Abstract
Background
Vulvovaginal candidiasis (VVC) is a common vaginitis in females. The commonly used diagnostic method, 10% potassium hydroxide (KOH) smear microscopy, makes it not very easy to recognize fungi.
Methods
Vaginal secretions were collected from clinically suspected VVC patients and divided into four groups and examined using KOH, CFW (Calcofluor White), FB 85(fluorescent brightener 85), and culture. The data were statistically analyzed.
Results
In total, 110 patients with suspected VVC were recruited. The positive rates of KOH, CFW, FB 85, and the culture method were 68.2%, 64.5%, 61.8%, and 77%, respectively. According to the McNemar test, there was no statistically significant difference between the KOH, CFW, and the FB 85 methods (p > 0.05). However, CFW had a shorter diagnosis time than the KOH method and had a statistically significant difference (p < 0.001). Moreover, CFW has the highest sensitivity, specificity, and accuracy. In morphological recognition, it was easier to recognize fungal structures with CFW and FB 85 than with the KOH.
Conclusions
The fluorescent method is a good method for the diagnosis of VVC. And the fungi can be found more quickly. Similar to CFW, FB 85 is also a potential good fluorescent reagent for the diagnosis of VVC and has potential value for application in clinical fungal infection diseases.
Keywords: CFW, FB85, fluorescent method, potassium hydroxide (KOH) smear microscopy, vulvovaginal candidiasis (VVC)
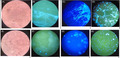
The images under fluorescence microscopy and light microscopy with four methods. a‐d) show C. albicans, and e‐h) show C. glabrata. Both the fungal color and the structure in FB 85 and CFW were clear and easily recognized from the background squamous cells compared with the KOH method. a) The KOH method with C. albicans under light microscopy. b) The CFW method with C. albicans under fluorescence microscopy. c) The FB 85 method with C. albicans under fluorescence microscopy. d) The culture method with C. albicans under fluorescence microscopy with CFW. e) The KOH method with C. glabrata under light microscopy. f) The CFW method with C. glabrata under fluorescence microscopy. g) The FB 85 method with C. glabrata under fluorescence microscopy. h) The culture method with C. glabrata under fluorescence microscopy with CFW.
1. INTRODUCTION
Vulvovaginal candidiasis (VVC) is a common vaginitis in females, which is estimated to be the second most common cause of vaginitis after bacterial vaginosis.1, 2, 3, 4 Vulvovaginal candidiasis affects around 70%–75% of all women during their childbearing age, and is considered to be an important public health problem.5, 6 The morbidity associated with VVC makes it a major cause of causing pain, great discomfort, mental distress, anxiety, altered self‐esteem, impairing work performance and interfering with sexual and affective relations.5, 7 The signs of VVC are typically characterized white clumpy discharge.5 The pathological characteristics of VVC is an acute inflammatory condition of the vulva and vaginal mucosa induced by the overgrowth of Candida organisms which normally exist as a quiescent vaginal commensalism.4, 8 VVC is most caused by Candida albicans, which is isolated in 85%–90% of all cases. Meanwhile, C. glabrata, as another pathogen, appears to be increasing.1, 7, 8, 9 The diagnosis criteria of VVC is the appearance of yeast hyphae in 10% potassium hydroxide (KOH) microscopic examination with typical symptoms and normal acidic vaginal pH. A fungal culture is recommended to confirm the diagnosis and is the gold standard too.10, 11 It also can be diagnosed by using Gram‐stained smear, antigen‐antibody reaction, PCR,12, 13 or DNA probe testing.14, 15, 16, 17 Among these diagnosed methods, the KOH smear is an easy and fast method, but the accuracy is affected by the skill of the technician, and the positive rate is not high. Meanwhile, KOH smear microscopy has a high rate of missed detection in the detection of fungal conidia and spores, as Geiger said, the false negative rate of KOH method for C. albicans was about 30%, but for the non‐C. albicans was 57%.18 Antigen‐antibody kit is expensive and the single kid is not popular used in China. The gene hybridization method has a high specificity, and PCR has a high sensitivity, but the procedures are complex and time consuming. PCR is not available as a diagnostic test and might not prove to be a clinically useful test. Even though the gold standard for diagnosis of VVC is culture, this method is not always practical because it takes up to 3–5 days to make the definitive results.
Calcofluor White is a fluorescent brightener that binds to cellulose in the cell wall of many plants and fungi. After ultraviolet irradiation, it shows strong bright blue fluorescence. The cell wall of fungi is rich in β‐glucans and chitin, so it is easy for CFW to bind to fungi and to detect fungi under a microscopy. Therefore, CFW has been used extensively for the diagnosis of microsporidial keratitis, superficial dermatophytes, onychomycosis, Candida in oral precancer and cancer, and pathological specimens.19, 20, 21, 22 However, no study has used CFW for the diagnosis of VVC. Moreover, fluorescent brightener 85, a new fluorescent reagent, was first found and used for the diagnosis of onychomycosis by the author,23 but there is no research about using FB 85 to detect fungi in VVC.
Therefore, in this study, we used CFW and FB 85 as two fluorescent reagents to diagnose VVC and compared them with the KOH smear method and fungal culture method to evaluate the advantages of CFW and FB 85 and the characteristics of microscopy. We also compared the diagnostic positive rate, sensitivity, specificity,consistency check (KAPPA), diagnosis time and stability of FB 85 and CFW.
2. MATERIALS AND METHODS
2.1. Specimen collection and methods
Inclusion criteria: According to the symptoms and the signs included vaginal discharge, irritation, itching, and/or odor, patients who were suspected of having VVC were recruited from the outpatient department of Obstetrics and Gynecology at Capital Medical University, Beijing Tiantan Hospital, from October 2018 to February 2019. Exclusion criteria: Patients were ruled out of the VVC by typical clinical symptoms. This study was approved by the Ethical Committee for Human Study, Beijing Tiantan Hospital, Capital Medical University. All subjects signed informed consent prior to the study.
In total, four methods were used to detect the fungus. After the vagina was opened with the dilator, two aseptic cotton swabs were used to collect the secretions from the posterior vaginal fornix and the vaginal wall: one for fungal culture, the other for fluorescent microscopy observation with CFW (Zhuhai Beisuo Biotechnology Co., Ltd) and FB 85 (Shandong Yousuo Chemical Technology Co., Ltd). The swabs were smeared on two separate slides, one with a drop of CFW, the other with a drop of 0.1% FB 85. The secretions gathered from the dilator were smeared on a third slide and examined using KOH under a light microscopy by adding a drop of 10% KOH to the slide. All three slides from the same person were covered with cover glass, and the extra liquid was suctioned with a cotton swab. Then, they were examined by three different technicians who were double blinded to the research. Microscopic observations were conducted using an Olympus CX23 LEDRFSIC microscopy (Olympus Co., Ltd) using either the 340–380 nm ultraviolet light mode or the visible light mode. The images under microscopy were taken using the HUAWEI phone P20. The diagnosis time was identified as how long it took to find the fungus, and it was also recorded.
The fungal culture method is as follows. First, the alcohol lamp was ignited, and the sterile cotton swab with secretion was smeared on two yeast chromogenic media (Jinan Baibo Biotechnology Co., Ltd). Then, the media were cultured in an incubator at 35 ± 1°C for 24 h. Plates with no growth after 24 h were re‐incubated for a further 24 h. Finally, fungal growth was observed and identified using colony characteristics after 24 h or 48 h. The colony characteristics were as follows: C. albicans: 2 mm emerald‐green‐smooth colony; C. tropicalis: 1.5 mm iron‐blue or bluish‐gray‐smooth colony; C. krusei: 4–5 mm light‐pink‐fuzzy‐velour colony; C.glabrata: 2 mm‐purple‐smooth colony; and other Candida: grayish‐white to pale‐pink colony. After identification, the identified time was also recorded.
2.2. Data analysis and statistical analysis
The positive result under fluorescence microscopy was the presence of filamentous fungal hyphae, pseudohyphae, or round or oval spores with bright blue peripheral fluorescence with the FB 85 or CFW method. The positive diagnosis with the KOH method was the presence of fungal hyphae, pseudohyphae, or spores with high refractivity.
The positive rates of the four methods were calculated, and fungal culture was the gold standard. We compared the positive rate between the FB 85 and KOH method, the CFW and KOH method. Statistical analysis was performed with the McNemar test using SPSS 21 (IBM SPSS Statistics).
The sensitivity, specificity and accuracy of KOH, CFW and FB 85 method were evaluated, with the culture method as gold standard. We also compared the consistency between the two fluorescent reagents and KOH method, using SPSS 21 for Kappa test, the consistency of Kappa >0.8 is very good.
The diagnosed time of the three methods were compared with t‐test using SPSS 21. The difference was statistically significant with p < 0.05.
Moreover,we also observed and compared the fade time of CFW and FB 85 in the room environment with a daylight lamp after 24 h.
3. RESULTS
3.1. The positive rate of the four methods
A total of 110 specimens were obtained with suspected VVC, aged 19–60 years, with an average age of 30.99 ± 7.77 years. The positive and negative rates of the four methods in 110 specimens are shown in Figure 1. In the 110 specimens, 68.2% (75/110) were positive by the KOH method, 64.5% (71/110) were positive by the CFW fluorescent method, 61.8% (68/110) were positive by the FB 85 fluorescent method, and 77% (77/110) were positive by the fungal culture method. According to the McNemar test, there is no statistically significant difference between the CFW and KOH method (p = 1.000), the FB 85 and KOH method (p = 0.727), and the FB 85 and CFW methods (p = 0.250). Among the positive fungal cultures, 96.1% (74/77) were C. albicans, and 3.90% (3/77) were C. glabrata.
FIGURE 1.
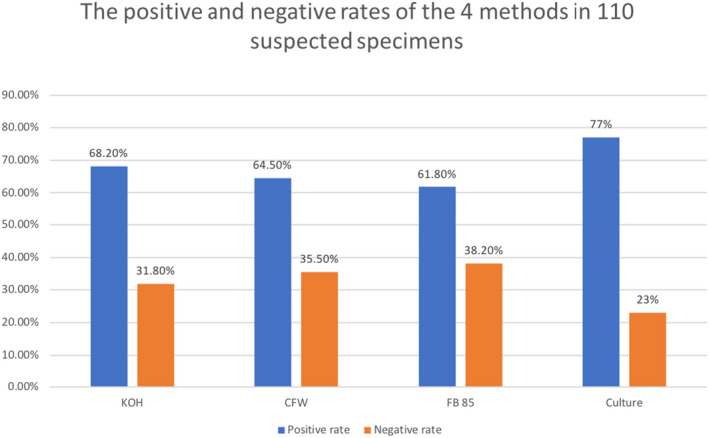
The positive and negative rates of the four methods in 110 specimens
3.2. The sensitivity, specificity, accuracy and consistency of the three methods
The sensitivity, specificity and accuracy of the KOH method, CFW method and FB85 method were evaluated with culture method as the gold standard. The results were shown in Table 1. In the 110 specimens, the specificity of the three methods was 100%. The sensitivity, and accuracy of CFW were the highest, followed by KOH method and FB 85 was the lowest.
TABLE 1.
The sensitivity, specificity and accuracy of KOH method, CFW method and FB85 method
| Sensitivity | Specificity | Accuracy | |
|---|---|---|---|
| KOH method | 90.9% | 100% | 93.6% |
| CFW method | 92.2% | 100% | 94.5% |
| FB85 method | 88.3 | 100% | 91.8% |
Then, the consistency between CFW and KOH, FB85 and KOH method were compared. The consistency test results of CFW with KOH method: Kappa = 0.901, greater than 0.8, p < 0.001, it can be seen that the consistency of the two methods is very good. The consistency test results of FB 85 with KOH method: Kappa = 0.845, greater than 0.8, p < 0.001, which shows that the consistency of the two methods is very good too. Therefore, the two fluorescent methods (CFW and FB85) are in good agreement with the common VVC detection method KOH method.
3.3. The images under fluorescence microscopy and light microscopy with four methods
The images under fluorescence microscopy and light microscopy with the four methods are shown in Figure 2 from two different species infections, C. albicans (Figure 2A‐D) and C. glabrata (Figure 2E‐H). The images of FB 85 (Figure 2C,G) were similar to those of CFW (Figure 2B,F), but had slightly different color. The fungal color for FB85 was blue, which did not look like the blue‐green color for CFW. It was because there was no KOH in FB 85. However, both the fungal color and the form in FB 85 and CFW were easily recognized from the background squamous cells. The fungal structures were clear, especially compared with the KOH method (Figure 1A,E), In KOH method, it is easy to confuse the fungal structure with the edge of squamous cells in light microscopy. For the VVC caused by C. glabrata, yeast form is always observed, which is very clear with CFW method (Figure 2F) and FB 85 method (Figure 2G) but not easy to be found by light microscopy (Figure 2E). There was no significant difference in morphology between C. albicans and C. glabrata after culture under fluorescence microscopy, and both of them were very bright spores (Figure 2D,H).
FIGURE 2.
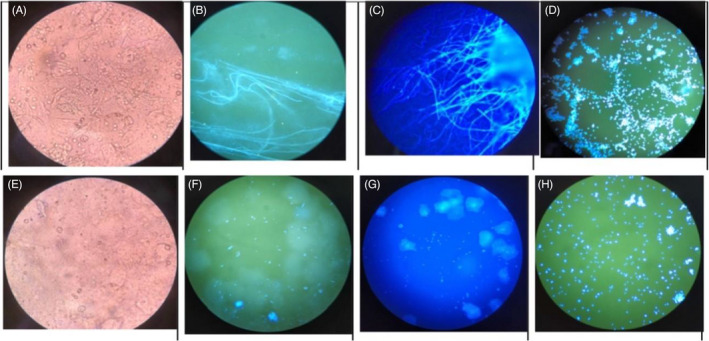
The images under fluorescence microscopy and light microscopy with four methods. A‐D, show C. albicans, and E‐H, show C. glabrata. Both the fungal color and the structure in FB 85 and CFW were clear and easily recognized from the background squamous cells compared with the KOH method. A, The KOH method with C. albicans under light microscopy. B, The CFW method with C. albicans under fluorescence microscopy. C, The FB 85 method with C. albicans under fluorescence microscopy. D, The culture method with C. albicans under fluorescence microscopy with CFW. E, The KOH method with C. glabrata under light microscopy. F, The CFW method with C. glabrata under fluorescence microscopy. G, The FB 85 method with C. glabrata under fluorescence microscopy. H, The culture method with C. glabrata under fluorescence microscopy with CFW
3.4. The diagnosis time for three methods and fade time of two fluorescent reagents under daylight
We also recorded the diagnosis time for the positive specimens. The average diagnosis times for the KOH, CFW, and FB 85 methods were 24.33 ± 22.64 s, 5.46 ± 10.27 s, and 19.46 ± 22.74 s, respectively. According to the t‐test, CFW had a shorter diagnosis time than the KOH method, and had a statistically significant difference (p < 0.001). But there was no statistically significant difference between the FB 85 and KOH methods (p > 0.05). There was also a statistically significant difference between the CFW and FB 85 methods (p < 0.001).
Moreover, we also observed a fade time of two fluorescent reagents under daylight in the room environment. For 21 CFW specimens, 76.2% (14/21) of the specimens had a relatively clear fungal structure with a strong crystalline or blurry background after the stained slide was placed in the room environment for 24 h (Figure 3A at 0 h and Figure 3B at 24 h). For 25 FB 85 specimens, 56% (14/25) of the specimens had a clear structure (Figure 3C at 0 h and Figure 3D at 24 h). However, due to the crystals and the faint fluorescence, it was harder to distinguish the fungi from the 24 h specimen than the fresh specimen.
FIGURE 3.
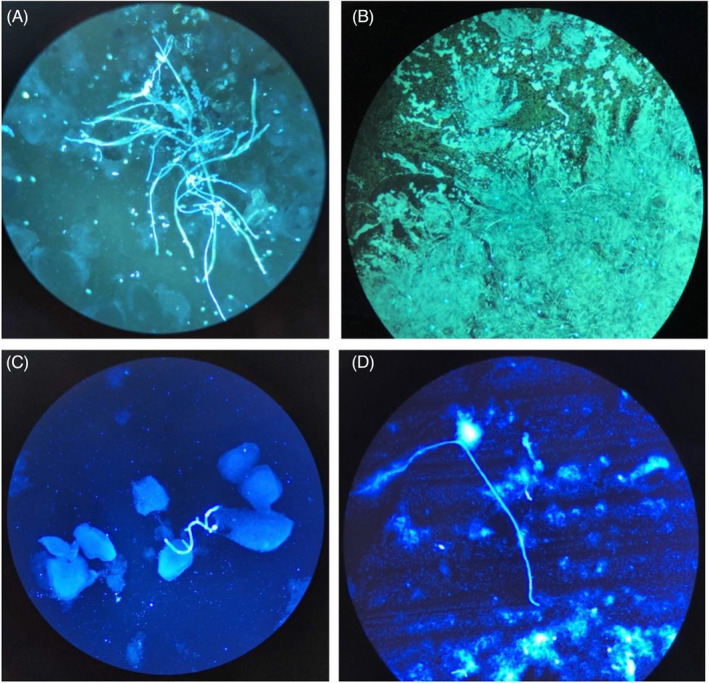
The images of CFW and FB 85 under fluorescence microscopy at 0 h and 24 h. A, B, show the CFW method, and C, D, show the FB 85 method. A, 0 h with CFW; B, 24 h with CFW of the same specimen. C, 0 h with FB 85; D, 24 h with FB 85 of the same specimen
4. DISCUSSION
Vulvovaginal candidiasis is a common vaginitis in women and is mainly caused by C. albicans. In our research, we analyzed the positive diagnostic rates and the species in 110 suspected VVC patients. There were 96.1% C. albicans and 3.9% C. glabrata. Our study is similar to other countries' studies.7, 24, 25, 26 In Gonçalves B's summary, the most common Candida species associated with VVC are C. albicans, C. glabrata, C. tropicalis, C. aparapsilosis and C. krusei. Typically, a single species is identified, but two or more species have been found in some women with VVC (1%–10%). In Chinese, Tunisian, and Iranian studies show a predominance of C. albicans (65.1%–90.4%).
In addition, among the 110 samples tested, although there was no statistically significant difference between the three methods for the diagnostic positive rate, CFW had a shorter diagnosis time than the KOH method (p < 0.001). There was also a statistically significant difference between the CFW and FB 85 methods (p < 0.001). But there was no statistically significant difference between the FB 85 and KOH methods (p > 0.05).
In the author Yue's21 previous study, she used CFW to evaluate the accuracy and efficiency of onychomycosis diagnosis and found there was no statistically significant difference between the CFW methods and KOH method. In our research, we found the similar result. Even though there was no statistically significant difference between the two fluorescent methods and the KOH method. When we compared the fungal structure under light microscopy and fluorescence microscopy with either CFW or FB 85, it was easy to find fungi with CFW or FB 85 in fluorescence microscopy. That is why the diagnosis time of CFW is much shorter than that of the KOH method and is a statistically significant difference. And this finding is the same as previous study.21
In the author Yue's23 another study, she was the first to use FB 85 to evaluate the accuracy and efficiency for the diagnosis of onychomycosis in clinic and found that FB 85 had a higher positive rate than the KOH method, which had a statistically significant difference. However, in our study, both the FB 85 and KOH methods had similar positive rates and had no statistical significance difference. We analyzed the reason for this difference. First, maybe it is easier to find fungi in the VVC specimen than in the superficial skin specimen. Second, there was no KOH and Even blue in the FB 85 reagent in this study, and the slides were not heated by an alcohol lamp for a while, which causes the poor dissolution of the background cells and the highlighted color of the background cells in FB 85 method. Especially compared with the CFW reagent, the absence of KOH in FB 85 reagent also caused the delay in diagnosis time for FB 85 method in this study. Third, the level of the skill of the three technicians in this study is different. The technicians for the KOH method and CFW method are skilled, but the technician for FB 85 is new, that is why the diagnosis time with FB 85 was delayed as well. Therefore, next, we need to mix KOH and Even blue to make the FB 85 reagents more suitable for clinical specimens and accumulating more experience in clinical.
The results of the two fluorescent reagents were in good agreement with those of KOH method. This indicates that fluorescent reagent can replace KOH method to detect fungi in vaginal secretions.
By comparing the morphology of the same specimen under light microscope and after fluorescent microscopy with two fluorescent reagents, it can be clearly seen that the filamentous with spores and long hypha of C. albicans with fluorescent reagent is easier to be observed than with KOH. Moreover, due to the C. glabrata is mostly appeared with yeast‐like spores or short hyphae, therefore, the C. glabrata can be easily distinguished with fluorescent reagent than with KOH.
5. CONCLUSION
In conclusion, we can find pathogenic fungi more quickly with fluorescent method in diagnosis of VVC. Both CFW and FB 85 are good reagents. FB 85 is a new fluorescent reagent for the diagnosis of VVC and other fungal affection diseases and has potential value for clinical applications. However, more research is needed to make it more suitable for clinical applications.
CONFLICT OF INTEREST
The authors declare there are no competing interests.
AUTHOR CONTRIBUTIONS
All authors have read and approved the final manuscript. Yue X participated in the experimental designing, collaboration and drafting of the manuscript. Zhao YZ participated in the experimental design and the lab examination. Yu ZX processed the lab examination.
EMPLOYMENT OR LEADERSHIP
None declared.
ACKNOWLEDGEMENTS
We thank the study participants for making this study possible.
Zhao Y, Yu Z, Yue X. Evaluating the accuracy and diagnostic value of CFW and a new fluorescent reagents, fluorescent brightener 85, for the diagnosis of vulvovaginal candidiasis. J Clin Lab Anal. 2021;35:e23891. 10.1002/jcla.23891
Funding information
The authors declare no specific funding for this work
1. DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current day are available from the corresponding author on reasonable request.
REFERENCES
- 1.Dovnik A, Golle A, Novak D, et al. Treatment of vulvovaginal candidiasis: a review of the literature. Acta Dermatovenerol Alp Pannonica Adriat. 2015;24(1):5‐7. [DOI] [PubMed] [Google Scholar]
- 2.Jeanmonod R, Jeanmonod D. Vaginal candidiasis (Vulvovaginal Candidiasis). In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. [Google Scholar]
- 3.Willems HME, Ahmed SS, Liu J, et al. Vulvovaginal candidiasis: a current understanding and burning questions. J Fungi (Basel). 2020;6(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidel PL Jr, Barousse M, Espinosa T, et al. An intravaginal live candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72(5):2939‐2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961‐1971. [DOI] [PubMed] [Google Scholar]
- 6.Aniebue UU, Nwankwo TO, Nwafor MI. Vulvovaginal candidiasis in reproductive age women in Enugu Nigeria, clinical versus laboratory‐assisted diagnosis. Niger J Clin Pract. 2018;21(8):1017‐1022. [DOI] [PubMed] [Google Scholar]
- 7.Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42(6):905‐927. [DOI] [PubMed] [Google Scholar]
- 8.Mtibaa L, Fakhfakh N, Kallel A, et al. Vulvovaginal candidiasis: etiology, symptomatology and risk factors. J Mycol Med. 2017;27(2):153‐158. [DOI] [PubMed] [Google Scholar]
- 9.Bitew A, Abebaw Y. Vulvovaginal candidiasis: species distribution of candida and their antifungal susceptibility pattern. BMC Womens Health. 2018;18(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendling W, Brasch J, Cornely OA, et al. Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses. 2015;58(Suppl 1):1‐15. [DOI] [PubMed] [Google Scholar]
- 11.Workowski KA, Berman SM. CDC sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2002;35(Suppl 2):S135‐S137. [DOI] [PubMed] [Google Scholar]
- 12.Hasanvand S, Azadegan Qomi H, Kord M, et al. Molecular epidemiology and in vitro antifungal susceptibility of candida isolates from women with vulvovaginal candidiasis in northern cities of Khuzestan province. Iran. Jundishapur J Microbiol. 2017;10(8):e12804. [Google Scholar]
- 13.Khanmohamadi M, Mehbod ASA, Noraeepour M, et al. Molecular identification of candida species isolated from women with vulvovaginal candidiasis: brief report. Tehran Univ Med J. 2017;75(7):538‐542. [Google Scholar]
- 14.Marot‐Leblond A, Nail‐Billaud S, Pilon F, et al. Efficient diagnosis of vulvovaginal candidiasis by use of a new rapid immunochromatography test. J Clin Microbiol. 2009;47(12):3821‐3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilkit M, Guzel AB. The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: a mycological perspective. Crit Rev Microbiol. 2011;37(3):250‐261. [DOI] [PubMed] [Google Scholar]
- 16.Paladine HL, Desai UA. Vaginitis: diagnosis and treatment. Am Fam Physician. 2018;97(5):321‐329. [PubMed] [Google Scholar]
- 17.Dessai F, Nyirenda M, Sebitloane M, et al. Diagnostic evaluation of the BD Affirm VPIII assay as a point‐of‐care test for the diagnosis of bacterial vaginosis, trichomoniasis and candidiasis. Int J STD AIDS. 2020;31(4):303‐311. [DOI] [PubMed] [Google Scholar]
- 18.Geiger AM, Foxman B, Sobel JD. Chronic vulvovaginal candidiasis: characteristics of women with candida albicans, C glabrata and no candida. Genitourin Med. 1995;71(5):304‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph J, Murthy S, Garg P, et al. Use of different stains for microscopic evaluation of corneal scrapings for diagnosis of microsporidial keratitis. J Clin Microbiol. 2006;44(2):583‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motamedi M, Sharifi Lari M, Pakshir K, et al. Comparing real‐time PCR and calcofluor‐white with conventional methods for rapid detection of dermatophytes: across‐sectional study. J Microbiol Methods. 2019;161:84‐86. [DOI] [PubMed] [Google Scholar]
- 21.Yue X, Shi X, Chen W, et al. Evaluate the accuracy of CFW fluorescent staining for the diagnosis of suspected onychomycosis. Chin J Mycol. 2018;13(1):8‐10. [Google Scholar]
- 22.Bhavasar RS, Goje SK, Takalkar AA, et al. Detection of candida by calcofluor white. Acta Cytol. 2010;54(5):679‐684. [DOI] [PubMed] [Google Scholar]
- 23.Yue X, Wang A, Wang H, et al. Evaluation of a new fluorescent reagent, fluorescent brightener 85, for the diagnosis of suspected onychomycosis compared with potassium hydroxide. Mycoses. 2018;61(4):279‐282. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad A, Khan AU. Prevalence of candida species and potential risk factors for vulvovaginal candidiasis in Aligarh, India. Eur J Obstet Gynecol Reprod Biol. 2009;144(1):68‐71. [DOI] [PubMed] [Google Scholar]
- 25.Amouri I, Sellami H, Borji N, et al. Epidemiological survey of vulvovaginal candidosis in Sfax, Tunisia. Mycoses. 2011;54:499‐505. [DOI] [PubMed] [Google Scholar]
- 26.Amouri I, Sellami H, Abbes S, et al. Microsatellite analysis of candida isolates from recurrent vulvovaginal candidiasis. J Med Microb. 2012;61:1091‐1096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current day are available from the corresponding author on reasonable request.


