ABSTRACT
Factors leading to the wide range of manifestations associated with Mycoplasma pneumoniae infection are unclear. We investigated whether M. pneumoniae genotypes are associated with specific clinical outcomes. We compared M. pneumoniae loads and genotypes of children with mucocutaneous disease to those of children with pneumonia, family members with upper respiratory tract infection (URTI), and carriers from a prospective cohort study (n = 47; 2016 to 2017) and to those of other children with mucocutaneous disease from a case series (n = 7; 2017 to 2020). Genotyping was performed using macrolide resistance determination, P1 subtyping, multilocus variable-number tandem-repeat analysis (MLVA), and multilocus sequence typing (MLST). Comparisons were performed with a pairwise Wilcoxon rank sum test and a Fisher exact test with corrections for multiple testing, as appropriate. M. pneumoniae loads did not statistically differ between patients with mucocutaneous disease and those with pneumonia or carriers. Macrolide resistance was detected in 1 (1.9%) patient with mucocutaneous disease. MLVA types from 2016 to 2017 included 3-5-6-2 (n = 21 [46.7%]), 3-6-6-2 (n = 2 [4.4%]), 4-5-7-2 (n = 14 [31.1%]), and 4-5-7-3 (n = 8 [17.8%]), and they correlated with P1 subtypes and MLST types. MLVA types were not associated with specific outcomes such as mucocutaneous disease, pneumonia, URTI, or carriage. They were almost identical within families but varied over geographic location. MLVA types in patients with mucocutaneous disease differed between 2016 to 2017 (3-5-6-2, n = 5 [62.5%]) and 2017 to 2020 (4-5-7-2, n = 5 [71.4%]) (P = 0.02). Our results suggest that M. pneumoniae genotypes may not determine specific clinical outcomes.
KEYWORDS: antimicrobial resistance (AMR), community-acquired pneumonia (CAP), multilocus variable-number tandem-repeat analysis (MLVA), Mycoplasma pneumoniae-induced rash and mucositis (MIRM), whole-genome sequencing (WGS)
INTRODUCTION
Mycoplasma pneumoniae is a common cause of community-acquired pneumonia (CAP) in children (1). Although the infection is generally mild, patients of every age can develop severe and fulminant disease (2–4). Apart from the respiratory tract, M. pneumoniae can cause a wide range of extrapulmonary manifestations (5, 6). We recently described the occurrence of M. pneumoniae-induced mucocutaneous disease among CAP patients, which was associated with increased systemic inflammation and morbidity and a higher risk of long-term sequelae (7). In contrast, M. pneumoniae can be carried in the upper respiratory tract (URT) without causing any symptoms in up to 56% of healthy children (8–10). However, factors leading to the wide range of clinical outcomes are unclear.
M. pneumoniae is one of the smallest self-replicating organisms and has a reduced genome size of 816,394 bp (11, 12) containing 694 protein-coding regions (open reading frames [ORFs]) in the current genome annotation (MPN001 to MPN694) (13, 14). M. pneumoniae is considered a genetically highly stable organism based on the limited sequence diversity between strains (8, 15). Sequence variations are mainly limited to the gene encoding the major adhesion P1 protein (MPN141) (16). M. pneumoniae isolates can be classified into 2 major genetic groups (subtypes 1 and 2) based on sequence differences in repetitive elements (RepMPs) in the P1 protein gene (i.e., RepMP2/3 and RepMP4) (8, 15, 16). RepMP2/3 and RepMP4 are not unique to the P1 protein gene but are also found at other sites within the bacterial genome (consisting of 8% of the total genome) (15, 16). Parts of these RepMPs are considered a reservoir for homologous DNA recombination that results in several subtype variants (16). It has been speculated that the cyclic occurrence of M. pneumoniae epidemics every few years may be facilitated by a shift from one P1 subtype to the other (17). Whole-genome sequencing (WGS) allowed the identification of even more regions in the genome, including RepMPs and other genes, that have high discriminatory power (8, 18). Several typing methods have recently been developed based on these regions (reviewed in reference 8).
Despite these advances in genotyping methods to characterize different M. pneumoniae strains, it is still unclear whether there is a relationship between M. pneumoniae genotypes and specific clinical outcomes. The basis for investigating this relationship is a correct diagnosis of M. pneumoniae infection in patients with different outcomes associated with M. pneumoniae. However, current diagnostic tests, including PCR of URT specimens and serology, are not able to reliably differentiate M. pneumoniae-infected symptomatic patients from asymptomatic carriers (8–10). We recently demonstrated in a prospective cohort study of CAP among children that the measurement of specific peripheral blood IgM antibody-secreting cells (ASCs) by an enzyme-linked immunospot (ELISpot) assay improves the diagnosis of M. pneumoniae infection (9). This test differentiated between M. pneumoniae infection and carriage.
Using this cohort, here, we compared M. pneumoniae isolates from patients with M. pneumoniae-induced mucocutaneous disease, CAP, and URT infection (URTI) and asymptomatic children (carriers) by several genotyping methods to investigate if genotype differences may be associated with differences in clinical outcomes.
MATERIALS AND METHODS
Ethics statement.
The local ethics committee approved the protocol for this study (no. 2016-00148). Written informed consent was obtained from all parents and from children 14 years of age or older.
Patients.
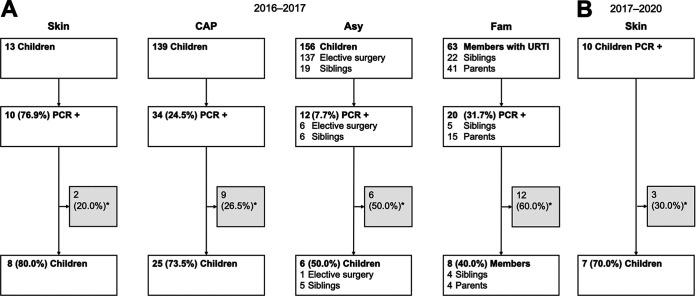
We identified patients from a prospective cohort study (2016 to 2017) and a case series (2017 to 2020) who had respiratory specimens and/or M. pneumoniae DNA extracts available for genotyping and compared M. pneumoniae genotypes between the different outcomes. The derivation set is shown in Fig. 1. More detailed information on the patients excluded due to insufficient samples available for genotyping is provided in Table S1 in the supplemental material.
FIG 1.
Enrollment flow diagram. (A) Prospective cohort study from 2016 to 2017. (B) Case series from 2017 to 2020. The respective patient groups are indicated above each flow diagram. *, no respiratory specimens and/or DNA extracts for genotyping were available (Table S1 in the supplemental material provides more detailed information on excluded patients). Asy, asymptomatic children; CAP, community-acquired pneumonia; Fam, family members with upper respiratory tract infection; Skin, mucocutaneous disease; URTI, upper respiratory tract infection.
(i) Prospective cohort study from 2016 to 2017.
Children aged 3 to 18 years with mucocutaneous disease (n = 13), with CAP (n = 139), and without symptoms (n = 156) were enrolled from 1 May 2016 to 30 April 2017 at the University Children’s Hospital Zurich. Children <3 years of age were excluded to reduce the probability of viral infection, as it is highest in this age group (1, 19). In all children, pharyngeal swab specimens were taken. Blood samples were collected if additional consent was given. CAP was clinically defined as the presence of fever of >38.5°C and tachypnea according to British Thoracic Society guidelines (19). Asymptomatic controls included healthy children undergoing elective surgical procedures and siblings of patients with CAP without recent (<1 week) respiratory tract infections (RTIs). Mucocutaneous disease was assessed by a dermatologist and defined as any eruptive lesion that involved skin and/or mucous membranes occurring during the CAP episode (7). In addition, family members with URTI (not fulfilling clinical CAP criteria, with no presence of acute cough and/or bronchitis) available for sampling at the presentation of index patients were also enrolled (20). Blood samples were not collected from those family members.
Out of these groups, patients positive for M. pneumoniae DNA by PCR (21) of pharyngeal swab specimens were examined for the availability of additional respiratory specimens and/or M. pneumoniae DNA extracts for genotyping in this study. M. pneumoniae infection was defined as a positive M. pneumoniae-specific IgM ASC ELISpot result, as previously described (9, 22).
(ii) Case series from 2017 to 2020.
Children aged 3 to 18 years with M. pneumoniae-induced mucocutaneous disease during the following 3 years (1 May 2017 to 30 April 2020) were prospectively enrolled as an additional comparison group according to the following inclusion criteria: mucocutaneous disease defined as described above and diagnosed by a dermatologist, radiologically confirmed CAP using criteria for radiographic pneumonia (23, 24), detection of M. pneumoniae DNA by PCR (21) of pharyngeal swab specimens, and a positive M. pneumoniae-specific IgM ASC ELISpot result (9, 22).
Study procedures.
Swabs were taken from the posterior pharynx using flocked nylon fiber tip swabs (Copan Diagnostics, Murrieta, CA). DNA isolation was performed with the QIAamp DNA minikit (Qiagen, Hilden, Germany), and extracts were stored at −80°C. Aliquots of DNA extracts were sent to the German reference center for mycoplasma (Dresden, Germany).
Genotyping.
To determine if different genotypes were associated with specific clinical outcomes, extensive molecular characterization of M. pneumoniae DNA was performed with the following PCR approaches and sequencing using the primers detailed in Table S2.
(i) Macrolide resistance.
M. pneumoniae strains were tested for macrolide resistance using PCR and sequencing of the 23S rRNA gene as previously described (25).
(ii) P1 subtyping.
Molecular subtyping of M. pneumoniae strains was performed as previously described (26), based on amplification by nested PCR and sequencing of RepMP2/3 and RepMP4 of the P1 gene (MPN141).
(iii) Multilocus variable-number tandem-repeat analysis.
Highly discriminatory 4-locus multilocus variable-number tandem-repeat analysis (MLVA) was performed to better understand the epidemiological relationships of M. pneumoniae strains using previously described methods (27, 28). The naming of profiles was based on a string of allele numbers in the order Mpn13 (intergenic region), Mpn14 (MPN501), Mpn15 (MPN524), and Mpn16 (MPN613), showing the actual number of repeats at each locus (28).
(iv) Multilocus sequence typing.
Multilocus sequence typing (MLST) analysis for global comparison of strains was based on amplification by nested PCR and sequencing of 8 housekeeping genes (ppa [MPN528], pgm [MPN628], gyrB [MPN003], gmk [MPN246], glyA [MPN576], atpA [MPN600], arcC [MPN307], and adk [MPN185]) as described at the MLST database website (https://pubmlst.org/mpneumoniae) (29).
Sample size and statistical analysis.
To identify M. pneumoniae genotypes that may contribute to a specific clinical outcome, patients with mucocutaneous disease (cases) from the prospective cohort study were compared to control subjects with CAP (controls with M. pneumoniae infection but lower severity than mucocutaneous disease), URTI (controls with M. pneumoniae detection but significantly lower severity than mucocutaneous disease and CAP), and carriage (asymptomatic controls) in an unmatched case-control study design. Patients with M. pneumoniae-induced mucocutaneous disease from the case series could not be compared directly to controls from the prospective cohort study as the time periods varied, which is critical in terms of cyclic epidemics of M. pneumoniae. Sample size estimation was limited by very few preliminary data about M. pneumoniae genotypes in patients with specific clinical outcomes such as M. pneumoniae-induced mucocutaneous disease and carriage. Assuming that P1 subtype 2 predominates in patients with M. pneumoniae-induced mucocutaneous disease (Stevens-Johnson syndrome [SJS]) (proportion of cases with exposure, 0.60 [30]) compared to M. pneumoniae CAP (proportion of controls with exposure, 0.10 [31]), we calculated a sample size of 32 children (control-to-case ratio, 3 [7]) to achieve 80% power and 5% 2-sided significance (32). A pairwise Wilcoxon rank sum test was used to compare medians, and a Fisher exact test was used to compare proportions with corrections for multiple testing. A 2-tailed P value of <0.05 was considered to be statistically significant. Statistical computing was conducted in R (version 4.0.0).
RESULTS
Overall, 54 participants were included (median age of children, 8.7 years; interquartile range, 6.3 to 11.8 years). The groups consisted of 8 children with mucocutaneous disease, 25 children with CAP, 6 asymptomatic children, and 8 family members with URTI (4 siblings and 4 parents) of the prospective cohort study from 2016 to 2017 (Fig. 1A) and 7 children with mucocutaneous disease of the case series as an additional comparison group during the following 3 years from 2017 to 2020 (Fig. 1B). The clinical diagnosis of CAP was radiologically confirmed in the included patients with mucocutaneous disease and CAP using criteria for radiographic pneumonia (23, 24). M. pneumoniae infection was established in all patients with mucocutaneous disease and CAP with the M. pneumoniae-specific IgM ASC ELISpot assay. Baseline characteristics and genotyping results are summarized in Table 1.
TABLE 1.
Results of molecular characterization of M. pneumoniae strainsa
| Group | Patient | Age (yrs) | Presentation | MR phenotype | P1 type | MLVA type |
MLST type |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | 16 | ppa | pgm | gyrB | gmk | glyA | atpA | arcC | adk | ST | ||||||
| 2016–2017: prospective cohort study | ||||||||||||||||||
| Skin | 1 | 3.8 | Rash | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 |
| 2 | 4.1 | Rash | WT | 2e | 3 | 6 | 6 | 2 | 2 | 3 | 2 | 2 | 2 | 4 | 1 | 5 | Newb | |
| 3 | 10.3 | MIRM | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 4 | 9.5 | Urticaria | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 5 | 5.4 | Rash | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 6 | 14.6 | MIRM | WT | 1 | 4 | 5 | 7 | 3 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 7 | 12.8 | Urticaria | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 8 | 7.9 | Rash | WT | 1 | 4 | 5 | 7 | 3 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| CAP | 9 | 7.9 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 |
| 10 | 12.0 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 11 | 6.0 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 12 | 8.9 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 13 | 11.4 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 14 | 7.6 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 15 | 5.5 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 16 | 13.5 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 17 | 10.2 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 18 | 10.3 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 19 | 10.1 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 20 | 11.0 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 21 | 15.6 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 22 | 3.0 | CAP | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 23 | 10.2 | CAP | WT | 1 | 4 | 5 | 7 | 3 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 24 | 8.6 | CAP | WT | 1 | 4 | 5 | 7 | 3 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 25 | 7.7 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 26 | 5.2 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 27 | 8.2 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 28 | 8.3 | CAP | WT | 1 | 4 | 5 | 7 | 3 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 29 | 13.5 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 30 | 4.3 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 31 | 4.3 | CAP | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 32 | 6.3 | CAP | WT | 1 | 4 | 5 | 7 | 3 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 33 | 6.7 | CAP | NA | NA | 4 | 5 | 7 | 2 | NA | 3 | NA | 1 | NA | NA | NA | NA | NA | |
| Asy | 34 | 7.6 | Asymptomatic | WT | 2c | 3 | 5 | 6 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 1 | 5 | Newb |
| 35 | 6.1 | Asymptomatic | WT | 2c | 3 | 5 | 6 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 1 | 5 | Newb | |
| 36 | 7.2 | Asymptomatic | WT | 1 | 4 | 5 | 7 | 2 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 37 | 4.7 | Asymptomatic | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 38 | 16.7 | Asymptomatic | WT | 1 | 4 | 5 | 7 | 3 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 39 | 10.6 | Asymptomatic | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| Fam | 40 | 34.7 | URTI | WT | NA | 3 | 6 | 6 | 2 | 2 | 3 | 2 | 2 | 2 | 4 | 1 | 1 | 2 |
| 41 | 13.5 | URTI | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 42 | 41.6 | URTI | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 43 | 3.6 | URTI | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
| 44 | 43.8 | URTI | WT | 1 | 4 | 5 | 7 | 3 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 45 | 6.4 | URTI | WT | 1 | 4 | 5 | 7 | 2 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 46 | 10.9 | URTI | WT | 1 | NA | 5 | NA | 2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 47 | 48.1 | URTI | WT | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 2017–2020: case series | ||||||||||||||||||
| Skin | 48 | 10.0 | Urticaria | WT | 1 | 4 | 5 | 7 | 2 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 |
| 49 | 12.7 | Urticaria | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 50 | 15.3 | MIRM | WT | 1 | 4 | 5 | 7 | 2 | 1 | 5 | 1 | 1 | 1 | 3 | 1 | 1 | 17 | |
| 51 | 8.2 | Urticaria | WT | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 52 | 12.0 | NSAGU | A2058Gc | 1 | 4 | 5 | 7 | 2 | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | |
| 53 | 16.1 | MIRM | WT | 2 | 3 | 6 | 6 | 2 | 2 | 3 | 2 | 2 | 2 | 4 | 1 | 1 | 2 | |
| 54 | 12.3 | MIRM | WT | 2c | 3 | 5 | 6 | 2 | 2 | 3 | 2 | 2 | 4 | 4 | 1 | 5 | 14 | |
Abbreviations: Asy, asymptomatic children (carriers); CAP, community-acquired pneumonia; Fam, family members with upper respiratory tract infection; MIRM, M. pneumoniae-induced rash and mucositis; MLST, multilocus sequence typing; MLVA, multiple-locus variable-number tandem-repeat analysis (MLVA); MR, macrolide resistance; NA, not available (limited sample volume); NSAGU, non-sexually acquired genital ulceration (Lipschütz ulcers); P1, major adhesion M. pneumoniae protein; Rash, maculopapular skin eruption; Skin, M. pneumoniae-induced mucocutaneous disease; ST, sequence type; URTI, upper respiratory tract infection; WT, wild type.
Not included in the MLST database (http://pubmlst.org/mpneumoniae).
Overlap A/G.
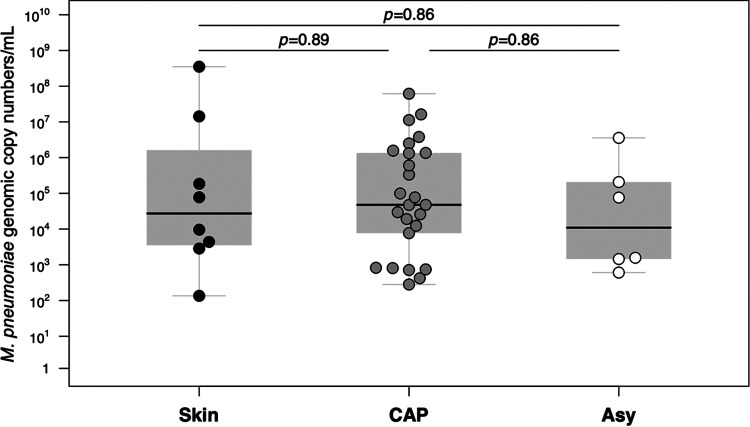
M. pneumoniae DNA levels did not statistically differ between patients with mucocutaneous disease and CAP and carriers (Fig. 2). There was also no difference for M. pneumoniae DNA levels between inpatients and outpatients with CAP (P = 0.09). An A2058G mutation associated with macrolide resistance was detected in 1 (1.9%) specimen of a patient with M. pneumoniae-induced mucocutaneous disease. The patient had not received prior macrolide treatment (33).
FIG 2.
M. pneumoniae DNA levels in pharyngeal swab samples. Shown is a comparison between patients with M. pneumoniae-induced mucocutaneous disease (“Skin”) and community-acquired pneumonia (“CAP”) and asymptomatic children (carriers) (“Asy”) from 2016 to 2017. The median is shown as a black line across the gray box that represents the lower and upper quartiles. Whiskers extend to the maximum and minimum values within 1.5 times the interquartile range above and below the third and first quartiles, respectively. Differences in medians are shown with the corresponding P values (pairwise Wilcoxon rank sum test with corrections for multiple testing).
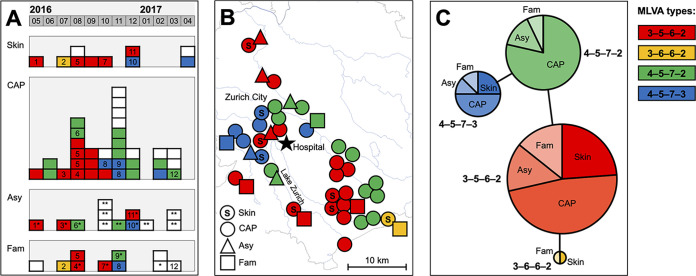
During the prospective cohort study from 2016 to 2017, P1 subtypes 1 and 2 were equally detected, but P1 subtype 2 dominated in the first 6 months (n = 20/26 [76.9%]), and P1 subtype 1 dominated in the second 6 months (n = 16/18 [88.9%]) (P < 0.01) (see Fig. S1 in the supplemental material). M. pneumoniae DNA levels were not different between P1 subtypes 1 and 2 (P = 0.06). Four MLVA types were observed, 3-5-6-2 (n = 21/45 [46.7%]), 3-6-6-2 (n = 2/45 [4.4%]), 4-5-7-2 (n = 14/45 [31.1%]), and 4-5-7-3 (n = 8/45 [17.8%]), with their temporal distribution depicted in Fig. 3A. MLVA types were almost identical within families (n = 10/11 [90.9%]) but varied over geographic location (city and surrounding area, 4-5-7-3, n = 8/15 [53.3%]; southeast, 3-5-6-2, n = 12/21 [57.1%]; P < 0.01) (Fig. 3B).
FIG 3.
Temporal, geographic, and genetic distribution of M. pneumoniae strains based on MLVA from 2016 to 2017. (A) M. pneumoniae strains over time in patients with M. pneumoniae-induced mucocutaneous disease (“Skin”) and community-acquired pneumonia (“CAP”), asymptomatic children (carriers) (“Asy”), and family members with upper respiratory tract infection (“Fam”). Numbers indicate strains belonging to the same family. White squares represent M. pneumoniae strains from which no respiratory specimens and/or M. pneumoniae DNA extracts were available for MLVA typing. *, siblings; **, healthy controls (enrolled during elective surgery). (B) Regional distribution of MLVA types. (C) Minimum-spanning tree. Each circle represents one MLVA type, and the size of the circle is proportional to the number of isolates. The pie chart shows the distribution of different patient groups within MLVA types. The distance between MLVA types corresponds to the number of allelic changes.
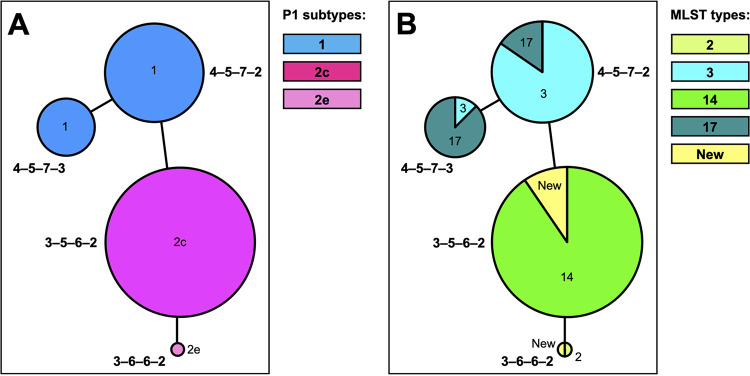
There was no association of MLVA types with clinical outcomes such as mucocutaneous disease, CAP, URTI, and carriage (Fig. 3C). Genotyping results correlated between MLVA typing and P1 subtyping (3-5-6-2 and 3-6-6-2 with P1 subtype 2 and variants; 4-5-7-2 and 4-5-7-3 with P1 subtype 1) (Fig. 4A) and also between MLVA and MLST typing (Fig. 4B and Fig. S2 in the supplemental material).
FIG 4.
Minimum-spanning tree based on MLVA, with color representing the P1 subtype (A) or the MLST type (B). Each circle represents one MLVA type, and the size of the circle is proportional to the number of isolates. The distance between MLVA types corresponds to the number of allelic changes.
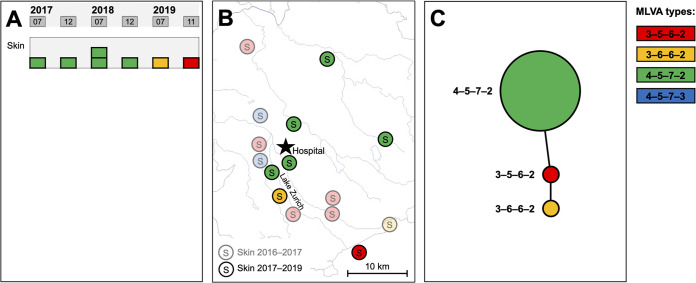
The most frequent MLVA type in patients with mucocutaneous disease was 3-5-6-2 (n = 5/8 [62.5%]). To evaluate the association of this particular MLVA type with this specific outcome, we additionally investigated M. pneumoniae strains from patients with mucocutaneous disease during the following 3 years. The predominant MLVA type during the later time period was 4-5-7-2 (n = 5/7 [71.4%]), and it also clustered geographically (city and surrounding area) (Fig. 5). MLVA types in patients with mucocutaneous disease differed significantly between 2016 to 2017 and 2017 to 2020 (P = 0.02).
FIG 5.
Temporal, geographic, and genetic distribution of M. pneumoniae strains based on MLVA from patients with M. pneumoniae-induced mucocutaneous disease from 2017 to 2020. (A) M. pneumoniae strains over time. (B) Regional distribution of MLVA types in comparison to those of patients with M. pneumoniae-induced mucocutaneous disease enrolled from 2016 to 2017. (C) Minimum-spanning tree. Each circle represents one MLVA type, and the size of the circle is proportional to the number of isolates. The distance between MLVA types corresponds to the number of allelic changes.
DISCUSSION
This study investigated the relationship between M. pneumoniae genotypes and specific clinical outcomes. Extensive molecular characterization of M. pneumoniae resulted in no specific genotype associated with mucocutaneous disease, CAP, URTI, or carriage.
These findings were corroborated by results from three previous studies (34–36). One study analyzed M. pneumoniae strains isolated from children in the Netherlands (2008 to 2012) with upper (n = 4) and lower (n = 9) respiratory tract infection (RTI) and asymptomatic children (n = 3) by WGS (34). Comparison of these sequences revealed no specific genotype associated with RTI or carriage. A study from Sweden (2005 to 2006) including adults and children compared M. pneumoniae strains between outpatients (n = 21) and inpatients (n = 24) with RTI by P1 subtyping (35). There was no subtype associated with disease severity, but M. pneumoniae DNA levels from pharyngeal swab specimens were significantly higher among inpatients. We did not observe differences in M. pneumoniae loads in pharyngeal swab specimens between clinical outcomes or inpatients and outpatients. This is supported by another observational study in which M. pneumoniae DNA levels in pharyngeal and nasopharyngeal samples also did not differ between RTI and carriage (10). Another study investigated M. pneumoniae strains during an outbreak in the United States (2013) from children with mucocutaneous disease (SJS) (n = 5) and compared them to M. pneumoniae strains from banked PCR-positive respiratory specimens (n = 69) (30, 36). Five MLVA types were observed, including 3 among the 5 patients with mucocutaneous disease (3-5-6-2 [n = 1], 3-6-6-2 [n = 2], and 4-5-7-2 [n = 2]). The different MLVA types observed among patients with mucocutaneous disease and the fact that cases with similar MLVA types were not closely clustered geographically led to the conclusion that a specific MLVA type may not be related to mucocutaneous disease (30). However, the diagnosis of M. pneumoniae infection in those studies was limited. We attempted to overcome these limitations by investigating a considerable sample size, particularly of patients with mucocutaneous disease and carriers, which derives from a well-defined cohort diagnosed with M. pneumoniae infection by improved diagnostics. All patients with CAP and mucocutaneous disease tested positive with the M. pneumoniae-specific IgM ASC ELISpot assay, which differentiates infection from carriage (9).
M. pneumoniae is responsible for 8 to 28% of CAP cases in children (1, 37–39). The increased rate of M. pneumoniae infections in children aged 3 to 18 years (28.9%) during the prospective cohort study was related to the coinciding M. pneumoniae epidemic in Europe from 2016 to 2017 (40–43). We observed an alternating predominance of P1 subtype 2 and 1 strains from 2016 to 2018, which can be related to the cyclic epidemics of M. pneumoniae. However, cocirculation of both subtypes and variants has been observed in our study and elsewhere (17). Also, macrolide-resistant M. pneumoniae (MRMp) has now been reported in Switzerland among children and adults (9%; 2014 to 2017) (44). Here, we detected only a single MRMp strain in a patient with mucocutaneous disease (2%; 2018). MRMp has been reported to be associated with more frequent mucocutaneous and nervous system disease in M. pneumoniae CAP patients, possibly as a result of stronger and more persistent inflammatory stimulation by MRMp because of treatment failure (45).
In consideration of these findings, it may be possible that not a specific genotype but an overall increased rate of M. pneumoniae infections leads to a concurrent increase in specific clinical outcomes and/or more severe disease. Host factors may determine the development of specific outcomes following exposure to M. pneumoniae. Several pathogenic processes have been proposed to play a role in the development of more severe disease and/or extrapulmonary manifestations, such as cell-mediated immunity in M. pneumoniae CAP (46), cell- or antibody-mediated epithelial injury in M. pneumoniae-induced mucocutaneous disease (7, 47), and antibody-mediated neuronal damage in M. pneumoniae-associated nervous system disease (48, 49).
Our study has several limitations. First, the study is geographically confined to the region of Zurich, Switzerland. No reference data about M. pneumoniae genotypes are available for Switzerland from the M. pneumoniae epidemic in Europe during the 2016–2017 study period (20). Second, our genotype analysis focused on selected genes, where sequence variation occurs (8). It is possible that WGS could have identified relevant differences between strains. However, the M. pneumoniae genome has an overall low sequence diversity, and genomic variation outside these regions is very uncommon (8, 34). Third, children younger than 3 years of age were excluded to reduce the probability of viral infection; therefore, the association between M. pneumoniae genotypes and clinical outcomes in children of that age is unknown. However, younger children with M. pneumoniae infection may be more likely to have RTIs other than CAP (50), which supports the hypothesis that host rather than bacterial factors determine clinical outcomes. Fourth, as this is a retrospective study (case-control study and case series), we cannot rule out that unintended selection bias occurred. Finally, the numbers of analyzed M. pneumoniae strains are still relatively small, which may lead to difficulty in interpreting the “negative” results (51). As an option for meaningful interpretation of negative results (52), we presented a sample size estimate, and the study met the target sample size. This makes it seem less likely that the negative results are inconclusive and uninformative (51), which is further supported by similar results from previous studies as discussed above (34–36). Although it may be too early to draw any definite conclusions, this study, together with previously reported data, provides valuable information on the impact of specific genotypes on the development of clinical outcomes.
Conclusion.
Our results suggest that M. pneumoniae genotypes vary over time and geographic location but may not determine specific clinical outcomes. It is hypothesized that host factors determine which children exposed to M. pneumoniae are more likely to develop pneumonia, extrapulmonary manifestations, or carriage. Further studies are required to identify such host factors that lead to the wide range of clinical outcomes associated with M. pneumoniae infection.
ACKNOWLEDGMENTS
We thank the children and their parents who contributed to this study, the emergency department staff and the outpatient clinic staff for recruiting participants, and the microbiology laboratory staff for processing samples.
P.M.M.S. was supported by a Walter und Gertrud Siegenthaler fellowship and the career development program Filling the Gap of the University of Zurich. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We have no conflict of interest.
P.M.M.S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design, P.M.M.S. Acquisition of data, P.M.M.S., E.P., M.S., M.T., C.B., and R.D. Analysis and interpretation of data, P.M.M.S. and R.D. Drafting of the manuscript, P.M.M.S. Critical revision of the manuscript for important intellectual content, all authors. Statistical analysis, P.M.M.S. Obtained funding, P.M.M.S. and C.B. Administrative, technical, or material support, P.M.M.S., C.B., and R.D.
Footnotes
Supplemental material is available online only.
Contributor Information
Patrick M. Meyer Sauteur, Email: patrick.meyer@kispi.uzh.ch.
Daniel J. Diekema, University of Iowa College of Medicine
REFERENCES
- 1.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, Zhu Y, Patel A, Hymas W, Chappell JD, Kaufman RA, Kan JH, Dansie D, Lenny N, Hillyard DR, Haynes LM, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, Wunderink RG, Edwards KM, Pavia AT, McCullers JA, Finelli L, CDC EPIC Study Team . 2015. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 372:835–845. 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waites KB, Talkington DF. 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 17:697–728. 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannan TR, Hardy RD, Coalson JJ, Cavuoti DC, Siegel JD, Cagle M, Musatovova O, Herrera C, Baseman JB. 2012. Fatal outcomes in family transmission of Mycoplasma pneumoniae. Clin Infect Dis 54:225–231. 10.1093/cid/cir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer Sauteur PM, Kleger G-R, Albrich WC. 2021. Acute respiratory distress syndrome during the COVID-19 pandemic: not only SARS-CoV-2. New Microbes New Infect 40:100836. 10.1016/j.nmni.2020.100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narita M. 2010. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 16:162–169. 10.1007/s10156-010-0044-x. [DOI] [PubMed] [Google Scholar]
- 6.Narita M. 2016. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol 7:23. 10.3389/fmicb.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer Sauteur PM, Theiler M, Büttcher M, Seiler M, Weibel L, Berger C. 2020. Frequency and clinical presentation of mucocutaneous disease due to Mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol 156:144–150. 10.1001/jamadermatol.2019.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. 2017. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev 30:747–809. 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer Sauteur PM, Seiler M, Truck J, Unger WWJ, Paioni P, Relly C, Staubli G, Haas T, Gysin C, Bachmann LM, van Rossum AMC, Berger C. 2019. Diagnosis of Mycoplasma pneumoniae pneumonia with measurement of specific antibody-secreting cells. Am J Respir Crit Care Med 200:1066–1069. 10.1164/rccm.201904-0860LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spuesens EB, Fraaij PL, Visser EG, Hoogenboezem T, Hop WC, van Adrichem LN, Weber F, Moll HA, Broekman B, Berger MY, van Rijsoort-Vos T, van Belkum A, Schutten M, Pas SD, Osterhaus AD, Hartwig NG, Vink C, van Rossum AM. 2013. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 10:e1001444. 10.1371/journal.pmed.1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himmelreich R, Hilbert H, Plagens H, Pirkl E, Li BC, Herrmann R. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res 24:4420–4449. 10.1093/nar/24.22.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dandekar T, Huynen M, Regula JT, Ueberle B, Zimmermann CU, Andrade MA, Doerks T, Sanchez-Pulido L, Snel B, Suyama M, Yuan YP, Herrmann R, Bork P. 2000. Re-annotating the Mycoplasma pneumoniae genome sequence: adding value, function and reading frames. Nucleic Acids Res 28:3278–3288. 10.1093/nar/28.17.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wodke JA, Alibes A, Cozzuto L, Hermoso A, Yus E, Lluch-Senar M, Serrano L, Roma G. 2015. MyMpn: a database for the systems biology model organism Mycoplasma pneumoniae. Nucleic Acids Res 43:D618–D623. 10.1093/nar/gku1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lluch-Senar M, Delgado J, Chen WH, Llorens-Rico V, O’Reilly FJ, Wodke JA, Unal EB, Yus E, Martinez S, Nichols RJ, Ferrar T, Vivancos A, Schmeisky A, Stulke J, van Noort V, Gavin AC, Bork P, Serrano L. 2015. Defining a minimal cell: essentiality of small ORFs and ncRNAs in a genome-reduced bacterium. Mol Syst Biol 11:780. 10.15252/msb.20145558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spuesens EB, Oduber M, Hoogenboezem T, Sluijter M, Hartwig NG, van Rossum AM, Vink C. 2009. Sequence variations in RepMP2/3 and RepMP4 elements reveal intragenomic homologous DNA recombination events in Mycoplasma pneumoniae. Microbiology 155:2182–2196. 10.1099/mic.0.028506-0. [DOI] [PubMed] [Google Scholar]
- 16.Vink C, Rudenko G, Seifert HS. 2012. Microbial antigenic variation mediated by homologous DNA recombination. FEMS Microbiol Rev 36:917–948. 10.1111/j.1574-6976.2011.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs E, Ehrhardt I, Dumke R. 2015. New insights in the outbreak pattern of Mycoplasma pneumoniae. Int J Med Microbiol 305:705–708. 10.1016/j.ijmm.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Xiao L, Ptacek T, Osborne JD, Crabb DM, Simmons WL, Lefkowitz EJ, Waites KB, Atkinson TP, Dybvig K. 2015. Comparative genome analysis of Mycoplasma pneumoniae. BMC Genomics 16:610. 10.1186/s12864-015-1801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A, British Thoracic Society Standards of Care Committee . 2011. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66(Suppl 2):ii1–ii23. 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 20.Meyer Sauteur PM, Krautter S, Ambroggio L, Seiler M, Paioni P, Relly C, Kellenberger C, Haas T, Gysin C, Bachmann LM, van Rossum AMC, Berger C. 2020. Improved diagnostics help to identify clinical features and biomarkers that predict Mycoplasma pneumoniae community-acquired pneumonia in children. Clin Infect Dis 71:1645–1654. 10.1093/cid/ciz1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardegger D, Nadal D, Bossart W, Altwegg M, Dutly F. 2000. Rapid detection of Mycoplasma pneumoniae in clinical samples by real-time PCR. J Microbiol Methods 41:45–51. 10.1016/s0167-7012(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 22.Meyer Sauteur PM, Trück J, van Rossum AMC, Berger C. 2020. Circulating antibody-secreting cell response during Mycoplasma pneumoniae childhood pneumonia. J Infect Dis 222:136–147. 10.1093/infdis/jiaa062. [DOI] [PubMed] [Google Scholar]
- 23.Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, Greenberg D, Lagos R, Lucero M, Madhi SA, O’Brien KL, Obaro S, Steinhoff MC. 2005. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 24.Fancourt N, Deloria Knoll M, Barger-Kamate B, de Campo J, de Campo M, Diallo M, Ebruke BE, Feikin DR, Gleeson F, Gong W, Hammitt LL, Izadnegahdar R, Kruatrachue A, Madhi SA, Manduku V, Matin FB, Mahomed N, Moore DP, Mwenechanya M, Nahar K, Oluwalana C, Ominde MS, Prosperi C, Sande J, Suntarattiwong P, O’Brien KL. 2017. Standardized interpretation of chest radiographs in cases of pediatric pneumonia from the PERCH study. Clin Infect Dis 64:S253–S261. 10.1093/cid/cix082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumke R, von Baum H, Luck PC, Jacobs E. 2010. Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect 16:613–616. 10.1111/j.1469-0691.2009.02968.x. [DOI] [PubMed] [Google Scholar]
- 26.Dumke R, Schnee C, Pletz MW, Rupp J, Jacobs E, Sachse K, Rohde G, Capnetz Study Group . 2015. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011-2012. Emerg Infect Dis 21:426–434. 10.3201/eid2103.140927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degrange S, Cazanave C, Charron A, Renaudin H, Bebear C, Bebear CM. 2009. Development of multiple-locus variable-number tandem-repeat analysis for molecular typing of Mycoplasma pneumoniae. J Clin Microbiol 47:914–923. 10.1128/JCM.01935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumke R, Jacobs E. 2011. Culture-independent multi-locus variable-number tandem-repeat analysis (MLVA) of Mycoplasma pneumoniae. J Microbiol Methods 86:393–396. 10.1016/j.mimet.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Brown RJ, Holden MT, Spiller OB, Chalker VJ. 2015. Development of a multilocus sequence typing scheme for molecular typing of Mycoplasma pneumoniae. J Clin Microbiol 53:3195–3203. 10.1128/JCM.01301-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins LKF, Olson D, Diaz MH, Lin X, Demirjian A, Benitez AJ, Winchell JM, Robinson CC, Bol KA, Glode MP, Dominguez SR, Miller LA, Kutty PK. 2017. Epidemiology and molecular characteristics of Mycoplasma pneumoniae during an outbreak of M. pneumoniae-associated Stevens-Johnson syndrome. Pediatr Infect Dis J 36:564–571. 10.1097/INF.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsukawa C, Kenri T, Shibayama K, Takahashi K. 2019. Genetic characterization of Mycoplasma pneumoniae isolated in Osaka between 2011 and 2017: decreased detection rate of macrolide-resistance and increase of p1 gene type 2 lineage strains. PLoS One 14:e0209938. 10.1371/journal.pone.0209938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahim NK, Negida A, Fahim AK. 2019. Sample size calculation guide—part 3: how to calculate the sample size for an independent case-control study. Adv J Emerg Med 3:e20. 10.22114/AJEM.v0i0.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer Sauteur PM, Stahli N, Theiler M, Hurlimann R, Berger C. 2020. Mycoplasma pneumoniae-induced non-sexually acquired genital ulceration (Lipschütz ulcers). Arch Dis Child 105:517–518. 10.1136/archdischild-2019-317676. [DOI] [PubMed] [Google Scholar]
- 34.Spuesens EBM, Brouwer RWW, Mol KHJM, Hoogenboezem T, Kockx CEM, Jansen R, Van IJcken WFJ, Van Rossum AMC, Vink C. 2016. Comparison of Mycoplasma pneumoniae genome sequences from strains isolated from symptomatic and asymptomatic patients. Front Microbiol 7:1701. 10.3389/fmicb.2016.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson AC, Bjorkman P, Welinder-Olsson C, Widell A, Persson K. 2010. Clinical severity of Mycoplasma pneumoniae (MP) infection is associated with bacterial load in oropharyngeal secretions but not with MP genotype. BMC Infect Dis 10:39. 10.1186/1471-2334-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson D, Watkins LKF, Demirjian A, Lin X, Robinson CC, Pretty K, Benitez AJ, Winchell JM, Diaz MH, Miller LA, Foo TA, Mason MD, Lauper UL, Kupfer O, Kennedy J, Glode MP, Kutty PK, Dominguez SR. 2015. Outbreak of Mycoplasma pneumoniae-associated Stevens-Johnson syndrome. Pediatrics 136:e386–e394. 10.1542/peds.2015-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, Ziegler T, Kauppila J, Leinonen M, McCrackenGH, Jr.. 2004. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113:701–707. 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 38.Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, ARPEC Project Group . 2016. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother 71:1106–1117. 10.1093/jac/dkv418. [DOI] [PubMed] [Google Scholar]
- 39.Wallihan RG, Suarez NM, Cohen DM, Marcon M, Moore-Clingenpeel M, Mejias A, Ramilo O. 2018. Molecular distance to health transcriptional score and disease severity in children hospitalized with community-acquired pneumonia. Front Cell Infect Microbiol 8:382. 10.3389/fcimb.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gullsby K, Olsen B, Bondeson K. 2019. Molecular typing of Mycoplasma pneumoniae strains in Sweden from 1996 to 2017 and the emergence of a new P1 cytadhesin gene, variant 2e. J Clin Microbiol 57:e00049-19. 10.1128/JCM.00049-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurkela S, Puolakkainen M, Hokynar K, Nieminen T, Saxen H, Mannonen L, Pietikainen R. 2019. Mycoplasma pneumoniae outbreak, southeastern Finland, 2017-2018: molecular epidemiology and laboratory diagnostic lessons. Eur J Clin Microbiol Infect Dis 38:1867–1871. 10.1007/s10096-019-03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Public Health England. 2018. Annual summary of respiratory Mycoplasma pneumoniae laboratory surveillance data, England and Wales, 2017. Public Health England, London, United Kingdom. https://www.gov.uk/government/collections/mycoplasma-pneumoniae. Accessed 20 January 2021. [Google Scholar]
- 43.National Institute for Public Health and the Environment (RIVM). 2018. Annual report surveillance of influenza and other respiratory infections in the Netherlands: winter 2017/2018. RIVM, Utrecht, the Netherlands. https://www.rivm.nl/bibliotheek/rapporten/2018-0049.pdf. Accessed 20 January 2021. [Google Scholar]
- 44.Wagner K, Imkamp F, Pires VP, Keller PM. 2019. Evaluation of Lightmix Mycoplasma macrolide assay for detection of macrolide-resistant Mycoplasma pneumoniae in pneumonia patients. Clin Microbiol Infect 25:383.e5–383.e7. 10.1016/j.cmi.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z. 2014. More complications occurred in macrolide-resistant Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother 58:1034–1038. 10.1128/AAC.01806-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pánisová E, Unger WWJ, Berger C, Meyer Sauteur PM. 2021. Mycoplasma pneumoniae-specific IFN-γ-producing CD4+ effector-memory T cells correlate with pulmonary disease. Am J Respir Cell Mol Biol 64:143–146. 10.1165/rcmb.2020-0237LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schalock PC, Dinulos JG. 2009. Mycoplasma pneumoniae-induced cutaneous disease. Int J Dermatol 48:673–680. 10.1111/j.1365-4632.2009.04154.x. [DOI] [PubMed] [Google Scholar]
- 48.Meyer Sauteur PM, Huizinga R, Tio-Gillen AP, Roodbol J, Hoogenboezem T, Jacobs E, van Rijn M, van der Eijk AA, Vink C, de Wit MC, van Rossum AM, Jacobs BC. 2016. Mycoplasma pneumoniae triggering the Guillain-Barré syndrome: a case-control study. Ann Neurol 80:566–580. 10.1002/ana.24755. [DOI] [PubMed] [Google Scholar]
- 49.Meyer Sauteur PM, Jacobs BC, Spuesens EB, Jacobs E, Nadal D, Vink C, van Rossum AM. 2014. Antibody responses to Mycoplasma pneumoniae: role in pathogenesis and diagnosis of encephalitis? PLoS Pathog 10:e1003983. 10.1371/journal.ppat.1003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon O, Oster Y, Michael-Gayego A, Marans RS, Averbuch D, Engelhard D, Moses AE, Nir-Paz R. 2019. The clinical presentation of pediatric Mycoplasma pneumoniae infections—a single center cohort. Pediatr Infect Dis J 38:698–705. 10.1097/INF.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 51.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376. 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 52.Gaskill BN, Garner JP. 2020. Power to the people: power, negative results and sample size. J Am Assoc Lab Anim Sci 59:9–16. 10.30802/AALAS-JAALAS-19-000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2 and Fig. S1 and S2. Download JCM.00748-21-s0001.pdf, PDF file, 443 KB (443KB, pdf)