due to citrate calorie provision also reduces the vitamin and mineral load. The high protein feed used as first line on our unit requires patients to receive 1339 kcal (males) and 1205 kcal (females) to be nutritionally adequate; all patients in this study received this. However, it should be noted that this does not reflect any increased micronutrient requirements as a result of CVVHDF which needs to be investigated further.
In our study, there was a large variation in calorie provision/h between 6.1 and 14.3 kcal/h; this was due to changes in blood flow, filtration fraction and citrate dose settings on the haemofilter. Citrate dose was the most stable with an average of 1.5 settings per patient and a range of 2.5–4 mmol/L, the difference between calorie provision at the maximum and minimum citrate dose was 108 kcal/day. Filtration fraction changed on the filter most frequently with an average of 2.35 settings per patient with a maximum of 7 settings per patient; collection of filtration fraction data was limited, as it was often not recorded electronically and so could only be collected by the dietitians from the machine when visiting the ICU to review patients. This introduced potential error in our analysis as assumptions that the filtration factor was not changed were made. However, changes in filtration fraction had the smallest impact on calorie provision; therefore, these assumptions are unlikely to have had a significant effect on our results. At extremes of filtration fraction, the calorie provision changed from 8.2 kcal to 9.4 kcal/h. Individuals had an average of 1.95 settings of blood flow in our study with a range of 80 to 200 ml/min; this had the biggest impact on calorie load with a difference of 9 kcal/h between the lowest and highest blood flow. This is equivalent to 216 kcal/day.
Conclusions
TSC solutions provided an average calorie load of 197 kcal/day in our study; however, this value is lower than that reported when using ACD solutions or high lactate replacement fluids. The calorie provision from citrate haemofiltration needs to be calculated regularly as part of a specialist nutritional assessment as a change in filtration settings can have a significant impact on calories delivered and should be taken into account when prescribing feeding regimens for critically ill patients to prevent overfeeding and associated complications and to ensure nutritional adequacy.
Figure 2.

Calculation to estimate citrate calories entering blood stream during CVVHDF.
Acknowledgement
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, University Hospital Southampton NHS Foundation Trust or the Department of Health.
Contributors statement
AR and BJ conceived the project. AR drafted the manuscript. AR completed the data collection and analysis. BJ edited, read and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Alice R Rogers https://orcid.org/0000-0003-3177-9515
References
- 1.Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008; 5: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 2.Koeze J, Keus F, Dieperink W, et al. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol 2017; 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411–1423. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RL, Pascaul MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66: 1613–1621. [DOI] [PubMed] [Google Scholar]
- 5.Oudemans-van Straaten HM, Kellum JA, Bellomo R. Clinical review: anticoagulation for continuous renal replacement therapy – heparin or citrate. Crit Care 2011; 15: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morabito S, Pistolesi V, Tritapepe L, et al. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin J Am Soc Nephrol 2014; 9: 2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oudemans-van Straaten HM, Ostermann M. Bench to bedside review: citrate for continuous renal replacement therapy from science to practice. Crit Care 2012; 16: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.New A, Nystrom E, Frazee E, et al. Continuous renal replacement therapy: a potential source of calories in the critically ill. Am J Clin Nutr 2017; 105: 1559–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Presier JC, van Zanten ARH, Berger MM, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care 2015; 19: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler TR. Parenteral Nutrition in the critically ill patient. N Engl J Med 2009; 361: 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiaccadori E, Regolisti G, Maggiore U. Specialized nutritional support interventions in critically ill patients on renal replacement therapy. Curr Opin Clin Nutr Metab Care 2013; 16: 217–224. [DOI] [PubMed] [Google Scholar]
- 12.Balik M, Zakharcehnko M, Leden P, et al. Bioenergetic gain of citrate anticoagulated continuous hemodiafiltration—a comparison between 2 citrate modalities and unfractionated heparin. J Crit Care 2013; 28: 87. [DOI] [PubMed] [Google Scholar]
- 13.Weijs P, Looijaard W, Beishuizen A, et al. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care 2014; 18: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zusman O, Theilla M, Cohen J, et al. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care 2016; 20: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Improving global outcomes (KDIGO) Acute Kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter 2012; 2(Suppl 1): 95–100. [Google Scholar]
- 16.Jonckheer J, Herbert S, Demol J, et al. The impact of continuous venovenous haemofiltration on indirect calorimetry. Clin Nutr 2018; 37: S1. [Google Scholar]


