Abstract
Purpose
The purpose of this study was to explore the predictive value of the ratio of the product of neutrophils and hemoglobin to lymphocytes (NHL) in patients with non‐muscular invasive bladder cancer (NMIBC).
Materials and Methods
We retrospectively collected clinical and pathological data of patients with NMIBC who underwent transurethral resection of bladder tumor (TURBT) at our hospital between 2013 and 2018. The ratio of neutrophils to lymphocytes (NLR), the Systemic Immune Inflammation Index (SII), and NHL were obtained based on routine blood settlement within a week before surgery. The receiver operating characteristic curve was used to determine the optimal cutoff value of each index, and different groups were grouped accordingly. Kaplan‐Meier survival curve and Cox regression model were used to study the factors affecting the prognosis of NMIBC patients.
Results
There was significant difference in recurrence‐free survival (RFS) rate between the high NLR group and the low NLR group, the high SII group and the low SII group, and the high NHL group and the low NHL group. Cox univariate regression analysis showed that tumor number, tumor size, tumor pathological grade, tumor pathological stage, NLR, SII, and NHL were related to postoperative RFS in patients with NMIBC. The tumor number, tumor pathological grade, SII, and NHL were independent predictors of RFS in multivariate analysis.
Conclusions
The preoperative clinical inflammatory indexes NLR, SII, and NHL have certain predictive value for postoperative RFS in NMIBC patients.
Keywords: neutrophil‐lymphocyte ratio, non‐muscular invasive bladder cancer, recurrence, systemic immune inflammation index
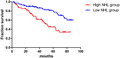
The recurrence‐free survival (RFS) rates of the high neutrophil hemoglobin product to lymphocyte ratio (NHL) group and the low NHL group were 54.0% and 75.9%, respectively, with significant difference between the two groups (p < 0.001),NHL have certain predictive value for postoperative RFS in non‐muscular invasive bladder cancer patients.
1. INTRODUCTION
Bladder cancer is the ninth most common malignant tumor worldwide and the most common malignant tumor of the urinary system.1 Non‐muscular invasive bladder cancer (NMIBC) accounts for about 70–80% of all bladder cancer cases and has a high recurrence rate after operation.2 Therefore, it is critical to find biomarkers to efficiently and reliably predict prognosis for the treatment of NMIBC patients.
In recent years, with the improvement of living standards, the incidence of metabolic diseases such as obesity and type 2 diabetes has been increasing year by year, which has seriously affected people's health. And some studies have shown that obesity and type 2 diabetes mellitus on prognosis of bladder cancer may also have certain effect, its mechanism might be caused by obesity, and type 2 diabetes mellitus metabolic balance disorders interfere with the body's inflammatory response and immune system, which creates a suitable microenvironment of tumor growth, thus to further promote the occurrence and development of tumor.3, 4 At the same time, numerous studies have proven that the inflammatory response induced by tumor tissue and the body's immune system play an important role in the occurrence and development of tumors.5 Among them, the ratio of neutrophils to lymphocytes (NLR) has been widely studied, and NLR was shown to play an important role in predicting the recurrence and progression of various tumors after operation.6, 7, 8 For urinary tract tumors, relevant studies have shown that NLR is not only related to upper urinary tract urothelial carcinoma after surgery,9 but also has a certain correlation with the prognosis of NMIBC.10 The Systemic Immune Inflammation Index (SII) has also been shown to have good predictive value in the prognosis of patients with hepatocellular carcinoma and renal clear cell carcinoma.11, 12 However, there are few studies on the prognostic value of SII in NMIBC.
The first symptom of bladder cancer is gross hematuria, and preoperative hemoglobin level is also related to tumor prognosis.13, 14 Therefore, we combined neutrophils, lymphocytes, and hemoglobin to create an indicator: the ratio of the product of neutrophils and hemoglobin to lymphocytes (NHL), and explore the predictive value of NHL, NLR, and SII on the prognosis of patients with NMIBC.
2. MATERIALS AND METHODS
2.1. Clinical data
The participants were mainly patients with bladder cancer who underwent transurethral bladder tumor resection (TURBT) at our hospital between January 2013 and December 2018. Inclusion criteria were as follows: (1) TURBT was performed at our hospital and confirmed as non‐myenteric invasive bladder cancer for the first time after operation; (2) there was no urinary tract infection or systemic infection before operation, and the blood routine examination was normal within one week; (3) no chemotherapy before operation; and (4) complete follow‐up. Exclusion criteria were as follows: (1) Serious complications occurred during perioperative period; (2) concomitant malignant tumors in other parts; (3) severe diseases such as cardiac and pulmonary insufficiency that affected the postoperative recovery of the patients; (4) concomitant systemic autoimmune diseases, blood diseases, rheumatic diseases, and other diseases affecting blood inflammatory markers.
2.2. Research methods
Clinical data of the participants, including age, gender, tumor number, tumor size, tumor pathological grade, and tumor pathological stage, were collected. Relapse‐free survival (RFS) was used as the main index of survival analysis. RFS data were obtained mainly through outpatient review or follow‐up by telephone. RFS was defined as the time from the first pathological confirmation of NMIBC to the first recurrence after operation, and the deadline for follow‐up was June 01, 2020. NLR, SII, and NHL were calculated based on the neutrophil count, lymphocyte count, platelet, and hemoglobin from the patient's blood routine test results within 1 week.
2.3. Statistical methods
All statistical analyses were performed using SPSS 26.0 version statistical software (IBM) and GraphPad Prism version 5.0 (GraphPad Software Inc.). With recurrence as the outcome, the receiver operating characteristic (ROC) curves of NLR, SII, and NHL were drawn, combined with the maximum Youden index to determine the best cutoff value of each index, and grouped accordingly. The clinical data of different groups were compared. Count data were compared using χ2 test or Fisher exact probability method. Kaplan‐Meier method was used to draw survival curves. Log‐rank test was used to compare the RFS rates among different NLR, SII, and NHL groups, and Cox regression model was used to analyze the risk factors affecting prognosis. A P‐value <0.05 indicated that the difference was statistically significant.
3. RESULTS
3.1. General information of participants
A total of 216 cases were enrolled in this study. The median patient age was 59 years (range 25–87 years). Among them, 120 cases were ≥60 years old, 96 cases were <60 years old, 164 cases were males, and 52 cases were females. The average follow‐up period was 59.41 months (range 2–89 months).
3.2. Determination of the optimal critical value of NLR, SII, and NHL
With recurrence as the outcome, the ROC curves of NLR, SII, and NHL were drawn (Figure 1A), and the best cutoff value of each index was determined by the most approximate Youden index. The areas under the ROC curves of NLR, SII, and NHL were 0.653, 0.644, and 0.659, respectively. When each reached the maximum value of the Youden index, the best critical value was selected as follows: the NLR cutoff value was 1.615 (sensitivity 0.757 and specificity 0.542); SII cutoff value was 276.685 (sensitivity 0.865 and specificity 0.408); and NHL cutoff value was 252.645 (sensitivity 0.622 and specificity 0.620). NLR was divided into the low NLR group (95 cases) and the high NLR group (121 cases) according to the limit of 1.615. SII was divided into the low SII group (68 cases) and the high SII group (148 cases) according to the limit of 276.685. NHL was divided into the low NHL group (116 cases) and the high NHL group (100 cases) according to the limit of 252.645.
FIGURE 1.
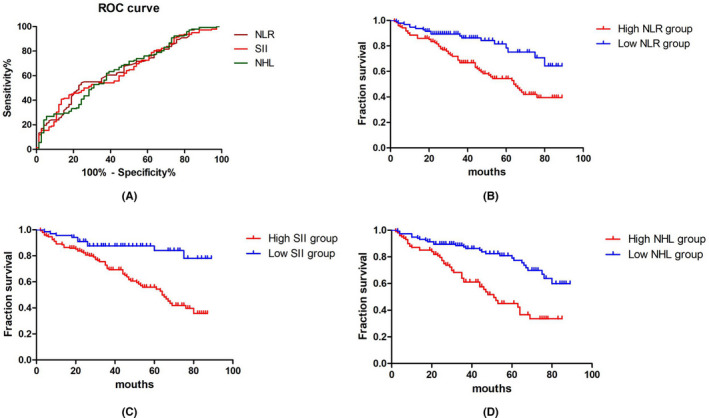
A, ROC curves of the best cutoff values of NHL, SII, and NHL. NLR: neutrophil‐lymphocyte ratio; SII: systemic immune inflammation index; NHL: neutrophil hemoglobin product to lymphocyte ratio. B, Comparison of survival curves between the high NLR group and the low NLR group. C, Comparison of survival curves between the high SII group and the low SII group. D, Comparison of survival curves between the high NHL group and the low NHL group. Key: ROC: Receiver operating characteristic, NLR: neutrophil‐lymphocyte ratio, SII: systemic immune inflammation index, NHL: neutrophil hemoglobin product to lymphocyte ratio
3.3. The relationship between patient clinical data and NLR, SII, and NHL
The patient's age (χ2 = 8.840, p < 0.01) and tumor pathological grade (χ2 = 4.090, p < 0.05) were significantly different between the high NLR group and the low NLR group, while smoking history (χ2 = 1.400, p = 0.237), gender (χ2 = 1.100, p = 0.294), number of tumors (χ2 = 1.150, p = 0.283), tumor size (χ2 = 0.010, p = 0.919), and tumor pathological stage (χ2 = 3.160, p = 0.075) showed no significant difference between the two groups (p > 0.05). There was significant difference in tumor pathological grade (χ2 = 8.106, p < 0.01) between the high SII group and the low SII group, but no significant difference in smoking history (χ2 = 3.103, p = 0.078), age (χ2 = 1.240, p = 0.265), gender (χ2 = 0.020, p = 0.899), number of tumors (χ2 = 0.512, p = 0.474), tumor size (χ2 = 0.293, p = 0.588), and pathological stage (χ2 = 3.580, p = 0.058). There were significant differences between the high NHL group and the low NHL group in gender (χ2 = 10.340, p < 0.01) and tumor pathological stage (χ2 = 10.611, p < 0.01), while smoking history (χ2 = 0.570, p = 0.451), age (χ2 = 2.240, p = 0.135), number of tumors (χ2 = 0.076, p = 0.782), tumor size (χ2 = 0.362, p = 0.547), and tumor pathological grade (χ2 = 0.724, p = 0.395) showed no significant difference between the two groups (Table 1).
TABLE 1.
Correlation between clinical data of patients and NLR, SII, and NHL
| High NLR group (n = 121) | Low NLR group (n = 95) | χ2 | p | High SII group (n = 148) | Low SII group (n = 67) | χ2 | p | High NHL group (n = 100) | Low NHL group (n = 116) | χ2 | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||||
| Smoking history | ||||||||||||
| Yes | 39 (32) | 38 (40) | 1.400 | 0.237 | 47 (32) | 30 (45) | 3.103 | 0.078 | 33 (33) | 44 (38) | 0.570 | 0.451 |
| No | 82 (68) | 57 (60) | 101 (68) | 37 (55) | 67 (67) | 72 (62) | ||||||
| Age (years) | ||||||||||||
| ≥60 | 78 (64) | 42 (44) | 8.840 | p < 0.01 | 86 (58) | 34 (50) | 1.240 | 0.265 | 61 (61) | 59 (51) | 2.240 | 0.135 |
| <60 | 43 (36) | 53 (56) | 62 (42) | 34 (50) | 39 (39) | 57 (49) | ||||||
| Gender | ||||||||||||
| Male | 95 (79) | 68 (72) | 1.100 | 0.294 | 112 (76) | 52 (76) | 0.020 | 0.899 | 86 (86) | 78 (67) | 10.340 | p < 0.01 |
| Female | 26 (21) | 26 (27) | 36 (24) | 16 (24) | 14 (14) | 38 (33) | ||||||
| Number of tumors | ||||||||||||
| Single | 86 (71) | 61 (64) | 1.150 | 0.283 | 103 (70) | 44 (65) | 0.512 | 0.474 | 69 (69) | 78 (67) | 0.076 | 0.782 |
| Multiple | 35 (29) | 34 (36) | 45 (30) | 24 (35) | 31 (31) | 38 (33) | ||||||
| Tumor size | ||||||||||||
| ≥3cm | 39 (32) | 30 (32) | 0.010 | 0.919 | 49 (33) | 20 (29) | 0.293 | 0.588 | 34 (34) | 35 (30) | 0.362 | 0.547 |
| <3cm | 82 (68) | 65 (68) | 99 (67) | 48 (71) | 66 (66) | 81 (70) | ||||||
| Pathological grade | ||||||||||||
| Low‐grade | 79 (65) | 74 (78) | 4.090 | p < 0.05 | 96 (65) | 57 (84) | 8.106 | p < 0.01 | 68 (68) | 85 (73) | 0.724 | 0.395 |
| High‐grade | 42 (35) | 21 (22) | 52 (35) | 11 (16) | 32 (32) | 31 (27) | ||||||
| Pathological stage | ||||||||||||
| Ta | 96 (79) | 84 (88) | 3.160 | 0.075 | 120 (81) | 62 (91) | 3.580 | 0.058 | 75 (75) | 106 (91) | 10.611 | p < 0.01 |
| T1 | 25 (21) | 11 (12) | 28 (19) | 6 (9) | 25 (25) | 10 (9) | ||||||
Abbreviations: NLR, neutrophil‐lymphocyte ratio; SII, systemic immune inflammation index; NHL, neutrophil hemoglobin product to lymphocyte ratio.
3.4. Relationship between preoperative peripheral blood NLR, SII, and NHL levels and recurrence
The Kaplan‐Meier method was used to draw the survival curves (Figure 1B–D), and the log‐rank test was used to compare the RFS among the different NLR, SII, and NHL groups. The RFS rates of the high NLR group and the low NLR group were 53.7% and 81.1%, respectively, with significant difference between the two groups (p < 0.001). The RFS rates of the high SII group and the low SII group were 56.8% and 85.3%, respectively, with significant difference between the two groups (p < 0.001). The RFS rates of the high NHL group and the low NHL group were 54.0% and 75.9%, respectively, with significant difference between the two groups (p < 0.001).
3.5. The relationship between NLR, SII, NHL, and PFS in patients with NMIBC
The patient's smoking history, age, gender, tumor stage, tumor grade, tumor size, tumor number, NLR, SII, and NHL were used as detection indicators, and the Cox proportional hazard risk regression model was used to analyze the results. The results showed that smoking history (HR = 1.698, p = 0.044), tumor number (HR = 2.072, p = 0.002), tumor size (HR = 0.572, p = 0.020), tumor pathological grade (HR = 2.485, p < 0.001), tumor pathological stage (HR = 1.981, p = 0.022), NLR (HR = 0.328, p < 0.001), SII (HR = 0.288, p < 0.001), and NHL (HR = 0.357, p < 0.001) were the risk factors affecting postoperative RFS in patients with NMIBC (Table 2).
TABLE 2.
Univariate analysis of influencing factors on RFS of patients
| Univariate analysis | ||
|---|---|---|
| HR (95% CI) | p | |
| Smoking history | ||
| Yes or no | 1.698 (1.015–2.838) | 0.044 |
| Gender | ||
| Male or Female | 1.808 (0.646–1.807) | 0.769 |
| Age(years) | ||
| ≥60 or <60 | 0.808 (0.507–1.286) | 0.369 |
| Number of tumors | ||
| Single or multiple | 2.072 (1.300–3.301) | 0.002 |
| Tumor size | ||
| ≥3 cm or <3 cm | 0.572 (0.356–0.917) | 0.020 |
| Pathological grade | ||
| Low‐grade or high‐grade | 2.485 (1.566–3.945) | <0.001 |
| Pathological stage | ||
| Ta or T1 | 1.981 (1.102–3.559) | 0.022 |
| NLR | ||
| ≥1.615 or <1.615 | 0.328 (0.192–0.559) | <0.001 |
| SII | ||
| ≥276.685 or <276.685 | 0.288 (0.148–0.561) | <0.001 |
| NHL | ||
| ≥252.645 or <252.645 | 0.357 (0.222–0.575) | <0.001 |
Abbreviations: NLR, neutrophil‐lymphocyte ratio; SII, systemic immune inflammation index; NHL, neutrophil hemoglobin product to lymphocyte ratio.
The significant indexes of univariate analysis were analyzed by multivariate analysis. The results showed that the number of tumors (HR = 2.121, p = 0.002), pathological grade of tumor (HR = 1.879, p = 0.010), SII (HR = 0.466, p = 0.043), and NHL (HR = 0.434, p = 0.002) were independent risk factors for RFS in patients with NMIBC (Table 3).
TABLE 3.
Multiple analysis of influencing factors on RFS of patients
| Multiple analysis | ||
|---|---|---|
| HR (95% CI) | p | |
| Smoking history | ||
| Yes or no | 1.466 (0.870–2.471) | 0.151 |
| Number of tumors | ||
| Single or multiple | 2.121 (1.307–3.442) | 0.002 |
| Tumor size | ||
| ≥3 cm or <3 cm | 0.755 (0.463–1.231) | 0.260 |
| Pathological grade | ||
| Low‐grade or high‐grade | 1.879 (1.166–3.028) | 0.010 |
| Pathological stage | ||
| Ta or T1 | 1.117 (0.584–2.134) | 0.738 |
| NLR | ||
| ≥1.615 or <1.615 | 0.605 (0.271–1.349) | 0.219 |
| SII | ||
| ≥276.685 or <276.685 | 0.466 (0.222–0.976) | 0.043 |
| NHL | ||
| ≥252.645 or <252.645 | 0.434 (0.255–0.736) | 0.002 |
Abbreviations: NLR, neutrophil‐lymphocyte ratio; SII, systemic immune inflammation index; NHL, neutrophil hemoglobin product to lymphocyte ratio.
4. DISCUSSION
The immune system and inflammatory response of tumor patients have been shown to be closely related to the occurrence and development of tumors.15, 16 As the key components of host immune system and immune response, neutrophils, and lymphocytes play an important role in tumor development. In the process of tumor development, neutrophils can produce various factors, such as tumor necrosis factor (TNF), vascular endothelial growth factor (VEGF), and interleukins (ILs), to stimulate tumor cell proliferation and angiogenesis.17, 18 Meanwhile, the tumor tissue can also stimulate the production of neutrophils by releasing factors such as IL‐1 and TNF‐α.19 The two processes complement each other and jointly promote the occurrence and development of tumors. As the main component of the body's immune response, lymphocytes mainly induce target cell lysis and apoptosis to play an anti‐tumor effect.20 The lymphocyte function in invasive diseases was found to be significantly lower than that of the control group.21 Therefore, the decrease in lymphocyte levels may indicate the decline of the body's anti‐tumor response, which will further promote the development of tumor. Based on the neutrophil count and lymphocyte count, the proposed NLR reflects the dynamic balance of the host's inflammatory response and anti‐tumor effect, and therefore has a certain predictive effect on the prognosis of tumors. Many studies have proven that NLR is correlated with the prognosis of patients with malignant tumors, such as colorectal cancer, prostate cancer, and renal cancer.
The role of NLR in the prognosis of patients with NMIBC has also been further verified by scholars at home and abroad. Mano et al. suggested that NLR ≥2.41 can be used as an independent predictor of postoperative disease recurrence in patients with NMIBC (HR = 1.75; 95% CI: 1.05–2.92; p = 0.032).22 Guan Qingjun et al. found that when the critical value of NLR was 2.75, the risk of recurrence in the high NLR group was higher than that in the low NLR group, and high NLR was also an independent risk factor for postoperative recurrence.23 However, Liu Zhiyu et al. found that although NLR ≥2.6 was associated with postoperative recurrence, it was not an independent risk factor for postoperative recurrence.24 Similarly, the results of this study showed that when the NLR cutoff value was 1.615, high NLR was associated with postoperative recurrence, but was not an independent risk factor for postoperative recurrence (HR = 0.615, 95% CI: 0.275–1.376, p = 0.237). In addition, the cutoff value of NLR in this study was somewhat different from the above studies. Considering the patient's own health status, the different tolerance levels of different regions and ethnic groups to tumors, and this was a single‐center retrospective study, there may be certain differences leading to selectivity bias.
Cancer patients usually suffer from anemia and thrombocytosis, but the mechanism remains unclear. Thrombocytosis is correlated with the progression of bladder cancer,25 and the mechanism may be as follows: In the tumor microenvironment, platelets can release VEGF to promote the formation of blood vessels, and aggregate with tumor cells to protect them from the body's immune system damage, and further promote the occurrence and development of tumors.26 Studies have shown that anti‐platelet drugs may reduce overall cancer mortality.27 Hara et al. found that hemoglobin concentration is closely related to the overall survival rate of patients after radical cystectomy. A meta‐analysis showed that patients’ preoperative hemoglobin levels were negatively correlated with postoperative all‐cause mortality (ACM) and cancer‐specific mortality (CSM).28 The mechanism of the correlation between anemia and tumor progression may be that anemia can cause hypoxia and induce the up‐regulation of hypoxia‐inducible factors, thereby inhibiting apoptosis and increasing the spread of cancer cells.29
The Systemic Immune Inflammation Index (SII), which combines neutrophil count, lymphocyte count, and platelet count, is a recently proposed prognostic indicator. This indicator has been shown to be associated with poor prognosis in patients with liver cancer, lung cancer, and renal cancer.11, 12, 30 However, there are very few studies on the prognostic role of SII in patients with NMIBC after surgery. In this study, the cases were divided into the high SII group and the low SII group based on the 276.685 limit. The RFS rate of the high SII group was significantly lower than that of the low SII group (p < 0.01). Cox multivariate regression analysis showed that high SII was an independent risk factor for recurrence after NMIBC. This result was consistent with the other results. Given the role of neutrophils, lymphocytes, and hemoglobin in tumor progression, we combined the three clinical indicators to propose a new parameter for predicting tumor prognosis: the ratio of the product of neutrophils and hemoglobin to lymphocytes (NHL), and determined the optimal cutoff value of NHL by ROC curve and approximately equal index: 252.645, and divided it into the high NHL group and the low NHL group. The RFS rate of the high NHL group was significantly lower than that of the low NHL group (p<0.01). Cox single and multivariate analyses suggested that high NHL was related to postoperative recurrence and was an independent risk factor for postoperative recurrence. The results of this study suggested that NHL may be correlated with postoperative deficiencies in patients with NMIBC.
The results of this study showed that patients in the high NLR group, the high SII group, and the high NHL group had lower RFS rates than those in the low NLR group, the low SII group, and the low NHL group. Hence, patients with high NLR, SII, and NHL levels have a higher risk of recurrence after surgery, so the detection of peripheral blood indicators before TURBT in patients with NMIBC has positive clinical significance for judging postoperative recurrence. In addition, the results of Cox univariate regression analysis in this study suggested that postoperative recurrence in patients with NMIBC was not only related to NHL, SII, and NHL but also to smoking history, tumor number, tumor size, tumor pathological grade, and tumor pathological stage. However, Cox multivariate regression analysis showed that only tumor number, tumor pathological grade, SII, and NHL were independent risk factors for postoperative recurrence. The EORTC risk rating scale31 proposed in 2007 provides a new basis for the prediction of tumor recurrence and progression.32 Some scholars in China have conducted further research on the score and found that the score is suitable for promotion in the country since it has good predictive value for the long‐term prognosis of tumors.33, 34 A foreign retrospective study has shown that the scoring system has a good predictive effect on short‐term recurrence and progression of bladder tumors.32 However, due to the loss of some case information, no further research on the EORTC score was conducted in this study. The results of this study may help in the clinical diagnosis and treatment of NMIBC patients with a high number of tumors, high‐grade postoperative diagnosis, and high preoperative SII and NHL, by planning appropriate treatment and follow‐up to reduce the follow‐up time interval or appropriately shorten the review time.
This study had certain limitations: At present, there are many studies on postoperative recurrence in patients with high‐grade T1 tumors treated with Bacille Calmette‐Guerin (BCG). Relevant studies have shown that the risk factors for postoperative recurrence are not only related to tumor size, obesity, and presence of carcinoma in situ, but also related to preoperative inflammatory indicators (such as: neutrophils, eosinophils, and basophils, etc.).35 Since this was a retrospective study, a strict follow‐up plan was not established in advance. Moreover, there is poor medical compliance in China. During the follow‐up, some patients were intolerant to chemotherapy drugs or BCG and could not provide a complete history of chemotherapy. However, in order to ensure the authenticity of the data in this study, the history of postoperative infusion chemotherapy was not included in the final analysis. This may have a certain impact on the results of this study. We will establish a strict follow‐up plan for the patients in the future. This study included a small sample size and was a single‐center retrospective study, so selection bias was inevitable. The predictive value of NLR, SII, and NHL for the prognosis of NMIBC patients needs further verification by multi‐center and large sample data. This study only retrospectively analyzed the predictive value of NHL for postoperative recurrence in NMIBC patients. Future prospective studies combined with the EORTC risk score table are needed. Therefore, the clinical significance of NHL needs to be further verified by expanding the sample size and conducting prospective studies.
5. CONCLUSIONS
NLR, SII, and NHL have a certain predictive effect on postoperative recurrence of patients with NMIBC. As a new prognostic indicator, the predictive value of NHL for postoperative recurrence of NMIBC patients requires prospective, multi‐center, large sample studies for further verification.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Ruining Zhao and Jiahao Shan performed research conception and design. Xiaobo Yang, Zhongyu Yuan, Haoran Xu, Ziyang Liu, Xiaojie Zhou, and Wenzhuo Ma involved in data acquisition: Lihong Nie and Jiahao Shan statistically analyzed the study. Ruining Zhao and Hongbin Shi obtained the funding. Hongbin Shi approved the final manuscript.
ACKNOWLEDGMENTS
No.
Zhao R, Shan J, Nie L, et al. The predictive value of the ratio of the product of neutrophils and hemoglobin to lymphocytes in non‐muscular invasive bladder cancer patients with postoperative recurrence. J Clin Lab Anal. 2021;35:e23883. 10.1002/jcla.23883
Ruining Zhao and Jiahao Shan these authors contributed equally to this work.
Funding information
This study was funded by the Ningxia Hui Autonomous Region Focus on Research and Development projects (2020BFH03001), the Natural Science Foundation of Ningxia (NZ17166, NZ17164, 2020AAC03155), and the National Natural Science Foundation of China (No. 81460148, No. 31560288)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11‐30. [DOI] [PubMed] [Google Scholar]
- 2.Marko B, Maximilian B, Richard Z, et al. EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639‐653. [DOI] [PubMed] [Google Scholar]
- 3.Ferro M, Vartolomei MD, Russo GI, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol. 2019;37(3):507‐514. [DOI] [PubMed] [Google Scholar]
- 4.Ferro M, Katalin MO, Buonerba C, et al. Type 2 diabetes mellitus predicts worse outcomes in patients with high‐grade T1 bladder cancer receiving bacillus Calmette‐Guerin after transurethral resection of the bladder tumor. Urol Oncol. 2020;38(5):459‐464. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paramanathan A, Saxena A, Morris DL. A systematic review and meta‐analysis on the impact of pre‐operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 7.Kubo T, Ono S, Ueno H, Shinto E, Yamamoto J, Hase K. Impact of the perioperative neutrophil‐to‐lymphocyte ratio on the long‐term survival following an elective resection of colorectal carcinoma. Int J Colorectal Dis. 2014;29(9):1091‐1099. [DOI] [PubMed] [Google Scholar]
- 8.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil‐to‐lymphocyte ratio in predicting short‐ and long‐term mortality in breast cancer patients. Ann Surg Oncol. 2012;19(1):217‐224. [DOI] [PubMed] [Google Scholar]
- 9.Vartolomei MD, Kimura S, Ferro M, et al. Is neutrophil‐to‐lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta‐analysis of 4385 patients. World J Urol. 2018;36(7):1019‐1029. [DOI] [PubMed] [Google Scholar]
- 10.Vartolomei MD, Ferro M, Cantiello F, et al. Validation of neutrophil‐to‐lymphocyte ratio in a multi‐institutional cohort of patients with T1G3 non‐muscle‐invasive bladder cancer. Clin Genitourin Cancer. 2018;16(6):445‐452. [DOI] [PubMed] [Google Scholar]
- 11.Xiaoteng YU, Cuijian Z, Ding P, et al. Significance of systemic immune inflammation index on the prognosis of patients with renal clear cell carcinoma. Chin J Clin (Electronic Edition). 2018;12(09):483‐487. [Google Scholar]
- 12.Hu B, Yang X‐R, Xu Y, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212‐6222. [DOI] [PubMed] [Google Scholar]
- 13.Lee S‐M, Russell A, Hellawell G. Predictive value of pretreatment inflammation‐based prognostic scores (neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio, and lymphocyte‐to‐monocyte ratio) for invasive bladder carcinoma. Korean J Urol. 2015;56(11):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sejima T, Morizane S, Yao A, et al. Prognostic impact of preoperative hematological disorders and a risk stratification model in bladder cancer patients treated with radical cystectomy. Int J Urol. 2014;21(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 15.Elinav E, Nowarski R, Thaiss CA, Hu BO, Jin C, Flavell RA. Inflammation‐induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759‐771. [DOI] [PubMed] [Google Scholar]
- 16.Roxburgh CSD, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110(6):1409‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitzman SA, Gordon L. Inflammation and cancer: role of phagocyte‐generated oxidants in carcinogenesis. Blood. 1990;76(4):655‐663. [PubMed] [Google Scholar]
- 18.Kusumanto YH, Dam WA, Hospers GAP, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283‐287. [DOI] [PubMed] [Google Scholar]
- 19.Fridlender ZG, Sun J, Mishalian I, et al. Transcriptomic analysis comparing tumor‐associated neutrophils with granulocytic myeloid‐derived suppressor cells and normal neutrophils. PLoS One. 2012;7(2):e31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang LIFangfang L, Li FUThe significance and evaluation methods of tumor stromal infiltrating lymphocytes in breast cancer. Chin J Pathol. 2016;45(04):282‐284. [DOI] [PubMed] [Google Scholar]
- 21.Zeljko K, Josip L, Danijel D, et al. Lymphocyte subsets, lymphocyte reactivity to mitogens, NK cell activity and neutrophil and monocyte phagocytic functions in patients with bladder carcinoma. Anticancer Res. 2003;23(6D):5185‐5189. [PubMed] [Google Scholar]
- 22.Roy M, Jack B, Ohad S, et al. Neutrophil‐to‐lymphocyte ratio predicts progression and recurrence of non‐muscle‐invasive bladder cancer. Urol Oncol. 2015;33(2):497. [DOI] [PubMed] [Google Scholar]
- 23.Qingjun G, Zhongjie S, Qianhe H, Jiefeng MA. The significance of preoperative peripheral blood NLR and PLR in the prognosis of transurethral non‐muscular invasive bladder tumor resection. J Clin Urol. 2018;33(07):545‐550. [Google Scholar]
- 24.Xiang G, Zhiyu L, Liang W, Zhihong D, Xin W, Kai C. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in the prognostic evaluation of non‐muscular invasive bladder cancer. J China Med Univ. 2019;48(07):638‐642. [Google Scholar]
- 25.Xia G, Kumar SR, Hawes D, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006;175(4):1245‐1252. [DOI] [PubMed] [Google Scholar]
- 26.Pinedo HM, Verheul H, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet (London, England). 1998;352(9142):1775‐1777. [DOI] [PubMed] [Google Scholar]
- 27.Rothwell PM, Wilson M, Price JF, Belch JFF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet (London, England). 2012;379(9826):1591‐1601. [DOI] [PubMed] [Google Scholar]
- 28.Xia L, Guzzo TJ. Preoperative anemia and low hemoglobin level are associated with worse clinical outcomes in patients with bladder cancer undergoing radical cystectomy: a meta‐analysis. Clin Genitourin Cancer. 2017;15(2):263‐272.e4. [DOI] [PubMed] [Google Scholar]
- 29.Erler JT, Cawthorne CJ, Williams KJ, et al. Hypoxia‐mediated down‐regulation of Bid and Bax in tumors occurs via hypoxia‐inducible factor 1‐dependent and ‐independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24(7):2875‐2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic Immune‐inflammation Index, Based on Platelet Counts and Neutrophil‐Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J Exp Med. 2015;236(4):297‐304. [DOI] [PubMed] [Google Scholar]
- 31.Kurth K‐H, Sylvester RJPrognostic factors in non‐muscle‐invasive bladder tumors I. Clinical prognostic factors: a review of the experience of the EORTC Genito‐Urinary Group II. Biologic prognostic markers. Eur Urol Suppl. 2007;14(6):789‐799. [Google Scholar]
- 32.Ather MH, Zaidi M. Predicting recurrence and progression in non‐muscle‐invasive bladder cancer using European organization of research and treatment of cancer risk tables. Urol J. 2009;6(3):189‐193. [PubMed] [Google Scholar]
- 33.Shengcai Z, Ming L, Yaoguang Z, Gang Z, Jianye W, Ben W. Long‐term follow‐up of Ta stage bladder cancer after transurethral resection and intravesical perfusion. Chin J Urol. 2005;5(26):334‐336. [Google Scholar]
- 34.Shuo L, Guang S, Wenlong M, Fengqi LI, Zhe W. The significance of the European Organization for Cancer Research and Treatment Risk Scale for the prognosis of Chinese non‐muscular invasive bladder cancer. Chin J Urol. 2011;04:232‐235. [Google Scholar]
- 35.Ferro M, Di Lorenzo G, Vartolomei MD, et al. Absolute basophil count is associated with time to recurrence in patients with high‐grade T1 bladder cancer receiving bacillus Calmette‐Guérin after transurethral resection of the bladder tumor. World J Urol. 2020;38(1):143‐150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


