Abstract
Background
To verify the differential expression of miR‐30c and miR‐142‐3p between tuberculosis patients and healthy controls and to investigate the performance of microRNA (miRNA) and subsequently models for the diagnosis of tuberculosis (TB).
Methods
We followed up 460 subjects suspected of TB, and finally enrolled 132 patients, including 60 TB patients, 24 non‐TB disease controls (TB‐DCs), and 48 healthy controls (HCs). The differential expression of miR‐30c and miR‐142‐3p in serum samples of the TB patients, TB‐DCs, and HCs were identified by reverse transcription–quantitative real‐time PCR. Diagnostic models were developed by analyzing the characteristics of miRNA and electronic health records (EHRs). These models evaluated by the area under the curves (AUC) and calibration curves were presented as nomograms.
Results
There were differential expression of miR‐30c and miR‐142‐3p between TB patients and HCs (p < 0.05). Individual miRNA has a limited diagnostic value for TB. However, diagnostic performance has been both significantly improved when we integrated miR‐142‐3p and ordinary EHRs to develop two models for the diagnosis of tuberculosis. The AUC of the model for distinguishing tuberculosis patients from healthy controls has increased from 0.75 (95% CI: 0.66–0.84) to 0.96 (95% CI: 0.92–0.99) and the model for distinguishing tuberculosis patients from non‐TB disease controls has increased from 0.67 (95% CI: 0.55–0.79) to 0.94 (95% CI: 0.89–0.99).
Conclusions
Integrating serum miR‐142‐3p and EHRs is a good strategy for improving TB diagnosis.
Keywords: diagnosis, electronic health records, microRNA, nomogram, tuberculosis
Integrating serum microRNAs and electronic health recordsis a good strategy for improving the diagnosis of tuberculosis (TB): we analyzed the expression of miR‐30c and miR‐142‐3p among TB patients, healthy controls and non‐TB disease controls. And integrating miRNA and electronic health records to develop diagnostic models of TB, we found integrating serum microRNAs and electronic health records is a good strategy for improving TB diagnosis.

1. INTRODUCTION
Tuberculosis (TB), the world's top infectious disease killer, is caused by Mycobacterium tuberculosis, with transmission occurring via the respiratory route.1 In 2019, although the TB incidence was decreased by 2.3%, an estimated 10 million people still fell ill with tuberculosis and 1.4 million people died from TB worldwide. Coupled with the threat of the COVID‐19 pandemic, it is not easy to reach global TB targets.2 Fortunately, TB is preventable and curable with prompt diagnosis and appropriate treatment. Early diagnosis of active tuberculosis is beneficial to reduce its death toll and prevent onward transmission of infection.3, 4 But so far, the common clinical diagnostic tools for tuberculosis exist with various deficiencies: smear microscopy suffers from poor sensitivity, mycobacterial culture has a long turn around time, GeneXpert Mtb/RIF test is not available in all laboratories, etc.5, 6
To develop TB diagnostic tools or biomarkers for patients with sputum‐scarce samples and extrapulmonary tuberculosis is one of the top 10 priorities for tuberculosis diagnostics development.3 More and more different types of biomarkers have been discovered,7 such as noncoding RNAs,8, 9, 10 proteins,11 and metabolites.12 MicroRNAs are small endogenous noncoding RNAs that regulate gene expression posttranscriptionally.13 And serum miRNAs are stable and detectable,14 which is a dominant characteristic of widely used biomarkers. In recent years, the development of transcriptome sequencing has allowed a surge of differential expression of miRNAs between TB subjects and healthy controls to be observed, including miR‐30c15, 16 and miR‐142‐3p.17, 18 But the absence of validation limits the applicability of the sequencing findings. Moreover, recent evidence suggested that integrating miRNAs and EHRs could improve the diagnosis for TB patients,19, 20 but there are a few reports on this yet.
Therefore, this study was performed to validate above miRNAs which were selected from miRNAs expression profiles and investigate the diagnostic potential of miRNAs and diagnostic models incorporating miRNA and EHRs data to identify TB cases.
2. MATERIALS AND METHODS
2.1. Sample collection
A total of 460 new inpatients suspected of TB were recruited from Henan Provincial Infectious Disease Hospital (Zhengzhou, China) between June 2020 and February 2021. Those cases had at least one of the clinical signs and symptoms: cough, expectoration, hemoptysis, fever, weight loss, night sweats, inappetence, and fatigue. The serum samples were collected from every eligible patient on the day of admission (or the next day) who were subsequently tracked via case information. Eventually, 84 patients fulfilled the inclusion and exclusion criteria. Forty‐eight healthy controls (HCs) came from physical examination donors and had no history of TB for the same period in the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
Tuberculosis patients were diagnosed relied on the diagnostic criteria of the Ministry of Health of China, and the TB‐DCs consisted of chronic obstructive pulmonary disease (COPD), lung cancer, and pneumonia. The inclusion criteria for TB patients were: naive patients with TB, anti‐TB therapy <14 days on admission, and age ≥15 years, and the exclusion criteria were: patients with the severe immunosuppressive disease, other infectious disease or cancer, and renal failure. The precise details are given in Figure 1.
FIGURE 1.
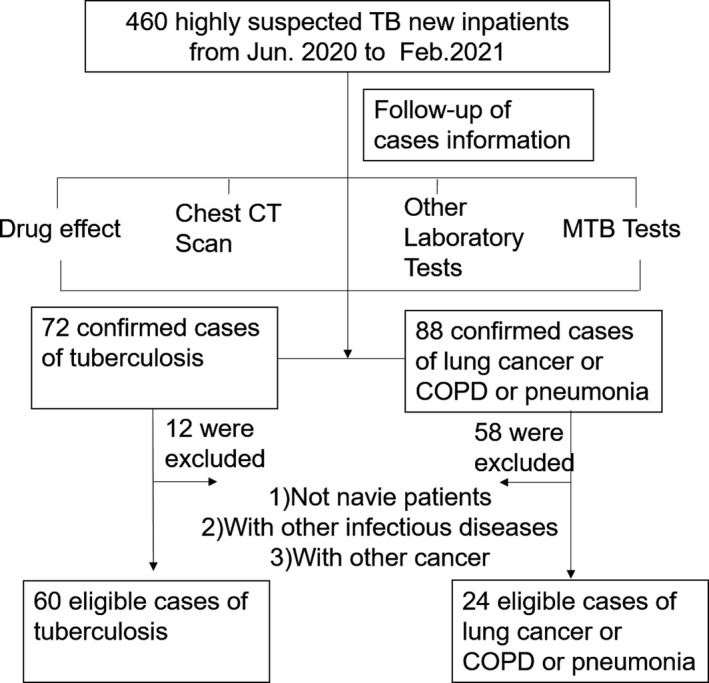
The flow diagrams of patient enrollment
Serum was harvested by centrifugation at 400 g, 10 min after blood samples were kept static at room temperature for 2 h. Then, the supernatant was transferred and stored at −80°C until use. Ethical approval was obtained from the ethics review board of the First Affiliated Hospital of Zhengzhou University (approval number: 2020‐KY‐300).
2.2. Sample size calculation
The sample size was calculated by the HyLown Power and Sample Size Calculators (http://powerandsamplesize.com/Calculators/). Based on our pre‐experiment, it was calculated that at least 22 subjects were needed in each group when the standard error α was 0.05 and the test efficiency β was 80%.
2.3. RNA extraction and reverse transcription–quantitative real‐time PCR
Total RNA was extracted using the BIOG cfRNA Easy Kit (Baidai). DNase I (Sangon Biotech) was used to remove contaminating genomic DNA. And then prolonging miRNAs by ploy(A) tail and subsequently converted into cDNA by miRNA First‐Strand cDNA Synthesis Kit (Sangon Biotech). All the kits were used following the manufacturer's instructions.
SYBR green reverse transcription–quantitative real‐time PCR (RT‐qPCR) assay (Sangon Biotech) was used for miRNA quantification. Three miRNAs were amplified using the following primers: miR‐142‐3p: ACGCCGTGTAGTGTTTCCTACTT (forward), miR‐30c: ACGGCACTGTAAACATCCTACAC (forward), and cel‐miR‐39‐3p: AACACGCTCACCGGGTGTAA (forward), and the reverse primer supplied by the kit was the universal primer. The total volume of reaction mixture was 20 µl, including 10 µl 2× SGExcel FastSYBR Mixture, 1 µl forward primer (10 µM), 1 µl general reverse primer (10 µM), 1 µl cDNA, and 7 µl ddH2O. The cycling program was as follows:pre‐denaturation at 95°C for 3 min, followed by 45 cycles at 95°C for 5 s, 60°C for 30 s, and 72°C for 30 s. Each sample was run in triplicate as well as the ddH2O negative control and blank control. RT‐qPCR data were analyzed by the 2−ΔΔ C t method, normalized against external controls (cel‐miR‐39‐3p).
2.4. Statistical analysis
Continuous variables are expressed as mean ± SD or mean (minimum–maximum) and compared using an unpaired, two‐tailed t‐test or Mann–Whitney U test. Categorical variables were reported as whole numbers and proportions and compared using the chi‐square test or Fisher's exact test. A 10% missing value threshold was applied to remove incomplete features, and mean imputation was performed to supplement missing data. The significance of each variable was assessed by univariate logistic regression analysis. All variables associated with TB at a significant level were candidates for stepwise multivariate analysis. Then, adding some variables linked with clinical symptoms based on clinical importance and scientific knowledge. Selected variables were used to create the models and incorporated in the nomograms to predict the probability of TB by using the rms package of R, version 4.0 (http://www.r‐project.org/).
For clinical use, the receiver operating characteristic curve (ROC) was used to evaluate the diagnostic value of miRNAs and the performance of the models. The calibration curves were generated to explore the performance characteristics of the nomograms.
In all analyses, p < 0.05 was considered to indicate statistical significance. All analyses were performed using SPSS Statistics 19.0 and R, version 4.0. Scatter diagrams and ROC were generated with GraphPad Prism 5.
3. RESULTS
3.1. Characteristics of prospectively enrolled participants
The characteristics of all participants are given in Table 1. There were no significant differences between patients with TB and HCs in age and gender, but TB patients were younger than their TB‐DCs (p < 0.0001). All clinical signs distinguished poorly between patients with TB and TB‐DCs, which puts more pressure on doctors. However, for hematological outcomes, there were some significant differences among the three groups, such as urea and mean platelet volume, which gives doctors insights into diagnosis.
TABLE 1.
Demographic and clinical features of enrolled participants
| Clinical features | TB patients | TB‐DCs | p a | HCs | p b |
|---|---|---|---|---|---|
| Total | 60 | 24 | / | 48 | / |
| Gender, male | 35 (58.3%) | 13 (54.2%) | 0.67 | 23 (47.9%) | 0.24 |
| Age (years) | 37.08 ± 18.153 | 60.33 ± 12.506 | 0.007 | 39.77 ± 11.265 | 0.35 |
| Radiologic pathologyc | 56 (93.3%) | 24 (100.0%) | 0.267 | 0 | < 0.001 |
| Fever | 13 (21.7%) | 5 (20.8%) | 0.32 | 0 | < 0.001 |
| Night sweat | 7 (11.7%) | 0 | 0.08 | 0 | < 0.001 |
| Coughing | 35 (58.3%) | 15 (62.5%) | 0.79 | 0 | < 0.001 |
| Expectoration | 29 (48.3%) | 11 (45.8%) | 0.78 | 0 | < 0.001 |
| Hemoptysis | 10 (16.7%) | 2 (8.3%) | 0.31 | 0 | < 0.001 |
| Fatigue | 13 (21.7%) | 3 (12.5%) | 0.32 | 0 | < 0.001 |
| Weight loss | 5 (8.3%) | 1 (4.2%) | 0.49 | 0 | 0.04 |
| Erythrocytes (×1012/L) | 4.34 ± 0.58 | 4.24 ± 0.44 | 0.45 | 4.66 ± 0.38 | < 0.001 |
| Hemoglobin (g/L) | 126.40 ± 18.77 | 125.10 ± 18.27 | 0.78 | 144.15 ± 14.24 | < 0.001 |
| Hematocrit | 0.39 (0.20–0.51) | 0.39 (0.27–0.46) | 0.94 | 0.43 (0.36–0.50) | < 0.001 |
| Platelets (×109/L) | 272 ± 87.91 | 214.92 ± 80.99 | 0.01 | 237.06 ± 46.54 | 0.01 |
| Leukocyte (×109/L) | 6.41 (2.72–16.82) | 6.14 (3.42–11) | 0.52 | 5.77 (4.01–9) | 0.15 |
| TBIL (µmol/L) | 14.42 (5.34–196.18) | 9.47 (3.70–28.60) | 0.38 | 10.79 (3.90–20.70) | 0.28 |
| ALT (U/L) | 23.93 (2–306) | 22.58 (7–72) | 0.04 | 17.10 (6–38) | 0.04 |
| AST (U/L) | 20.27 (6–90) | 25.75 (10–125) | 0.19 | 18.56 (11–32) | 0.17 |
| Albumin (g/L) | 38.52 ± 6.14 | 40.69 ± 6.81 | 0.17 | 46.96 ± 3.02 | < 0.001 |
| Urea (mmol/L) | 4.10 ± 1.32 | 5.05 ± 1.53 | 0.01 | 4.75 ± 1.24 | 0.01 |
| Creatinine (µmol/L) | 60.62 ± 16.14 | 67.12 ± 20.80 | 0.14 | 70.60 ± 13.36 | < 0.001 |
| UA (µmol/L) | 358.16 (178–710) | 244.33 (168–339) | < 0.001 | 296.54 (170–454) | 0.08 |
| ESR | 27.75 (2–117) | 19.47 (2–63) | 0.59 | 5.50 (2–8) | 0.29 |
| CRP (mg/L) | 16.85 (0.25–177.91) | 18.43 (1–244) | 0.13 | 0.36 (0.24–0.62) | < 0.001 |
| Glucose (mmol/L) | 5.29 (3.47–20.47) | 5.01 (3.63–9.75) | 0.95 | 4.87 (4.10–5.74) | 0.10 |
| MCV (fl) | 88.99 (77.70–103.40) | 90.91 (60.60–100.40) | 0.06 | 92.36 (84.90–100) | < 0.001 |
| MCH (pg) | 29.20 (23.30–34.20) | 29.50 (18–32) | 0.14 | 30.93 (26.90–34.20) | < 0.001 |
| MCHC (g/L) | 327.05 ± 11.62 | 323.80 ± 9.11 | 0.23 | 334.93 ± 9.04 | < 0.001 |
| RDW (×109/L) | 13.19 (11–20) | 13.45 (10.10–17.20) | 0.04 | 12.79 (11.40–13.80) | 0.84 |
| MPV (fl) | 10.57 (8.20–12.90) | 9.54 (0.40–16.80) | 0.01 | 9.31 (7.20–13.10) | < 0.001 |
| Plateletcrit | 0.28 (0.10–0.50) | 0.84 (0.11–15.50) | < 0.001 | 0.22 (0.13–0.30) | < 0.001 |
| PDW (fl) | 12.51 (8.70–19.30) | 16.50 (15.50–17.50) | < 0.001 | 16.21 (15.53–17.11) | < 0.001 |
| GGT (U/L) | 32.89 (8–292) | 26.50 (8–72) | 0.68 | 19.98 (6–48) | 0.03 |
| ALP (U/L) | 90.58 (49–351) | 75.29 (36–125) | 0.10 | 67.04 (32–109) | < 0.001 |
| DBIL (µmol/L) | 5.86 (1–141) | 4.35 (1.70–9.70) | 0.04 | 4.81 (1.90–8.30) | < 0.001 |
| LDH (U/L) | 182.57 (129–303) | 217.38 (10–431) | 0.03 | 168.80 (138–204) | 0.56 |
| T‐CHO (mmol/L) | 4 ± 0.91 | 4.03 ± 0.92 | 0.91 | 4.02 ± 0.61 | 0.23 |
| Triglycerides (mmol/L) | 1.13 (0.41–2.94) | 1.25 (0.52–4.61) | 0.62 | 1.02 (0.45–1.78) | 0.72 |
| HDL (mmol/L) | 1.03 ± 0.31 | 1.21 ± 0.34 | 0.05 | 1.42 ± 0.88 | 0.01 |
| LDL (mmol/L) | 2.70 ± 0.79 | 2.46 ± 0.75 | 0.26 | 2.63 ± 0.55 | 0.61 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; DBIL, direct bilirubin; ESR, erythrocyte sedimentation rate; GGT, γ‐glutamyl transpeptidase; HCs, healthy controls; HDL, high‐density lipoprotein; LDH, lactate dehydrogenase; LDL, low‐density lipoprotein; MCH, mean corpuscular hemoglobin; MCHC, mean red blood cell hemoglobin concentration; MCV, mean red blood cell volume; MPV, mean platelet volume; PDW, platelet distribution width; RDW, red blood cell distribution width; TB‐DCs, non‐TB disease controls; TBIL, total bilirubin; T‐CHO, total cholesterol; UA, uric acid.
p Value for the comparison of TB patients and TB‐DCs.
p Value for the comparison of TB patients and HCs.
Radiologic pathology refers to abnormal chest imaging, including at least one of the signs: shadow, calcification, cavity, fibrosis, and pleural effusion.
3.2. Differential expression and performance of serum miRNAs
The expression level of two candidate miRNAs was measured by RT‐qPCR. Comparison between TB patients and healthy subjects, the expression levels of miR‐30c and miR‐142‐3p were both decreased (p < 0.05) in TB patients. While, there was no significantly different expression of miR‐30c and miR‐142‐3p between TB patients and non‐TB disease controls (Figure 2).
FIGURE 2.
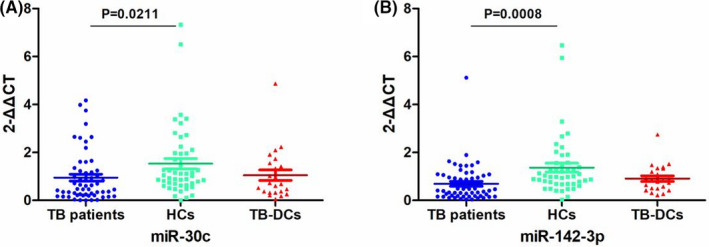
miRNAs expression among TB patients, HCs and TB‐DCs. The expression of (A) miR‐30c and (B) miR‐142‐3p among TB patients, HCs and TB‐DCs. HCs, healthy controls; TB, tuberculosis; TB‐DCs, non‐TB disease controls
Then, ROC analysis was performed to calculate the area under the curve (AUC) of each miRNA and combined miRNAs. The results indicated miR‐30c and miR‐142‐3p were both acceptable as an index of TB diagnosis (AUC = 0.67 and 0.75, respectively). Based on the ROC analysis, combinatorial serum miRNAs and single serum miRNA performed similarly (Figure 3).
FIGURE 3.
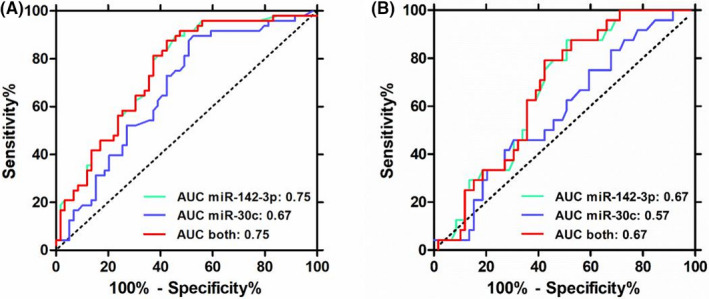
Receiver operating characteristic curve (ROC) of differential expressed serum miRNAs. ROC of miR‐142‐3p and miR‐30c alone or combined (A) between TB patients and HCs and (B) between TB patients and TB‐DCs. HCs, healthy controls; TB, tuberculosis; TB‐DCs, non‐TB disease controls
3.3. Features selection and nomogram
The results of the univariate logistic analysis are presented in the Table S1. Of texture features (deletion of severely missing variables), 35 features were reduced to 15 between TB patients and HCs on the basis of univariate logistic regression analysis, and 26 potential predictors to 6 between TB patients and TB‐DCs. Based on the stepwise multivariate analysis, the results indicated that large mean volume of platelet (MPV) (OR: 3.079, 95% CI: 1.743–5.438), low concentration of albumin (ALB) (OR: 0.656, 95% CI: 0.538–0.799) and hemoglobin (HB) (OR: 0.963, 95% CI: 0.917–1.011), and relatively less expression of miR‐142‐3p (OR: 0.43, 95% CI: 0.205–0.900) were associated with tuberculosis between TB patients and HCs (Table 2). Similarly, stepwise multivariate analysis identified large MPV (OR: 1.532, 95% CI: 1.022–2.297), high concentration of uric acid (UA) (OR:1.026, 95% CI: 1.009–1.044) and urea (OR: 0.581, 95% CI: 0.332–1.017), younger (OR: 0.926, 95% CI: 0.881–0.972) and relatively less expression of miR‐142‐3p (OR: 0.403, 95% CI: 0.171–0.950) were associated with TB between TB patients and TB‐DCs (Table 3).
TABLE 2.
Multivariate logistic regression analysis showing the association of variables with TB between TB patients and HCs
| Variable | OR (95% CI) | p Value |
|---|---|---|
| ALB | 0.656 (0.538–0.799) | <0.001 |
| MPV | 3.079 (1.743–5.438) | <0.001 |
| miR‐142‐3p | 0.43 (0.205–0.900) | 0.025 |
| HB | 0.963 (0.917–1.011) | 0.125 |
Abbreviations: ALB, albumin (g/L);HB, hemoglobin (g/L); MPV, mean platelet volume.
TABLE 3.
Multivariate logistic regression analysis showing the association of variables with TB between TB patients and TB‐DCs
| Variable | OR (95% CI) | p Value |
|---|---|---|
| Age | 0.926 (0.881–0.972) | 0.002 |
| UREA | 0.581 (0.332–1.017) | 0.057 |
| UA | 1.026 (1.009–1.044) | 0.002 |
| MPV | 1.532 (1.022–2.297) | 0.039 |
| miR‐142‐3p | 0.403 (0.171–0.950) | 0.038 |
Abbreviations: MPV, mean platelet volume; UA, uric acid (µmol/L);UREA, urea (mmol/L).
The diagnostic models that incorporated the features based on stepwise multivariate analysis were developed and presented as the nomograms (Figure 4).
FIGURE 4.
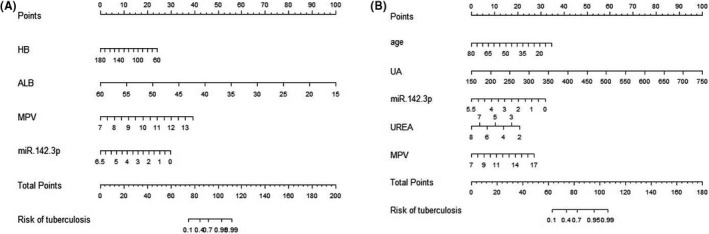
Nomogram for the prediction of TB patients. (A) Between TB patients and healthy controls. (B) Between TB patients and non‐TB disease controls. HB, hemoglobin (g/L); ALB, albumin (g/L); MPV, mean platelet volume; UREA, urea (mmol/L); UA, uric acid (µmol/L). TB; tuberculosis
3.4. Model performance
The Hosmer–Lemeshow test yielded a nonsignificant statistic (p = 0.909) between the group of TB patients and HCs and p = 0.499 between TB subjects and TB‐DCs, which suggested that there was no departure from perfect fit. The results of ROC were consistent with the above conclusion (Figure 5). The AUCs for the diagnostic models were 0.956 (95% CI: 0.923–0.991) and 0.938 (95% CI: 0.886–0.989), respectively. The calibration curves of both the models demonstrated prediction and observation in the group of TB patients and HCs were in good agreement, as well as in the group of TB patients and TB‐DCs (Figure 6).
FIGURE 5.
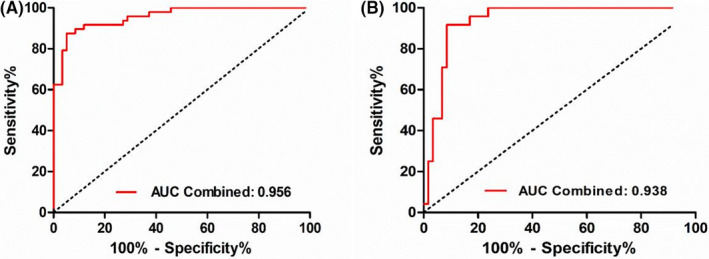
ROC analysis in diagnosing TB from HCs and TB‐DCs. ROC in diagnosing (A) TB from HCs and (B) TB from TB‐DCs. HCs, healthy controls; ROC, receiver operating characteristic curve; TB, tuberculosis; TB‐DCs, non‐TB disease controls
FIGURE 6.
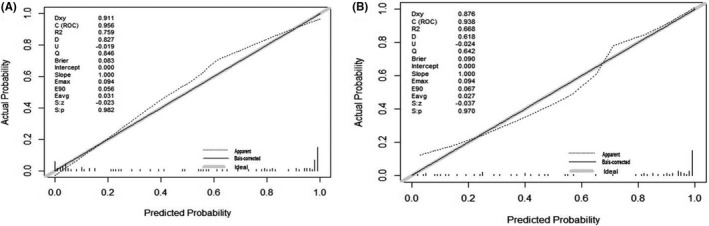
The calibration curve of the nomogram for the probability of TB. Calibration plot of the nomogram for the probability of TB in (A) TB patients and HCs and (B) TB patients and TB‐DCs. HCs, healthy controls; TB, tuberculosis; TB‐DCs, non‐TB disease controls
4. DISCUSSION
Several previous studies have noted the importance of miRNAs in TB about its roles in the disease pathogenesis,21, 22 diagnosis,23, 24 and treatment.25, 26 In this study, the differential expression of miR‐30c and miR‐142‐3p between TB patients and healthy subjects was validated by RT‐qPCR. Then, the models incorporating miRNA signatures and clinically available EHRs indicators were created, which vastly improved the diagnostic power.
We searched PubMed and “web of science” with “tuberculosis,” “serum,” and “miRNA” as the keywords, and the results showed that a total of 38 literature were retrieved. Then, we screened out nine articles whose purpose was to clarify the miRNAs with differential expression between active tuberculosis and health controls. Intersecting the available miRNA expression profiles in these literature, we harvested miR‐30c and miR‐142‐3p finally (data not shown). Spinelli et al27 found that miR‐30c is strongly downregulated in mononuclear cells of pulmonary TB patients compared to tuberculous pleurisy cases and identified miR‐30c as a specific correlate of pulmonary manifestations of TB. And miR‐142‐3p interacted with a functional SNP (rs13120371) in the 3′ untranslated region of the xCT gene which increases susceptibility to TB.28 However, these miRNAs for the TB diagnostic value were not evaluated in the above studies until now. Therefore, we selected miR‐30c and miR‐142‐3p to assess whether these serum miRNAs could act as putative candidate biomarkers in TB patients.
We found that the level of miR‐30c in serum from patients with tuberculosis was lower than that in the healthy group, and the same is true for miR‐142‐3p (Figure 2). Surprisingly, the expression tendency of miR‐30c is inconsistent with the original profiles, which implied that there was a higher expression in TB patients.15, 16 These differences can be explained in part by the proximity of different experimental methods and samples. For miR‐142‐3p, compared with latent M. tuberculosis infection, there was a same downward trend in CD4+ T cells and peripheral blood in TB patients, which implied that miR‐142‐3p may be involved in the development of tuberculosis.29 And Xu et al30 demonstrated that miR‐142‐3p was significantly decreased at various time points following BCG infection in RAW264.7 macrophage cells. These studies indicated that miR‐142‐3p had a low level in TB patients, which is consistent with our results. To plot ROC, we showed miR‐30c and miR‐142‐3p were potential biomarkers for TB (Figure 3). Macrophages, the forefront of innate immune defense, play a decisive role in host responses to Mycobacterium tuberculosis.31, 32 Several reports have demonstrated that downregulation of miR‐142‐3p was required for the generation of fully functional macrophage and dendritic cell,33 and miR‐142‐3p was a key candidate to be involved in the regulation of actin dynamics required in phagocytosis,34 which implied that miR‐142‐3p played a certain role in the pathogenesis of tuberculosis.
Individual miRNA has a limited diagnostic value for TB. However, when we integrated miR‐142‐3p and ordinary EHRs to develop two models for the diagnosis of tuberculosis, diagnostic performance has been both significantly improved. The AUC of the model for distinguishing tuberculosis patients from healthy controls has increased from 0.75 to 0.96 and the model for distinguishing tuberculosis patients from non‐TB disease controls has increased from 0.67 to 0.94 (Figures 3 and 5). The results illustrated that integrating miRNAs and EHRs could indeed improve the diagnosis for TB patients as proposed by prior findings.19 One unanticipated finding was that the feature of MPV included in the both of models was paid a little attention to the diagnosis of tuberculosis in the previous reports. Huang et al35 showed that patients with active intestinal tuberculosis had decreased MPV (p = 0.002), but red cell distribution width had better diagnostic value than MPV. However, MPV has been confirmed to be related to tuberculosis and inflammation.36 More specifically, MPV can be an inflammatory marker to determine the disease activity in TB patients,37 and platelet abnormalities in patients with tuberculous meningitis contribute to infarct and are associated with poor clinical outcomes.38 Besides, there was significantly higher MPV level in the active tuberculosis group and that decreased with anti‐TB therapy. And MPV correlations with radiological extent of TB was also significant but weaker, which indicated MPV may be related to the severity of the TB.39 Similarly, Dong et al40 also showed that activation of the coagulation pathway, shown by increased platelet distribution width, decreased MPV, and shortened prothrombin time, was risk factor for severe lung lesions in patients with TB. Therefore, more interests should be given to MPV in the diagnosis of TB. As nomogram for individualized prediction of incident tuberculosis and multidrug‐resistant tuberculosis is convenient,20, 41, 42 two nomograms were produced subsequently, with good discrimination (through AUC) and calibration (via Hosmer–Lemeshow and test calibration curve) (Figure 6). However, these results were not very encouraging due to the limitation of sample size, and further study should verify the nomograms and confirm its universality.
Overall, our results suggested that miR‐30c and miR‐142‐3p are potential biomarkers for TB diagnosis. The proposed nomograms were able to promote the diagnosis and differential diagnosis of TB and required further validation with large sample size in multicenter.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Jun‐Wei Zhao developed the concept and experimental design; Shu‐Hui Gao, Chun‐Bo Zhuang, and Zhen‐Zhen Zeng performed the experiments and data analysis; Yu‐Ling Zeng, Chun‐Guang Chen, and Yong‐Min Yu enrolled patients; Shu‐Hui Gao, Pei‐Hao Wen, Liang Ming, and Jun‐Wei Zhao wrote the manuscript. All authors contributed to revise the manuscript, provided intellectual input, and gave final approval.
Supporting information
Table S1
Gao S‐H, Chen C‐G, Zhuang C‐B, et al. Integrating serum microRNAs and electronic health records improved the diagnosis of tuberculosis. J Clin Lab Anal. 2021;35:e23871. 10.1002/jcla.23871
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Table S1 of this article.
REFERENCES
- 1.Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393(10181):1642‐1656. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Global tuberculosis report. 2020; https://www.who.int/teams/global‐tuberculosis‐programme/data
- 3.Walzl G, McNerney R, du Plessis N , et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18(7):e199‐e210. [DOI] [PubMed] [Google Scholar]
- 4.Turner CT, Gupta RK, Tsaliki E, et al. Blood transcriptional biomarkers for active pulmonary tuberculosis in a high‐burden setting: a prospective, observational, diagnostic accuracy study. Lancet Respir Med. 2020;8(4):407‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dicks KV, Stout JE. Molecular diagnostics for Mycobacterium tuberculosis infection. Annu Rev Med. 2019;70:77‐90. [DOI] [PubMed] [Google Scholar]
- 6.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors. July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376‐1395. [DOI] [PubMed] [Google Scholar]
- 7.Goletti D, Lee MR, Wang JY, Walter N, Ottenhoff THM. Update on tuberculosis biomarkers: from correlates of risk, to correlates of active disease and of cure from disease. Respirology. 2018;23(5):455‐466. [DOI] [PubMed] [Google Scholar]
- 8.Sabir N, Hussain T, Shah SZA, Peramo A, Zhao D, Zhou X. miRNAs in tuberculosis: new avenues for diagnosis and host‐directed therapy. Front Microbiol. 2018;9:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian Z, Liu H, Li M, et al. Potential diagnostic power of blood circular RNA expression in active pulmonary tuberculosis. EBioMedicine. 2018;27:18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fathizadeh H, Hayat SMG, Dao S, et al. Long non‐coding RNA molecules in tuberculosis. Int J Biol Macromol. 2020;156:340‐346. [DOI] [PubMed] [Google Scholar]
- 11.De Groote MA, Sterling DG, Hraha T, et al. Discovery and validation of a six‐marker serum protein signature for the diagnosis of active pulmonary tuberculosis. J Clin Microbiol. 2017;55(10):3057‐3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner J 3rd, Maertzdorf J, Sutherland JS, et al. Metabolite changes in blood predict the onset of tuberculosis. Nat Commun. 2018;9(1):5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513‐10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Yi Z, Wu X, Li J, Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J Clin Microbiol. 2011;49(12):4246‐4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Sun Z, Wei W, et al. Identification of serum microRNA biomarkers for tuberculosis using RNA‐seq. PLoS One. 2014;9(2):e88909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu H, Yang S, Jiang T, et al. Elevated pulmonary tuberculosis biomarker miR‐423‐5p plays critical role in the occurrence of active TB by inhibiting autophagosome‐lysosome fusion. Emerg Microbes Infect. 2019;8(1):448‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Y, Cui L, Ge Y, Shi Z, Zhao K. Altered serum microRNAs as biomarkers for the early diagnosis of pulmonary tuberculosis infection. BMC Infect Dis. 2012;12(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Liao S, Bai H, et al. Integrating exosomal microRNAs and electronic health data improved tuberculosis diagnosis. EBioMedicine. 2019;40:564‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Liao S, Bai H, et al. Long noncoding RNA and predictive model to improve diagnosis of clinically diagnosed pulmonary tuberculosis. J Clin Microbiol. 2020;58(7):e01973‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang T, Sui D, You D, et al. MiR‐29a‐5p inhibits proliferation and invasion and induces apoptosis in endometrial carcinoma via targeting TPX2. Cell Cycle. 2018;17(10):1268‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Zhang G, Liu X, Wang W, et al. Down‐regulation of miR‐20a‐5p triggers cell apoptosis to facilitate mycobacterial clearance through targeting JNK2 in human macrophages. Cell Cycle. 2016;15(18):2527‐2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miotto P, Mwangoka G, Valente IC, et al. miRNA signatures in sera of patients with active pulmonary tuberculosis. PLoS One. 2013;8(11):e80149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng ML, Zhou NK, Luo CH. MiRNA‐155 and miRNA‐132 as potential diagnostic biomarkers for pulmonary tuberculosis: a preliminary study. Microb Pathog. 2016;100:78‐83. [DOI] [PubMed] [Google Scholar]
- 25.Lv Y, Guo S, Li XG, Chi JY, Qu YQ, Zhong HL. Sputum and serum microRNA‐144 levels in patients with tuberculosis before and after treatment. Int J Infect Dis. 2016;43:68‐73. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Yang S, Liu CM, et al. Screening and identification of four serum miRNAs as novel potential biomarkers for cured pulmonary tuberculosis. Tuberculosis. 2018;108:26‐34. [DOI] [PubMed] [Google Scholar]
- 27.Spinelli SV, Fernández RDV, Zoff L, et al. miR‐30c is specifically repressed in patients with active pulmonary tuberculosis. Tuberculosis. 2017;105:73‐79. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Cai Y, Deng G, et al. Allelic‐specific regulation of xCT expression increases susceptibility to tuberculosis by modulating microRNA‐mRNA interactions. mSphere. 2020;5(2):e00263‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinsteuber K, Heesch K, Schattling S, et al. Decreased expression of miR‐21, miR‐26a, miR‐29a, and miR‐142‐3p in CD4⁺ T cells and peripheral blood from tuberculosis patients. PLoS One. 2013;8(4):e61609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu G, Zhang Z, Wei J, et al. microR‐142‐3p down‐regulates IRAK‐1 in response to Mycobacterium bovis BCG infection in macrophages. Tuberculosis. 2013;93(6):606‐611. [DOI] [PubMed] [Google Scholar]
- 31.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264(1):182‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan A, Singh VK, Hunter RL, Jagannath C. Macrophage heterogeneity and plasticity in tuberculosis. J Leukoc Biol. 2019;106(2):275‐282. [DOI] [PubMed] [Google Scholar]
- 33.Fordham JB, Naqvi AR, Nares S. Regulation of miR‐24, miR‐30b, and miR‐142‐3p during macrophage and dendritic cell differentiation potentiates innate immunity. J Leukoc Biol. 2015;98(2):195‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettencourt P, Marion S, Pires D, et al. Actin‐binding protein regulation by microRNAs as a novel microbial strategy to modulate phagocytosis by host cells: the case of N‐Wasp and miR‐142‐3p. Front Cell Infect Microbiol. 2013;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang S, Yi FM, Zhou R, et al. The utility of platelet, mean platelet volume, and red cell distribution width in the diagnosis of active Crohn's disease and intestinal tuberculosis. Saudi Med J. 2013;34(11):1161‐1166. [PubMed] [Google Scholar]
- 36.Korniluk A, Koper‐Lenkiewicz OM, Kamińska J, Kemona H, Dymicka‐Piekarska V. Mean Platelet Volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:e9213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MY, Kim YJ, Lee HJ, Cho SY, Park TS. Mean platelet volume in Mycobacterium tuberculosis infection. Biomed Res Int. 2016;2016:e7508763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma R, Mahapatro S, Kumar A, et al. Platelet dysfunction and coagulation assessment in patients of tuberculous meningitis. Neurol Sci. 2020;41(8):2103‐2110. [DOI] [PubMed] [Google Scholar]
- 39.Tozkoparan E, Deniz O, Ucar E, Bilgic H, Ekiz K. Changes in platelet count and indices in pulmonary tuberculosis. Clin Chem Lab Med. 2007;45(8):1009‐1013. [DOI] [PubMed] [Google Scholar]
- 40.Dong Z, Shi J, Dorhoi A, et al. Hemostasis and lipoprotein indices signify exacerbated lung injury in TB with diabetes comorbidity. Chest. 2018;153(5):1187‐1200. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Tu J. Nomogram to predict multidrug‐resistant tuberculosis. Ann Clin Microbiol Antimicrob. 2020;19(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Q, Zhao G, Wang X, et al. Nomogram for individualized prediction of incident multidrug‐resistant tuberculosis after completing pulmonary tuberculosis treatment. Sci Rep. 2020;10(1):13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available in the Table S1 of this article.


