Abstract
Background
In this study, we investigated the clinical value of serum Inhibin B alone or in combination with other hormone indicators in subfertile men.
Methods
This is a multicenter study involving 324 men from different cities in China. Testicular volume, routine semen analysis, serum Inhibin B, anti‐Müllerian hormone (AMH), follicle‐stimulating hormone (FSH), luteinizing hormone (LH), testosterone, estradiol, and prolactin were measured. Testicular tissue samples were also analyzed in 78 of 129 patients with azoospermia to distinguish impaired spermatogenesis from obstructive azoospermia.
Results
The concentration of Inhibin B, FSH, and AMH is related to spermatogenesis. For men with impaired spermatogenesis, including mild‐to‐moderate oligozoospermia (IMO) and severe oligozoospermia (ISO), serum levels of Inhibin B and FSH are highly correlated with sperm counting. However, in patients with idiopathic moderate oligozoospermia or severe oligozoospermia, there was no significant correlation between Inhibin B (or FSH) and sperm concentration. The upper cutoff value of Inhibin B to diagnose ISO is 58.25 pg/ml with a predictive accuracy of 80.65%. To distinguish between nonobstructive azoospermia (NOA) and obstructive azoospermia (OA), the area under the curve (AUC) for AMH + Inhibin B + FSH is very similar to Inhibin B (0.943 vs. 0.941). The cutoff level of Inhibin B to diagnose nonobstructive azoospermia is 45.9 pg/ml with a positive and negative prediction accuracy of 97.70% and 85.71%, respectively.
Conclusion
In summary, Inhibin B is a promising biomarker alone or in combination with other hormone indicators for the diagnosis of testicular spermatogenesis status, helping clinical doctors to distinguish NOA from OA.
Keywords: biomarker, Inhibin B, spermatogenesis, subfertile men
1. INTRODUCTION
Inhibin B is a testicular hormone produced by Sertoli cells, which has a negative regulatory role in follicle‐stimulating hormone (FSH) secretion by the pituitary gland.1, 2 Inhibin is a heterodimeric glycoprotein, consisting of two disulfide‐linked α and β subunits, which belongs to the superfamily of transforming growth factor β (TGFβ).2 In adult human testes, Marchetti et al.2 reported that the Inhibin α subunit and the corresponding mRNA were present in both Sertoli cells and Leydig cells, but not in the germ cells. However, in testes with normal spermatogenesis and with spermatogenic arrest (MA), βB subunit mRNA is expressed in germ cells from spermatogonia to round spermatids and in Sertoli cells. Inhibin βB subunit is found in germ cells from pachytene spermatocytes to round spermatids, but not in Sertoli cells. In testes with Sertoli‐cell‐only syndrome (SCO), high Inhibin βB subunit mRNA expression was observed in both Sertoli cells and Leydig cells, but βB subunit immunostaining was negative for Sertoli cells and weakly positive for Leydig cells.2
Men with obstructive azoospermia (OA) and spermatidic arrest (MA) showed normal serum Inhibin B concentrations, whereas for those men with SCO, severe hypospermatogenesis and spermatogenesis arrest, much lower mean Inhibin B concentrations were observed.3, 4, 5 Patients after testicular radiotherapy for carcinoma demonstrated a SCO pattern, with undetectable Inhibin B levels after irradiation. Male monkey study showed similar SCO pattern and sharp decline of Inhibin B after testicular irradiation as well.6, 7 The decrease in Inhibin B and the consequent rise in FSH concentrations have been found a few months after testicular irradiation.6
Although these early studies suggest that Inhibin B is closely related to spermatogenesis, in the clinical application of Inhibin B, there are many inconsistent research conclusions that limit its application. Wang et al.8 reported that the combined evaluation of Inhibin B, FSH, and luteinizing hormone (LH) is a more effective predictor for successful sperm retrieval in patients with nonobstructive azoospermia before decision‐making of an invasive procedure than any single factor. Su et al.9 reported that FSH may predict sperm retrieval rate and guide surgical approach in patients with nonobstructive azoospermia. However, Green et al.10 reported that serum levels of FSH and Inhibin B are not good predictors of sperm levels in male survivors of childhood cancer.
The above research results suggest that a hormone indicator may be used alone in clinical applications, or it may be used in combination with other hormone indicators. In this study, in order to confirm the clinical application value of Inhibin B alone or in combination with other hormones, we investigated the relationship between multiple serum hormone levels, sperm concentration, and different spermatogenic status.
2. METHODS
2.1. Subjects
This multicenter study was performed on 324 men (aged 22–45 years) from Wuhan Tongji Reproductive Medicine Hospital (n = 138), the First Affiliated Hospital of Wenzhou Medical University (n = 56), Taihe Hospital, Hubei University of Medicine (n = 52), the General Hospital of Ningxia Medical University (n = 26), JingHua Hospital of Shenyang (n = 26), Hunan Yueyang Meternal and Children Health‐Care Hospital (n = 16), and Hubei Province Human Sperm Bank (n = 10).
Infertility is defined as the inability of a sexually active, noncontracepting couple to achieve pregnancy in one year (WHO Laboratory Manual For The Examination And Processing Of Human Semen, 5th Edition).
Patients enrolled in this study were evaluated according to the guidelines of “WHO Laboratory Manual for the Examination and Processing of Human Sperm (5th edition).” Based on sperm concentration in semen, testicular biopsy/testicular sperm extraction result, patients were diagnosed with normospermia (NOR; n = 96, ≥15 million sperm/ml), idiopathic moderate oligozoospermia (IMO; n = 46, 5–15 million sperm/ml), idiopathic severe oligozoospermia (ISO; n = 53, 0–5 million sperm/ml), obstructive azoospermia (OA; n = 38), nonobstructive azoospermia (NOA; n = 91).
The inclusion criteria of male infertility group: age 22–45, healthy, normal sex life after marriage, no contraception for more than 1 year, azoospermia or oligozoospermia with two semen analyses (2 weeks apart).
The exclusion criteria of the male infertility group are as follows: the history of the following diseases or conditions: alcohol abuse or drug abuse, synthetic steroid hormone drug use or immunotherapy within three months before treatment, radiotherapy and chemotherapy, known endocrine diseases (eg, hyperprolactinemia, hypogonadotropic hypogonadism, or diabetes), cryptorchidism, testicular absence, swelling, and pain in testis, inflammation, malignant tumors, autoimmune disease, testicular torsion, orchiectomy, sperm extraction, varicocele ligation, vasectomy, organ transplantation, hepatorenal syndrome, hepatopulmonary syndrome, genetic disorders (eg, Y chromosome microdeletion), infection (eg, puberty orchitis), immune disorders, severe cardiovascular diseases, kidney diseases, liver diseases, sexually transmitted diseases, and other chronic diseases. The following patients were also excluded: patients with incomplete semen analysis data, genetics data, or other related data, and patients who withdrew voluntarily during the study.
2.2. Hormone analysis
Fasting peripheral blood samples were taken from 8 to 11 am and centrifuged at 900 g for 10 min. All samples were processed within 2 h of collection and assayed immediately or stored at −80℃ until further analysis. Serum levels of Inhibin B, anti‐Müllerian hormone (AMH), FSH, LH, testosterone, estradiol, and prolactin were measured by chemiluminescence immunoassay analyzer iFlash 3000 (Shenzhen YHLO Biotech). The within‐run coefficient of variance (CV) and the between‐run CV were <10%.
2.3. Semen analysis
After 2–7 days of abstinence, each patient's semen was collected by masturbation and incubated in a sterile container in a 37℃ water bath, and analyzed within 1 h after ejaculation. After the semen is liquefied, according to the WHO Manual of human semen inspection and processing laboratory (5th Version), the semen volume, color, transparency, pH value, viscosity, and semen liquefaction status were recorded. The sperm concentration and vitality were detected by semen analyzer. The semen samples with no sperm found were centrifuged at 3000 g for 15 min before microscopic examination.
2.4. Testicular evaluation
An accurate correlation has been found between volume measurements by ultrasound and by the Prader orchidometer (R(2) = 0.956),11 and the testicular volume was estimated with the scrotal ultrasonography or Prader orchidometer in this study. Testicular tissue samples were analyzed in 78 of 129 patients with azoospermia to distinguish impaired spermatogenesis from reproductive duct obstruction. Testicular sperm extraction (TESE) or testicular fine‐needle aspiration was performed as previously described separately.10 Based on testicular biopsy/testicular sperm extraction result, subjects were diagnosed with obstructive azoospermia (n = 38) and nonobstructive azoospermia (n = 40). For infertile men with azoospermia, when the scrotal ultrasonography estimates that the testicular volume is ≤10 ml, there is only a 1.6% chance of obstructive azoospermia.12 The remaining 51 patients with azoospermia did not accept testicular tissue sampling and classified as NOA, because either of the two testicles was ≤10 ml, and their FSH concentration was more than double above the upper limit of the reference range.
2.5. Statistical analysis
The correlations between each parameters of Inhibin B, AMH, FSH, LH, testosterone, estradiol, and prolactin were analyzed with Spearman's correlation. Student's t test was used for comparing the parameters from groups with different symptoms. Two‐sided p values less than 0.05 were considered significant. The cutoff value of each parameter on differentiating normal and impaired spermatogenesis was determined by receiver operating characteristic (ROC) curve. The areas under the curve (AUCs) were used for comparing the diagnostic values of each parameter. AUCs were estimated with the Wilcoxon Mann‐Whitney statistics. Statistical analysis was carried out with the software SAS 9.4 (for correlation analysis) and SPSS 19 (for ROC analysis).
2.6. Ethics approval
This study was approved by Center for Reproductive Medicine, Tongji Medical College, HUST Approval Report of Medical Scientific Research Ethics Committee, and all participants provided informed consent (Ethics No. [2018] ‐(04)).
3. RESULTS
3.1. Comparison of hormone levels among all groups
Based on data showed in Table 1 and Figure 1, the concentration of three hormones (Inhibin B, FSH, and LH, especially Inhibin B) is related to spermatogenesis. No significant difference for prolactin level was found between different groups (p > 0.05), and the significant difference was observed for the levels of E2, testosterone, AMH, Inhibin B, LH, and FSH (p < 0.01). There was no significant difference in hormone concentrations between the normal spermatogenesis group and the obstructive azoospermia group (Table 2).
TABLE 1.
Comparison of hormone levels among all groups
| Group | E2 (pg/ml) | Testosterone (ng/ml) | AMH (ng/ml) | Inhibin B (pg/ml) | LH (mIU/ml) | FSH (mIU/ml) | PRL (ng/ml) |
|---|---|---|---|---|---|---|---|
| NOR (n = 96) | |||||||
| Mean | 71.31 | 5.57 | 8.13 | 132.67 | 4.60 | 5.01 | 12.78 |
| SD | 18.49 | 1.80 | 4.49 | 49.55 | 3.36 | 2.98 | 11.83 |
| OA (n = 38) | |||||||
| Mean | 71.28 | 5.19 | 8.41 | 124.25 | 4.82 | 4.93 | 12.12 |
| SD | 20.48 | 1.81 | 4.20 | 51.22 | 2.72 | 3.00 | 5.48 |
| IMO (n = 46) | |||||||
| Mean | 72.18 | 5.39 | 8.30 | 96.38 | 5.10 | 8.05 | 10.60 |
| SD | 22.18 | 1.50 | 4.61 | 49.45 | 2.13 | 6.43 | 3.22 |
| ISO (n = 53) | |||||||
| Mean | 68.19 | 4.98 | 5.72 | 66.55 | 6.04 | 13.26 | 10.50 |
| SD | 18.96 | 1.82 | 3.55 | 40.08 | 2.41 | 9.80 | 4.89 |
| NOA (n = 91) | |||||||
| Mean | 59.95 | 4.22 | 5.41 | 25.22 | 9.71 | 19.71 | 11.25 |
| SD | 25.16 | 1.77 | 4.43 | 14.91 | 5.93 | 11.14 | 4.20 |
| Total (n = 324) | |||||||
| Mean | 67.76 | 5.04 | 6.99 | 85.09 | 6.35 | 11.00 | 11.57 |
| SD | 21.73 | 1.83 | 4.50 | 59.84 | 4.46 | 9.89 | 7.65 |
| Group comparison | |||||||
| F * | 4.02 | 7.10 | 7.09 | 82.68 | 21.30 | 44.73 | 1.10 |
| p | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.355 |
Abbreviations: IMO, idiopathic moderate oligozoospermia; ISO, idiopathic severe oligozoospermia; NOA, nonobstructive azoospermia; NOR, normospermia; OA, obstructive azoospermia.
F‐test for comparison of multiple groups’ means, p < 0.05 indicates statistical significance.
FIGURE 1.
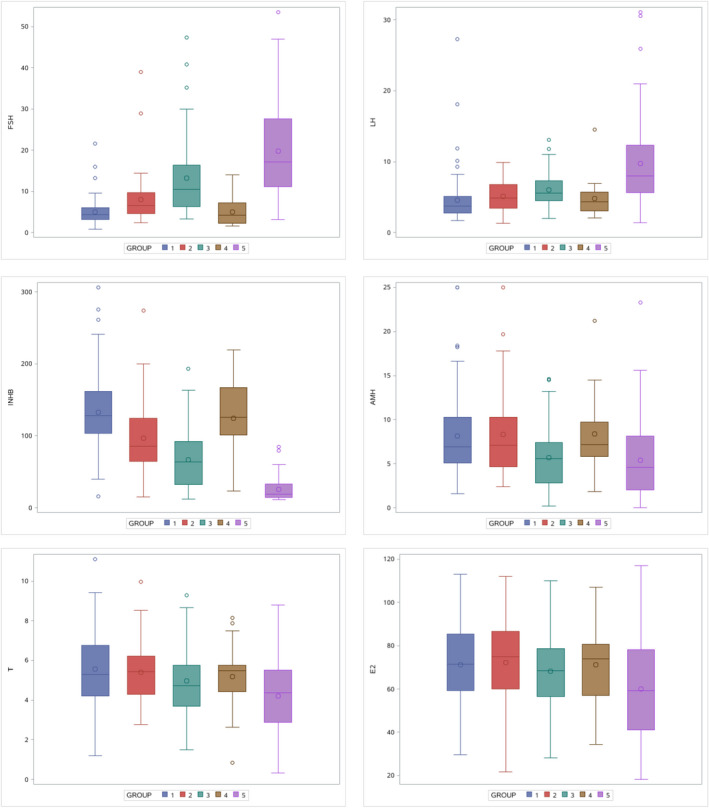
Differences in hormone distribution among groups. *Group 1: NOR (n = 96), Group 2: IMO (n = 46), Group 3: ISO (n = 53), Group 4: OA (n = 38), and Group 5: NOA (n = 91)
TABLE 2.
Comparison between normal control group and obstructive azoospermia group
| Inhibin B | AMH | FSH | E2 | Testosterone | LH | |
|---|---|---|---|---|---|---|
| T value | 0.673 | 0.250 | 0.104 | 0.006 | 0.835 | 0.270 |
| p Value | 0.502 | 0.803 | 0.918 | 0.995 | 0.405 | 0.788 |
3.2. Correlation between hormone levels and semen concentration
In oligozoospermia patients (including IMO and ISO), Inhibin B and FSH are highly correlated with sperm concentration (Table 3). In oligozoospermia patients with IMO, AMH still highly correlated with sperm concentration. However, in patients with IMO or ISO which have a narrower range of sperm concentration, there was no significant correlation between Inhibin B or FSH and sperm concentration.
TABLE 3.
Correlation analysis of hormone levels and semen concentration
| E2 | Testosterone | AMH | LH | Inhibin B | FSH | |
|---|---|---|---|---|---|---|
| Group 1 (n = 286) | ||||||
| Spearman_r | 0.197 | 0.252 | 0.267 | −0.502 | 0.755 | −0.692 |
| p | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Group 2 (n = 195) | ||||||
| Spearman_r | 0.048 | 0.090 | 0.172 | −0.319 | 0.530 | −0.543 |
| p | 0.504 | 0.212 | 0.016 | 0.000 | 0.000 | 0.000 |
| Group 3 (n = 99) | ||||||
| Spearman_r | −0.032 | 0.129 | 0.183 | −0.078 | 0.216 | −0.231 |
| p | 0.751 | 0.205 | 0.070 | 0.445 | 0.032 | 0.022 |
| Group 4 (n = 46) | ||||||
| Spearman_r | −0.227 | 0.011 | −0.360 | 0.274 | −0.218 | 0.188 |
| p | 0.130 | 0.940 | 0.014 | 0.066 | 0.146 | 0.211 |
| Group 5 (n = 53) | ||||||
| Spearman_r | −0.067 | 0.088 | 0.055 | −0.115 | 0.061 | −0.137 |
| p | 0.632 | 0.531 | 0.694 | 0.412 | 0.666 | 0.328 |
Group 1: NOR (n = 96), IMO (n = 46), ISO (n = 53) and NOA (n = 91), Group 2: NOR (n = 96), IMO (n = 46) and ISO (n = 53), Group 3: IMO (n = 46), ISO (n = 53), Group 4: IMO (n = 46), Group 5: ISO (n = 53).
3.3. Correlation between sperm concentration and total testicular volume
Distribution characteristics of total testicular volume under different spermatogenesis status were analyzed, and the total testicular volume was decreased with the severity of spermatogenesis function damage (Table 4). Through Spearman correlation analysis, there was a significant positive correlation between sperm concentration and total testicular volume (Figure 2). When the total testicular volume was <10 ml, there was significant damage to the spermatogenesis status of testis, making it very difficult to retrieve sperm by testicular biopsy or testicular sperm extraction.
TABLE 4.
Distribution characteristics of total testicular volume under different spermatogenesis status
| Group | Subgroup | N | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|---|
| NOR | 96 | 37.03 | 9.56 | 13.63 | 60.00 | |
| Oligozoospermia | IMO | 46 | 26.27 | 7.40 | 14.99 | 44.42 |
| ISO | 53 | 22.97 | 7.97 | 6.00 | 40.00 | |
| NOA | SCOS (Sertoli‐cell‐only syndrome) | 8 | 22.78 | 2.25 | 18.00 | 25.38 |
| Spermatogenic arrest | 32 | 16.88 | 5.68 | 7.75 | 30.00 | |
| No biopsy | 51 | 10.90 | 4.40 | 1.27 | 18.15 |
FIGURE 2.
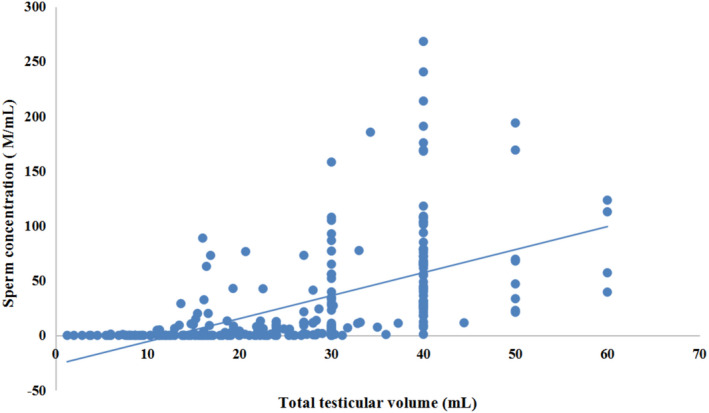
Correlation analysis of semen average concentration and total testicular volume. *Spearman correlation analysis (r = 0.750, p < 0.001) of samples under different spermatogenesis status (normal, mild and medium, severe oligospermia, nonobstructive azoospermia)
3.4. Serum concentrations of Inhibin B, FSH, and AMH in different spermatogenesis status
The order of AUC for diagnosing oligospermia is E2 + T + AMH + INHB + LH + FSH > AMH + INHB + FSH > INHB > FSH > LH > AMH > T > E2 (Table 5 and Figure 3). Considering that the serum levels of E2 and T are not different between NOR group and idiopathic oligozoospermia group (Table 6), it is suggested that AMH + INHB + FSH is suitable for diagnosing oligospermia. If a single hormone is used to distinguish between normal spermatogenesis and oligospermia, Inhibin B is better than other hormones. The cutoff value of Inhibin B is 97.1 pg/ml between normospermia (NOR) and idiopathic oligozoospermia (including IMO and ISO), with sensitivity and specificity of 79.2% and 72.7%, respectively (Table 6).
TABLE 5.
AUC of different hormone parameters in the diagnosis of idiopathic oligozoospermia
| Parameter | AUC | Standard errora | 95% Confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| E2 | 0.519 | 0.042 | 0.437 | 0.600 |
| T | 0.566 | 9.041 | 0.486 | 0.647 |
| AMH | 0.588 | 0.041 | 0.508 | 0.668 |
| Inhibin B | 0.802 | 0.032 | 0.739 | 0.865 |
| FSH | 0.781 | 0.033 | 0.763 | 0.881 |
| LH | 0.681 | 0.039 | 0.605 | 0.756 |
| Inhibin B + AMH + FSH | 0.822 | 0.030 | 0.763 | 0.881 |
| ALL 6 | 0.824 | 0.030 | 0.765 | 0.882 |
Under the nonparametric assumption.
FIGURE 3.
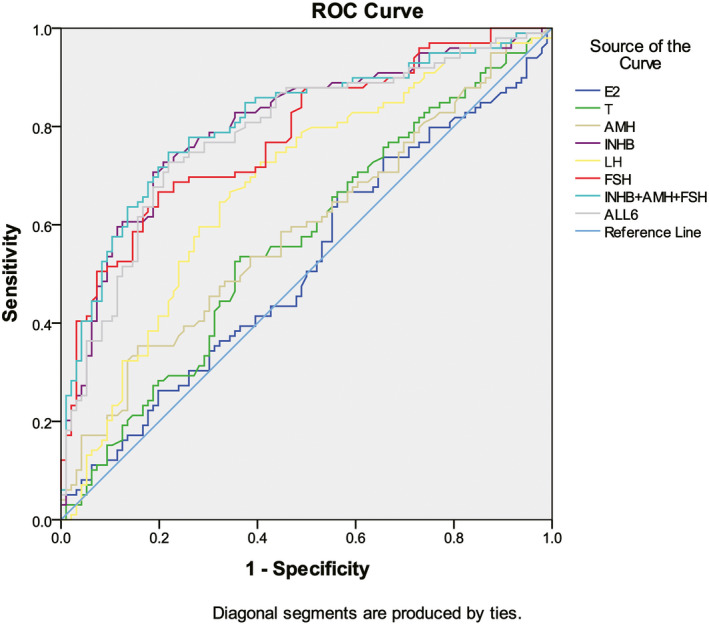
AUC of different hormone parameters in the diagnosis of idiopathic oligozoospermia
TABLE 6.
Comparison between NOR and idiopathic oligozoospermia (including IMO and ISO) with Inhibin B, AMH, FSH, and other parameters
| Inhibin B | AMH | FSH | E2 | T | LH | |
|---|---|---|---|---|---|---|
| T value | 7.57 | 1.93 | 6.185 | 0.453 | 1.587 | 2.442 |
| p Value | <0.001 | 0.055 | <0.001 | 0.651 | 0.114 | 0.016 |
| Cutoff | 97.1 | — | 6.245 | — | — | 4.565 |
| Sensitivity | 0.792 | — | 0.667 | — | — | 0.646 |
| Specificity | 0.727 | — | 0.802 | — | — | 0.677 |
| Youden index | 0.519 | — | 0.469 | — | — | 0.324 |
To distinguish between moderate oligozoospermia and severe oligozoospermia, AUC for AMH + Inhibin B + FSH is slightly higher than each single parameter (Figure 4, Table 7). The upper cutoff value of Inhibin B to diagnose severe oligozoospermia is 58.25 pg/ml (Table 8). There were 46 patients with IMO, including six patients with INHB less than 58.25 ng/ml and 40 patients with INHB >58.25 ng/ml. There were 53 patients with ISO, including 25 patients with INHB less than 58.25 ng/ml and 28 patients with INHB greater than 58.25 ng/ml. Therefore, Inhibin B has a positive predictive value of 80.65% for diagnosing ISO and a negative predictive value of 58.82%.
FIGURE 4.
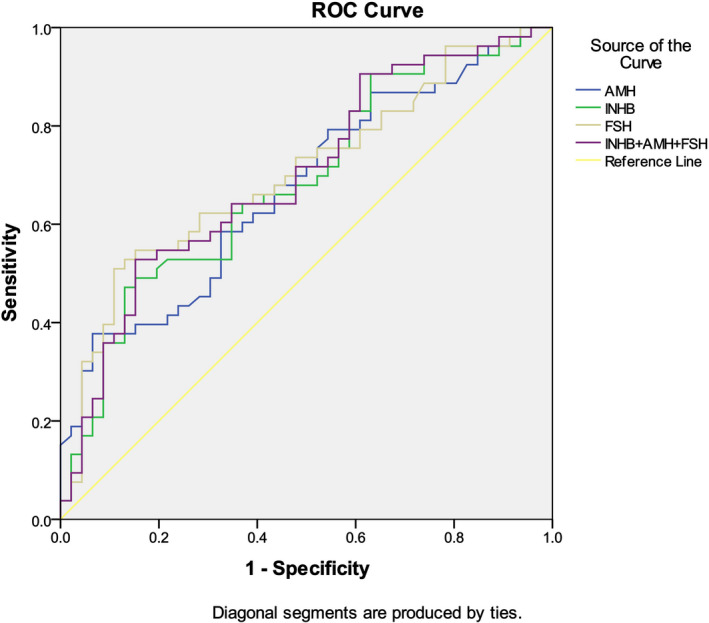
AUC of different parameters in the diagnosis of idiopathic severe oligozoospermia
TABLE 7.
AUC of different hormone parameters in the diagnosis of idiopathic severe oligozoospermia (ISO)
| Parameter | AUC | Standard errora | 95% Confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Inhibin B | 0.685 | 0.053 | 0.581 | 0.790 |
| FSH | 0.692 | 0.053 | 0.588 | 0.795 |
| AMH | 0.673 | 0.054 | 0.568 | 0.778 |
| Inhibin B + AMH + FSH | 0.714 | 0.051 | 0.613 | 0.814 |
Under the nonparametric assumption.
TABLE 8.
Comparison between idiopathic moderate oligozoospermia (IMO) and idiopathic severe oligozoospermia (ISO) with Inhibin B, AMH, FSH, and other parameters
| Inhibin B | AMH | FSH | E2 | Testosterone | LH | |
|---|---|---|---|---|---|---|
| T value | 3.313 | 3.143 | 3.167 | 0.963 | 1.217 | 2.029 |
| p Value | 0.001 | 0.002 | 0.002 | 0.338 | 0.226 | 0.045 |
| Cutoff | 58.25 | 3.935 | 10.80 | — | — | — |
| Sensitivity | 0.870 | 0.935 | 0.869 | — | — | — |
| Specificity | 0.472 | 0.377 | 0.491 | — | — | — |
| Youden index | 0.341 | 0.312 | 0.360 | — | — | — |
Using the same calculation method, when the upper cutoff value of AMH to diagnose ISO is 3.935 ng/ml, the positive predictive value is 86.96% and the negative predictive value is 56.58%. The upper cutoff value of FSH to diagnose ISO is 10.80 mIU/ml with a positive and negative prediction accuracy of 81.25% and 59.70%, respectively.
To distinguish between NOA and OA patients, AUC for AMH + Inhibin B + FSH is very similar to Inhibin B (0.943 vs. 0.941, Figure 5, Table 9). Since the specificity and sensitivity of Inhibin B are higher than those of AMH and FSH, it shows that Inhibin B is a better biomarker to predict NOA with cutoff value of 45.90 pg/ml (Table 10). There were 38 patients with OA, including two patients with INHB less than 45.90 pg/ml and 36 patients with INHB greater than 45.90 pg/ml. There were 91 patients with NOA, including 85 patients with INHB less than 45.90 pg/ml and six patients with INHB greater than 45.90 pg/ml. Therefore, the positive predictive value of Inhibin B to diagnose NOA is 97.70%, and the negative predictive value of that is 85.71%.
FIGURE 5.
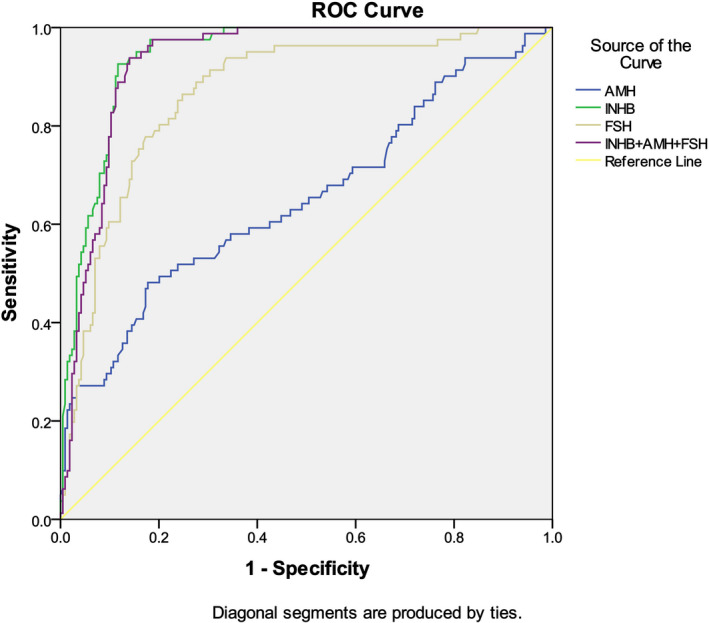
AUC of different parameters in the diagnosis of nonobstructive azoospermia
TABLE 9.
AUC of different parameters in the diagnosis of nonobstructive azoospermia
| Parameter | AUC | Standard errora | 95% Confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Inhibin B | 0.941 | 0.013 | 0.916 | 0.966 |
| AMH | 0.652 | 0.038 | 0.577 | 0.727 |
| FSH | 0.867 | 0.023 | 0.822 | 0.912 |
| Inhibin B + AMH + FSH | 0.943 | 0.012 | 0.919 | 0.967 |
Under the nonparametric assumption.
TABLE 10.
Cutoff and other index comparison between OA (obstructive azoospermia) and NOA (non‐obstructive azoospermia) for Inhibin B, AMH, and FSH
| Inhibin B | AMH | FSH | |
|---|---|---|---|
| p Value | <0.000 | 0.009 | <0.000 |
| Cutoff | 45.90 | 4.17 | 8.83 |
| Sensitivity | 0.926 | 0.822 | 0.864 |
| Specificity | 0.883 | 0.481 | 0.752 |
| Youden index | 0.809 | 0.304 | 0.617 |
The cutoff value of AMH to diagnose NOA is 4.17 ng/ml with a positive and negative prediction accuracy of 96.08% and 46.15%, respectively. The cutoff value of FSH to diagnose NOA is 8.83 mIU/ml with a positive and negative prediction accuracy of 96.39% and 76.09%, respectively.
The clinical application value of AMH/T was also analyzed and compared with that of Inhibin B in this study. It showed that AMH/T's AUC to distinguish nonobstructive azoospermia and obstructive azoospermia patients is lower than Inhibin B`s AUC (0.728 vs. 0.941; Figure 6, Table 11). We found that the AMH/T with cutoff value of <1.14 can distinguish nonobstructive azoospermia and obstructive azoospermia patients with predictive sensitivity and specificity are 89.47% and 63.64%, respectively, both of which are lower than that of Inhibin B (Table 12).
FIGURE 6.
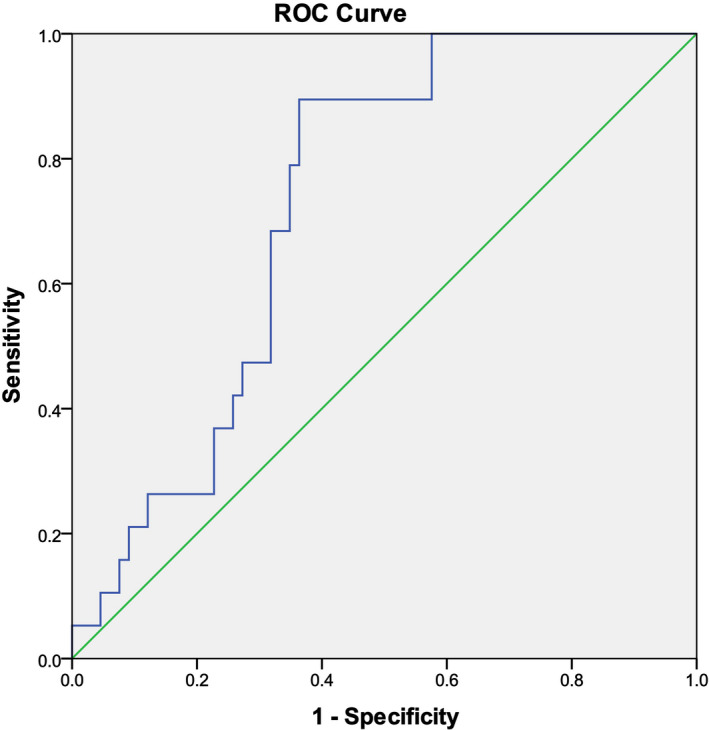
AUC of AMH/T in the diagnosis of nonobstructive azoospermia
TABLE 11.
AUC of Inhibin B and AMH/T in the diagnosis of nonobstructive azoospermia
| Parameter | AUC | Standard errora | 95% Confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Inhibin B | 0.941 | 0.013 | 0.916 | 0.966 |
| AMH/T | 0.728 | 0.055 | 0.621 | 0.819 |
Under the nonparametric assumption.
TABLE 12.
Cutoff and other index comparison between OA (obstructive azoospermia) and NOA (non‐obstructive azoospermia) for Inhibin B and AMH/T
| Inhibin B | AMH/T | |
|---|---|---|
| p Value | <0.000 | <0.000 |
| Cutoff | 45.90 | 1.14 |
| Sensitivity | 0.926 | 0.895 |
| Specificity | 0.883 | 0.636 |
| Youden index | 0.809 | 0.531 |
4. DISCUSSION
Prior to puberty, Inhibin B levels are most likely produced by Sertoli cells and independent on the presence of actively proliferating germ cells, while after puberty serum Inhibin B levels are closely related to the spermatogenesis status.13 In adult men, as a joint product of Sertoli cells and germ cells, Inhibin B levels reflect mainly the “amount” of spermatogenesis. Inhibin B was considered a direct biomarker of the Sertoli cell function and spermatogenesis.14, 15 In infertile men with elevated FSH, correlation between Inhibin B level and sperm count was more significant than FSH level to sperm count.16 In some studies, Inhibin B is a good biomarker for identifying spermatogenetic disorders.9, 10, 17
Following the conventional TESE, microsurgical testicular sperm extraction (micro TESE) technology has been more and more widely used in patients with NOA to obtain sperm from testis with the aid of intracytoplasmic sperm injection (ICSI).8 There are some controversial studies related to Inhibin B application in testicular sperm extraction. Eckardstein found that serum Inhibin B in combination with FSH is a more sensitive biomarker than serum FSH alone for evaluating impaired spermatogenesis in men, but it cannot predict accurately for TESE outcome.18 Ballescá suggested all azoospermic males should have serum Inhibin B detection prior to undergoing TESE.19 Vernaeve found that Inhibin B, either alone or in combination with serum FSH, fails to predict the presence of sperm in men with non‐obstructive azoospermia undergoing testicular sperm extraction.20 Mehmet found that Inhibin B was not an independent predictive factor and thus cannot reflect sperm retrieval.21
Because of above controversial study results, we tried to confirm the clinical value of serum Inhibin B alone or in combination with other hormone indicators in subfertile men and get some preliminary ideas.
For patients with different sperm concentration (including normal sperm concentration, mild‐to‐moderate oligospermia, and severe oligospermia), the concentrations of Inhibin B, FSH and AMH are closely related to testicular spermatogenesis. However, for each of these spermatogenic status, the correlation between the serum concentration of these three hormone indicators and the sperm concentration was weakened. For example, in patients with idiopathic oligozoospermia (including IMO and ISO), Inhibin B and FSH had good correlation with semen concentration. In IMO group or ISO group that have a narrower range of sperm concentration, this correlation was not significant anymore. In cryptorchid boys under age 4 years, there is a direct correlation between Inhibin B levels and the number of spermatogonia in testicular biopsies.22 In men with isolated GnRH deficiency, pulsatile GnRH administration was associated with significant increases in Inhibin B concentrations, which did not correlated with sperm concentration during therapy, and the constitutive secretion of Inhibin B (gonadotropin‐independent) is a biomarker of seminiferous tubule maturity such that the restoration of gonadotropin stimulation results in more rapid progression to mature spermatogenesis.23 Therefore, there may be a local regulatory mechanism of Inhibin B levels that is independent on gonadotropin and sperm concentration. As a joint product of testicular Sertoli and spermatogonia in adult men, we infer speculated that Inhibin B levels have has a direct correlation with the number and function of specific germ cell (ie, pachytene spermatocytes and round spermatids) in the testis, and the presence of mature sperm is not necessary for Inhibin B production. To distinguish different status of spermatogenesis in subfertility male patients, we found the AUC of Inhibin B + FSH or Inhibin B + FSH + AMH is slightly higher than Inhibin B alone, but difference is not significant.
To distinguish between moderate oligozoospermia and severe oligozoospermia, AUC for AMH + Inhibin B + FSH is 0.714 and slightly higher than each single parameter.
However, AUC to distinguish non‐obstructive azoospermia and obstructive azoospermia patients is 0.941 for Inhibin B with cutoff value of 45.90 pg/ml. These findings also suggest that the concentration of Inhibin B in the peripheral blood is related to the spermatogenesis ability of the testis.
Eight of the patients who underwent testicular biopsy were diagnosed with Sertoli‐cell‐only syndrome, but low levels of Inhibin B (range: 11.5–39.0 pg/ml, mean ± SD: 24.4 ± 10.5 pg/ml) were still detectable in their peripheral blood. This phenomenon may be due to the residual spermatogenic cells in the testicular tissue and the fact that Inhibin is partly secreted by extragonadal sources. For example, Salmenkivi et al.24 have found significant immunoreactivity of the medulla against βB in the inner layer of the cortex. In this study, 39 patients with NOA received Micro TESE. Thirteen patients had no sperm retrieved with Inhibin B levels (range: 2.30–138.50 pg/ml, mean ± SD: 38.96 ± 28.42 pg/ml, 95% confidence interval: 38.96 ± 4.86 pg/ml), and 26 patients had sperm retrieved with Inhibin B levels (range: 11.00–98.83 pg/ml, mean ± SD: 45.65 ± 23.88 pg/ml, 95% confidence interval: 45.65 ± 9.18 pg/ml). Therefore, we roughly estimated that it is not recommended to perform Micro TESE if Inhibin B level is less than 36.47 pg/ml. However, if Inhibin B is expected to predict the outcome of Micro TESE, a larger sample size needs to be collected in further research.
AMH also belongs to the transforming growth factor beta (TGF‐β) family, the same as Inhibin B, which is secreted by Sertoli cells in testes. AMH is critical for physiologic involution of the Mullerian ducts during sexual differentiation in the male fetus.25 The testis of idiopathic NOA men with germ cell aplasia may be representative of a prepubertal stage, which contributes to an impaired biochemical composition of the basal membrane, along with high levels of AMH.26 Alfano et al.27 reported that the levels of AMH and the ratio AMH‐to‐total testosterone (AMH/tT), with cutoff values of <4.62 ng/ml for AMH and <1.02 for AMH/tT, achieved independent predictor status for sperm retrieval at microTESE, with a predictive accuracy of 93% and 95%. Our study showed that AMH in severe oligozoospermia patient group had similar AUC as Inhibin B (0.673 vs. 0.685), but AMH's AUC to distinguish nonobstructive azoospermia and obstructive azoospermia patients is lower than Inhibin B’s AUC (0.652 vs. 0.941). We found that the AMH/T with cutoff value of <1.14 can distinguish nonobstructive azoospermia and obstructive azoospermia patients with predictive sensitivity and specificity are 89.47% and 63.64%, respectively. The AMH/T's cutoff value found in this study is basically consistent with the value reported by Alfano et al.
Further studies are needed to investigate these two TGF‐β family members in the application of spermatogenesis in male. In the future, Inhibin B combined with AMH and AMH/T may achieve higher predictive accuracy for sperm retrieval at microTESE.
In summary, Inhibin B is a promising biomarker for judging the state of testicular spermatogenesis, which can be a good biomarker for clinical doctors to screen non‐obstructive azoospermia patients from obstructive azoospermia patients. For azoospermia patients with normal Inhibin B, more spermatogenesis biomarkers as AMH FSH and testicular volume might be needed to distinguish different spermatogenesis states.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that would prejudice the impartiality of this work.
AUTHOR CONTRIBUTIONS
Xiangbin Kong and Zhen Ye analyzed the data, wrote the paper, and contributed to study design. Yaoping Chen, Huan Zhao, Jian Tu, Tianqing Meng, and Honggang Li performed the research. Yijun Gong, Liang Zheng, and Bangning Cheng designed the research study and collected the data. Chengliang Xiong, Zhijun Zhang, and Peng Xu designed the research study and revised the article. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
Inhibin B, anti‐Müllerian hormone (AMH), FSH, LH, testosterone, estradiol, and prolactin CLIA kits used in this study were kindly supplied by the manufacturers, namely, YHLO Biotech (Shenzhen, China).
Kong X, Ye Z, Chen Y, et al. Clinical application value of Inhibin B alone or in combination with other hormone indicators in subfertile men with different spermatogenesis status: A study of 324 Chinese men. J Clin Lab Anal. 2021;35:e23882. 10.1002/jcla.23882
Kong and Ye contributed equally.
Funding information
This work was supported by National Key Research and Development Program of China (2017YFC1002001) and the 12th Five‐Year Plan of National Science and Technology of China (2012BAI32B03)
Contributor Information
Zhijun Zhang, Email: zhangzhijun@taihehospital.com.
Peng Xu, Email: pengxu0616@126.com.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Pineau C, Sharpe RM, Saunders PTK, et al. Regulation of Sertoli cell inhibin production and of inhibin alpha‐subunit mRNA levels by specific germ cell types. Mol Cell Endocrinol. 1990;72(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 2.Marchetti C, Hamdane M, Mitchell V, et al. Immunolocalization of Inhibin and activin α and βB subunits and expression of corresponding messenger RNAs in the human adult testis. Biol Reprod. 2003;68(1):230‐235. [DOI] [PubMed] [Google Scholar]
- 3.Andersson AM, Muller J, Skakkebaek NE. Different roles of prepubertal and postpubertal germ cells and sertoli cells in the regulation of serum Inhibin B levels. J Clin Endocrinol Metab. 1998;83:4451‐4458. [DOI] [PubMed] [Google Scholar]
- 4.Foresta C, Bettella A, Petraglia F, et al. Inhibin B levels in azoospermic subjects with cytologically characterized testicular pathology. Clin Endocrinol. 1999;50:695‐701. [DOI] [PubMed] [Google Scholar]
- 5.Frydelund‐Larsen L, Krausz C, Leffers H, et al. Inhibin B: a marker for the functional state of the seminiferous epithelium in patients with azoospermia factor C microdeletions. J Clin Endocrinol Metab. 2002;87:5618‐5624. [DOI] [PubMed] [Google Scholar]
- 6.Petersen PM, Andersson AM, Rorth M, et al. Undetec table Inhibin B serum levels in men after testicular irradiation. J Clin Endocrinol Metab. 1999;84:213‐215. [DOI] [PubMed] [Google Scholar]
- 7.Foppiani L, Schlatt S, Simoni M, et al. Inhibin B is a more sensitive marker of spermatogenetic damage than FSH in the irradiated non‐human primate model. J Endocrinol. 1999;162:393‐400. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Huang XB. Combined evaluation of inhibin B, follicle stimulating hormone and luteinizing hormone improve sperm retrieval prediction in patients with non‐obstructive azoospermia. Adv Reprod Sci. 2013;1:1‐5. [Google Scholar]
- 9.Liu Y‐P, Qi L, Zhang N‐N, et al. Follicle‐stimulating hormone may predict sperm retrieval rate and guide surgical approach in patients with non‐obstructive azoospermia. Reprod Biol. 2020;20(4):573‐579. [DOI] [PubMed] [Google Scholar]
- 10.Green DM, Zhu L, Zhang N, et al. Lack of specificity of plasma concentrations of inhibin B and follicle‐stimulating hormone for identification of azoospermic survivors of childhood cancer: a report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31(10):1324‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goede J, Hack W, Sijstermans K, et al. Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr. 2011;76(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 12.Du J, Li FH, Guo YF, et al. Differential diagnosis of azoospermia and etiologic classification of obstructive azoospermia: role of scrotal and transrectal US. Radiology. 2010;256(2):493‐503. [DOI] [PubMed] [Google Scholar]
- 13.Pierik FH, Vreeburg JTM, Stijnen T, et al. Serum Inhibin B as a marker of spermatogenesis. J Clin Endocrinol Metab. 1998;83(9):3110‐3114. [DOI] [PubMed] [Google Scholar]
- 14.Bergmann M, Behre HM, Nieschlag E. Serum FSH and testicular morphology in male infertility. Clin Endocrinol. 1994;40(1):133‐136. [DOI] [PubMed] [Google Scholar]
- 15.Meachem SJ, Nieschlag E, Simoni M. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol. 2001;145(5):561‐571. [DOI] [PubMed] [Google Scholar]
- 16.Andersson AM, Toppari J, Haavisto AM, et al. Longitudinal reproductive hormone profiles in infants: peak of Inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83:675‐681. [DOI] [PubMed] [Google Scholar]
- 17.Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non‐obstructive azoospermia: a systematic review. Andrology. 2014;2(1):20‐24. [DOI] [PubMed] [Google Scholar]
- 18.Hayes FJ, Pitteloud N, DeCruz S, et al. Importance of Inhibin B in the regulation of FSH secretion in the human male. J Clin Endocrinol Metab. 2001;86:5541‐5546. [DOI] [PubMed] [Google Scholar]
- 19.Kumanov P, Nandipati K, Tomova A, et al. Inhibin B is a better marker of spermatogenesis than other hormones in the evaluation of male factor in fertility. Fertil Steril. 2006;86:332‐338. [DOI] [PubMed] [Google Scholar]
- 20.Nachtigall LB, Boepple PA, Seminara SB, et al. Inhibin B secretion in males with gonadotropin‐releasing hormone (GnRH) deficiency before and during long‐term GnRH replacement: relationship to spontaneous puberty, testicular volume, and prior treatment‐a clinical research center study. J Clin Endocrinol Metab. 1996;81:3520‐3525. [DOI] [PubMed] [Google Scholar]
- 21.von Eckardstein S , Simoni M, Bergmann M, et al. Serum Inhibin B in combination with serum follicle‐stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab. 1999;84(7):2496‐2501. [DOI] [PubMed] [Google Scholar]
- 22.Ballescá JL, Balasch J, et al. Serum Inhibin B determination is predictive of successful testicular sperm extraction in men with non‐obstructive azoospermia. Hum Reprod. 2000;15(8):1734‐1738. [DOI] [PubMed] [Google Scholar]
- 23.Jensen TK, Andersson AM, Hjollund NH, et al. Inhibin B as a serum marker of spermatogenesis: correlation to differences in sperm concentration and follicle‐stimulating hormone concentrations. A study of 349 Danish men. J Clin Endocrinol Metab. 1997;82:4059‐4063. [DOI] [PubMed] [Google Scholar]
- 24.Vernaeve V, Tournaye H, Schiettecatte J, et al. Serum inhibin B cannot predict testicular sperm retrieval in patients with non‐obstructive azoospermia. Hum Reprod. 2002;17:971‐976. [DOI] [PubMed] [Google Scholar]
- 25.Shrikhande L, Shrikhande B, Shrikhande A. AMH and its clinical implications. J Obstet Gynaecol India. 2020;70(5):337‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfano M, Pederzoli F, Locatelli I, et al. Impaired testicular signaling of vitamin A and vitamin K contributes to the aberrant composition of the extracellular matrix in idiopathic germ cell aplasia. Fertil Steril. 2019;111(4):687‐698. [DOI] [PubMed] [Google Scholar]
- 27.Alfano M, Ventimiglia E, Locatelli I, et al. Anti‐mullerian hormone‐to‐testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non‐obstructive azoospermia. Sci Rep. 2017;7(1):17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


