Purpose of review
Although the literature to date on COVID-19 outcomes in those with immune-mediated inflammatory disease has been largely reassuring there remain many unanswered questions. These include the impact of specific medications on outcomes and the antibody response after COVID-19 vaccination.
Recent findings
We summarized the current literature related to COVID-19 outcomes in immune-mediated inflammatory diseases in rheumatology, gastroenterology, dermatology, and neurology. Overall, we found either no difference or modest differences in risk for severe COVID-19 for people with immune-mediated diseases compared with the general population. When considering disease-specific factors, glucocorticoid use and underlying immune-mediated disease activity were generally associated with worse outcomes. Specific medications varied in associations: tumor necrosis factor inhibitors generally had lower odds for severe COVID-19 outcomes, whereas rituximab use generally had higher odds for severe outcomes. We also detailed the recent reports of antibody response to COVID-19 vaccination in people with immune-mediated inflammatory diseases.
Summary
Investigations of immune-mediated inflammatory diseases across several organ systems have offered important insight into the COVID-19 disease course. Overall, these studies have provided reassurance to patients and clinicians while also identifying groups who may be at higher risk for poor outcomes.
Keywords: coronavirus disease 2019, dermatology, gastroenterology, neurology, outcomes, rheumatology
INTRODUCTION
The novel coronavirus pandemic continues to have a tremendous impact on our daily life, particularly in those with immune-mediated disease. This is because of altered immunity from underlying disease and immunomodulating medications. The impact of the pandemic on outcomes has been described previously but constantly evolving information makes regular updates mandatory [1–3]. International collaborative studies, such as the COVID-19 Global Rheumatology Alliance and SECURE-IBD have efficiently generated timely data that has informed the rheumatology community [4–7,8▪▪,9▪▪].
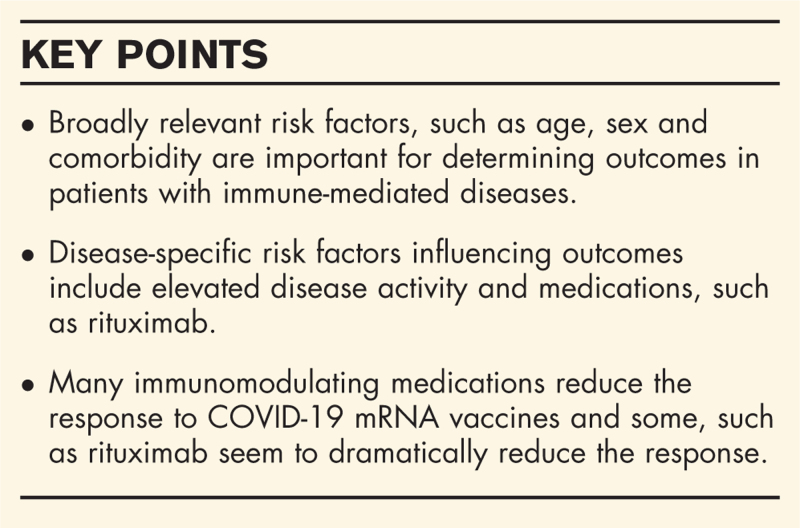
There is clear relevance to reviewing immune-mediated diseases across specialities as many therapies are used widely, for example, targeted cytokine inhibitors and B-cell depletion therapies are used across rheumatology, dermatology, gastroenterology and neurology. With increasingly large datasets being collected and randomized controlled trials of many immunosuppressing therapies in coronavirus disease 2019 (COVID-19) being completed, we are building a better picture of both the risks and benefits of immune-mediated therapies [10]. With more time and information, it is becoming clear that the risk factors that apply to the general population like age, sex, and comorbidity are critically important to outcomes in patients with immune-mediated disease. Although there are clearly some therapies that seem to stand out for their increased risk, for example, rituximab, the burden of increased risk can be attributed to risk factors that are widely relevant across all those in the community [11,12▪▪].
Box 1.
no caption available
RHEUMATIC DISEASES
Rheumatic diseases are broadly characterized by autoimmunity, systemic inflammation and fibrosis -- also identified to be prominent features of COVID-19 even early in the pandemic [13,14]. Throughout the pandemic, there has been intense interest in re-purposing immunomodulatory medications, such as hydroxychloroquine, tocilizumab and baricitinib as therapies for COVID-19 [10,15,16]. Some rheumatic disease manifestations, such as interstitial lung disease and acquired comorbid conditions, such as cardiovascular disease may place people with rheumatic diseases susceptible to infection and poor outcomes from COVID-19 [11,17]. Thus, studying the intersection of rheumatic diseases and COVID-19 has been of intense interest. However, this also offered challenges as rheumatic diseases are both uncommon and heterogeneous.
One of the first reports of rheumatic disease and COVID-19 was a case series mostly consisting of inflammatory arthritis who seemed to mostly have a mild disease course [18]. Another case series showed that most people with systemic lupus erythematosus (SLE) did not develop COVID-19 and only a very few had poor outcomes [19]. However, two comparative studies suggested that rheumatic disease patients may be at increased risk for mechanical ventilation compared with general population controls [20,21]. Risks for hospitalization and mortality were similar in both of those small studies. In a large nationwide English study OpenSAFELY, people with rheumatoid arthritis (RA), lupus, or psoriasis were identified to have a modest but statistically significantly increased risk for mortality (hazard ratio 1.19) [11]. However, the identification of these diseases using administrative codes alone may be prone to misclassification and the three conditions are quite heterogeneous. Thus, the associations of specific rheumatic diseases with COVID-19-related mortality is not clear. A large study analyzed multiple electronic health records compared people with rheumatic disease to age-matched and sex-matched comparators [12▪▪]. This study found that people with rheumatic diseases were at increased risk for many poor outcomes including hospitalization, intensive care unit admission, acute kidney injury and venous thromboembolism [12▪▪]. Most associations were attenuated or eliminated after adjustment for comorbidities, suggesting that these mediated the relationship between rheumatic diseases and poor COVID-19 outcomes.
These studies were mostly performed early in the pandemic when hospital systems were commonly overwhelmed and the clinical benefits of drugs, such as remdesivir and dexamethasone were not yet established [22,23]. Two studies showed that the excess risk of mechanical ventilation and other poor COVID-19 outcomes improved over calendar time [24,25]. This suggests that people with rheumatic disease may have similar outcomes to the general population later in the pandemic now with effective treatments and health system capacity. A nationwide study in Sweden showed that the excess relative risk of mortality for RA and other inflammatory joint diseases was relatively stable in 2020 compared with earlier years [26]. People with RA or other inflammatory joint diseases had slightly higher rates of severe COVID-19 outcomes, such as hospitalization, ICU admission, and mortality but these were infrequent and generally not statistically different than general population comparators [26]. Another matched comparative study of hospitalized patients suggested that severe COVID-19 outcomes were more common in connective tissue diseases, such as SLE than general population controls; inflammatory arthritis had similar outcomes to their controls [27]. A meta-analysis reported that autoimmune disease patients had two-fold odds of COVID-19 than controls [28]. Overall, the current literature suggests that rheumatic diseases may modestly increase risk of severe COVID-19 compared with the general population.
The COVID-19 Global Rheumatology Alliance (GRA) formed early in the pandemic and allowed physicians to voluntarily enter cases of COVID-19 in rheumatic patients [4–6]. After an initial, early descriptive report of 110 patients, the first large GRA paper with 600 patients investigated risk factors for hospitalization among rheumatic patients [29▪,30]. This verified general population risk factors for severe COVID-19, such as older age and comorbidities [29▪]. This also showed that baseline use of glucocorticoids were associated with increased odds of hospitalization [29▪]. Importantly, biologic and targeted synthetic disease-modifying antirheumatic drugs (DMARDs), particularly tumor necrosis factor inhibitors (TNFi) were associated with lower odds of hospitalized COVID-19 compared with no DMARDs [29▪]. Other reports also support this finding seen with TNFi [31]. Similar findings implicating glucocorticoids with worse COVID-19 outcomes were reported in a large single-center study in New York City [32]. This offered early reassurance to patients and clinicians that use of these medications were not clearly associated with poor outcomes.
A more recent, larger GRA study that included 3729 patients showed that higher baseline rheumatic disease activity was associated with higher odds of COVID-19-related mortality [33▪▪,34]. This study also showed that rituximab and sulfasalazine were each associated with higher odds of COVID-19-related mortality than methotrexate monotherapy. In another French cohort study, rituximab use in rheumatic diseases was also associated with higher risk of severe COVID-19 outcomes than rheumatic diseases not treated with rituximab [35]. These poor outcomes may be because of prolonged SARS-CoV-2 infection related to B-cell depletion and impaired antibody response. Some reports have suggested that immunocompromised patients, particularly those on rituximab, may be a reservoir for prolonged SARS-CoV-2 infection that may result in accelerated viral evolution that has resulted in variants that could increase virulence and evade vaccination efforts [36]. Thus, rheumatic patients and other immunocompromising states are likely to remain a central player as the pandemic continues to unfold. Finally, the differences in outcome based on race and ethnicity seen in the wider population has also been reflected in the rheumatic disease population, likely mediated by multiple medical and nonmedical factors [37].
GASTROENTEROLOGY
In contrast to rheumatic diseases, inflammatory bowel diseases (IBD), such as Crohn's disease and ulcerative colitis have relatively less heterogeneity. A recent large nationwide population-based matched retrospective study in Sweden showed that IBD patients were significantly more likely to be hospitalized for COVID-19 than matched comparators [38]. Another nationwide Danish study identified IBD patients with COVID-19 and compared with a population-based cohort [39]. This study found that IBD patients had lower prevalence of COVID-19 than the general population, offering reassurance but was limited by small numbers of IBD patients [39]. In a meta-analysis of 24 studies, SARS-CoV-2 infection risk in patients with IBD was similar to the general population [40]. COVID-19 outcomes for IBD patients were worse in ulcerative colitis compared with Crohn's disease.
Gastroenterologists formed a physician registry called Surveillance Epidemiology of Coronavirus Under Research Exclusion for IBD (SECURE-IBD) early in the pandemic, which was a model that the GRA adapted. The initial report in SECURE-IBD reported higher odds of hospitalization for COVID-19 in patients on baseline glucocorticoids and lower odds for IBD patients on TNFi [9▪▪]. A larger follow-up study reported that thiopurine monotherapy or in combination was strongly associated with severe COVID-19 outcomes compared with TNFi monotherapy [8▪▪]. This offered further reassurance to the safety of biologic DMARDs, such as TNFi as this finding has been observed across several diseases and organ systems [31]. It is not currently clear whether these findings are because of a possible protective effect of TNFi or whether the findings may be confounded. Trials are underway to investigate possible efficacy of TNFi for treating COVID-19 [41,42]. Another large study identified all people with IBD in the Veterans Affairs Healthcare System and found that vedolizumab and glucocorticoids were associated with severe COVID-19 outcomes [43].
Overall, the experience of IBD during the COVID-19 has mostly offered reassurance that patients have at best modestly increased risk for severe outcomes compared with the general population. The outcomes of those on TNFi also provides reassurance that this class of medication may be safely continued. Conversely, other medications used in IBD, such as glucocorticoids, thiopurines, and vedolizumab may be associated with more severe COVID-19 outcomes.
DERMATOLOGY
As in rheumatology and gastroenterology, there has been great interest and concern in the dermatology community concerning the risk of COVID-19 in patients with psoriasis, atopic dermatitis, and other immune-mediated conditions. As in rheumatology, early concern was informed by findings from the OpenSAFELY population-based study in England, which reported a 20% higher risk of COVID-19 death among patients with RA, lupus, or psoriasis (hazard ratio 1.19), a large, heterogeneous group [11]. Two large cohort studies sought to evaluate the risk of COVID-19 infection in patients with psoriasis compared with the general population. In one study, investigators estimated the incidence of COVID-19 in patients with psoriasis on systemic therapies in a previously established large, multicenter prospective cohort study [44]. Compared with the general population estimate, the investigators reported a nonstatistical significant trend toward a higher standard incidence rate for COVID-19 infection [SIR 1.58, 95% confidence interval (CI) 0.98–2.41]. Among other limitations, the number of confirmed infections (n = 21) and severe outcomes (n = 13 hospitalized, n = 1 death) was relatively small, which limited the study's power to estimate SIRs for infection and outcomes, such as hospitalization and death. Reassuringly, in a similar study conducted in a prospective psoriasis cohort in Italy, investigators found that psoriasis patients did not have a higher SIR for COVID-19 hospitalization or death [45]. In that study, the investigators also found no association between biologic DMARD use and a higher SIR compared with the general population. However, the number of infections was similarly small in this Italian cohort study as in the Spanish cohort study. Both studies were limited by their reliance on standardized incidence rates, which may not account for other potential confounders of the association of psoriasis with COVID-19 risk and outcomes.
In addition to prospective cohort studies, two physician-reported registries were established early on by the dermatology community. The design of SECURE-AD (atopic dermatitis) and PsoProtect (psoriasis) are similar to the SECURE-IBD and GRA registries previously discussed. The findings from the SECURE-AD registry will be particularly interesting because of the unique treatments used in atopic dermatitis compared with those used in psoriasis, IBD and rheumatic diseases. At the time of this publication, results from SECURE-AD have not yet been published. In contrast, results from the first 374 patients in the physician-reported PsoProtect registry confirmed several observations reported by the GRA and SECURE-IBD registries [46▪]. First, similar risk factors for worse disease were observed in the psoriasis population as in the general population, including older age, male sex, nonwhite ethnicity and comorbid lung disease. Second, patients who used biologic therapies had a 65% lower risk of hospitalization compared with those using nonbiologic therapies.
Observed differences in outcomes according to DMARD use in SECURE-IBD prompted the investigators to explore factors that may contribute to differences in outcomes according to treatment. In an analysis of 1626 patients who reported their experiences during the pandemic to a patient-facing psoriasis registry, patients on biologic treatments were 39% more likely than those on nonbiologic DMARDs to practice shielding (OR 1.39, 95% CI 1.23–1.56) [47]. These findings highlight the caution with which one should interpret estimates of the risk of COVID-19 in patients on various DMARDs as shielding practices may have differed between users of different treatments.
NEUROLOGY
Multiple sclerosis, myasthenia gravis and other neurological diseases, such as autoimmune encephalitis are all managed with immunosuppression, so, neurology faces similar challenges to rheumatology, dermatology and gastroenterology. To add a complicating factor, it has become evident that COVID-19 infection has numerous neurological manifestations [48].
French data in 347 multiple sclerosis patients demonstrated male gender, comorbidities and higher disability as measured by the Expanded Disability Severity Scale score (EDSS) were associated with worse COVID-19 outcome measured with a 7-point ordinal scale [49]. Of broader interest in the context of the hyper-inflammation of COVID-19, a higher proportion of multiple sclerosis patients not on disease-modifying therapy (DMT, 46%) developed severe COVID-19 compared with those taking DMT (16%) [10]. In univariate analysis, DMT therapies were protective of poorer outcomes but this finding was not evident in the multivariate model, noting the limitation of small numbers in this analysis.
B-cell-depleting agents are potentially a risk for poorer outcomes based on patients with rheumatic disease. There were 51 suspected or confirmed cases of COVID-19 found in the B-cell-depleting agent ocrelizumab multiple sclerosis clinical trials up until the end of July 2020 [50]. Disease severity was asymptomatic, mild or moderate in 68.6% and severe in 19.6%, with 6% dying and 6% outcome data missing. Of the total group, 31.4% were hospitalized. In the manufacturer postmarketing surveillance safety database, there were 307 postmarketing cases of COVID-19 with 86% (n = 263) confirmed and 14% suspected [50]. Of those 33% were hospitalized and 47% had asymptomatic, mild or moderate disease, with 6% dying.
As of mid-July 2020 in the OPTUM COVID-19 database there were 357 multiple sclerosis patients with confirmed COVID-19 [50]. There were 48 of these patients treated with ocrelizumab and 309 not treated with ocrelizumab. The outcomes were similar between the two groups with 76% and 75% hospitalized in the non-ocrelizumab and ocrelizumab groups, respectively. There were 1.6 and 2.1% who received invasive ventilation in the non-ocrelizumab and ocrelizumab groups, respectively. Finally 3.9 and 2.1% died in the non-ocrelizumab and ocrelizumab groups, respectively.
There have been other small case series published on other neurological diseases, such as myasthenia gravis where older patients made up 75% of the deaths again supporting the premise that widely relevant risk factors remain critical in disease sub-groups we worry about [51]. In summary, the small size of the reported cohorts limits the conclusions that can be drawn but specific therapies, such as B-cell-depleting therapies remain a concern, but not to the exclusion of the broadly relevant demographic and comorbidity risk factors.
VACCINE EFFICACY
The quick development of well tolerated and effective COVID-19 vaccines has not only been a tremendous scientific advance during the pandemic but also raises important questions about the efficacy of vaccination in patients with immune-mediated diseases, especially those on immunomodulation. Previous studies have established that several DMARD classes may be associated with a less robust immune response to vaccines for influenza, pneumococcus and other infections [52]. Unfortunately, patients with immune-mediated inflammatory conditions and those on immunomodulation were generally excluded from the initial COVID-19 vaccination trials leaving providers and patients with little guidance on how or when to vaccinate patients on DMARDs. Several reports have not only described a potentially dampened antibody response to SARS-CoV-2 mRNA vaccines among DMARD users but also highlight that the response likely varies across DMARD classes [53,54▪▪,55].
In the largest study to date, 133 adults with autoimmune diseases, including IBD (31.6%), RA (28.6%), spondyloarthritis (15%) and lupus (11%) were included [54▪▪]. Though the antibody response to two doses of SARS-CoV-2 mRNA vaccines was robust among many with autoimmune diseases, it was three-fold lower than the response observed in healthy controls. Similar reductions were observed in the neutralization activity of SARS-CoV-2 antibodies. In particular, glucocorticoid users, Janus Kinase inhibitor users, antimetabolite users and especially those with recent B-cell-depleting therapy exposure had significant reductions in antibody responses. Reductions in the antibody response among patients using TNF inhibitors were more modest.
Similar observations were made in a study of 123 patients with rheumatic diseases, including inflammatory arthritis (28%), lupus (20%) and Sjogren's syndrome (13%) [53]. Though 74% of patients had a detectable antibody response, a median of 22 days after the first dose of an mRNA vaccine, antibody responses were variable across DMARD classes. For instance, while nearly all TNFi users (n = 16 of 17) and methotrexate users (n = 10 of 13) had an antibody response, rituximab (n = 2 of 6) and mycophenolate mofetil (n = 3 of 11) users less often had an antibody response. The sample size of this study was small, limiting conclusions that can be drawn, especially as the response was assessed only after the first dose.
A report from a cohort of patients with a history of solid organ transplantation on medications commonly used (e.g. glucocorticoids, azathioprine, mycophenolate) described poor vaccine efficacy after the first dose of an mRNA vaccine [55]. Notably, those on mycophenolate mofetil, mycophenolic acid or azathioprine had a particularly poor response to the vaccine with only 9% mounting an antibody response following the first dose. Caution is needed whenever interpreting these data; however, as the generalizability of these findings to those with immune-mediated conditions is unclear and the immune response was only assessed following the first dose of the mRNA vaccine series, which typically includes two doses.
Additional studies are urgently needed to better define the efficacy of mRNA and other vaccines in each DMARD class, across different vaccine classes, the durability of the antibody response, the T-cell response to vaccination in this population, and the appropriate timing of vaccination in relation to DMARD use.
CONCLUSION
Patients with immune-mediated inflammatory disease have offered important insight into the COVID-19 disease course. Findings have generally suggested either no or modest differences in severity of COVID-19, which provides reassurance to patients and clinicians. However, subgroups of patients may be susceptible to poor outcomes, in particular, patients on rituximab as well as those requiring glucocorticoids because of elevated underlying disease activity. Some evidence suggests that immunocompromised patients may have prolonged viral infection that may result in viral evolution resulting in SARS-CoV-2 variants. COVID-19 vaccinations offer the possibility to protect immune-mediated inflammatory disease patients. However, the underlying altered immunity and immunomodulator use may blunt vaccine response.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
J.A.S. reports consultancy for Bristol-Myers Squibb, Gilead and Pfizer unrelated to this work.
Z.S.W. reports grant support from Bristol-Myers Squibb and Sanofi as well as personal fees from MedPace and Viela Bio unrelated to this work. P.C.R. reports personal fees from Eli Lilly, AbbVie, Pfizer, UCB Pharma, Novartis, Janssen and Gilead Sciences; grants from Pfizer, UCB Pharma, Novartis and Janssen; and nonfinancial support from Pfizer and Bristol Myers Squibb, all outside the submitted work.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Grainger R, Machado PM, Robinson PC. Novel coronavirus disease-2019 (COVID-19) in people with rheumatic disease: epidemiology and outcomes. Best Pract Res Clin Rheumatol 2020; 35:101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianfrancesco M, Yazdany J, Robinson PC. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr Opin Rheumatol 2020; 32:434–440. [DOI] [PubMed] [Google Scholar]
- 3.Hyrich KL, Machado PM. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol 2021; 17:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson PC, Yazdany J, Machado PM. Global research collaboration in a pandemic-challenges and opportunities: the COVID-19 Global Rheumatology Alliance. Curr Opin Rheumatol 2021; 33:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace ZS, Bhana S, Hausmann JS, et al. The Rheumatology Community responds to the COVID-19 pandemic: the establishment of the COVID-19 global rheumatology alliance. Rheumatology 2020; 59:1204–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liew JW, Bhana S, Costello W, et al. COVID-19 Global Rheumatology Alliance. The COVID-19 Global Rheumatology Alliance: evaluating the rapid design and implementation of an international registry against best practice. Rheumatology 2021; 60:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson PC, Yazdany J. The COVID-19 Global Rheumatology Alliance: collecting data in a pandemic. Nat Rev Rheumatol 2020; 16:293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪▪.Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021; 70:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes outcomes and risk factors in 1400 patients with inflammatory bowel disease.
- 9▪▪.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020; 159:481.e3–491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article described outcomes and risk factors in 525 inflammatory bowel disease patients.
- 10.Amigues I, Pearlman AH, Patel A, et al. Coronavirus disease 2019: investigational therapies in the prevention and treatment of hyperinflammation. Expert Rev Clin Immunol 2020; 16:1185–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.D'Silva KM, Jorge A, Cohen A, et al. COVID-19 outcomes in patients with Systemic Autoimmune Rheumatic Diseases (SARDs) compared to the general population: a US Multi-Center Comparative Cohort Study. Arthritis Rheumatol 2020; 73:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article examined outcomes in 2379 patients with rheumatic disease compared with controls to explore risk factors for poor outcomes.
- 13.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–793. [DOI] [PubMed] [Google Scholar]
- 14.Mehta P, Sattui SE, van der Geest KSM, et al. Giant cell arteritis and COVID-19: similarities and discriminators. a systematic literature review. J Rheumatol 2020; doi: 10.3899/jrheum.200766 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Putman M, Chock YPE, Tam H, et al. COVID-19 Global Rheumatology Alliance. Antirheumatic disease therapies for the treatment of COVID-19: a systematic review and meta-analysis. Arthritis Rheumatol 2021; 73:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konig MF, Kim AH, Scheetz MH, et al. COVID-19 Global Rheumatology Alliance. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis 2020; 79:1386–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito AJ, Menon AA, Ghosh AJ, et al. Increased odds of death for patients with interstitial lung disease and COVID-19: a case-control study. Am J Respir Crit Care Med 2020; 202:1710–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med 2020; 383:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Ruiz R, Masson M, Kim MY, et al. NYU WARCOV Investigators. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol 2020; 72:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot.’. Ann Rheum Dis 2020; 79:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye C, Cai S, Shen G, et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis 2020; 79:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horby P, Lim WS, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med 2020; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigel JH, Tomashek KM, Dodd LE, et al. ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med 2020; 383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorge A, D'Silva KM, Cohen A, et al. Temporal trends in severe COVID-19 outcomes in patients with rheumatic disease: a cohort study. Lancet Rheumatol 2021; 3:e131–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis 2021; 80:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower H, Frisell T, Di Giuseppe D, et al. ARTIS Study Group. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis 2021; doi: 10.1136/annrheumdis-2021-219845 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pablos JL, Galindo M, Carmona L, et al. RIER Investigators group. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020; 79:1544–1549. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis [Internet] 2020; doi: 10.1136/annrheumdis-2020-218946 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29▪.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. COVID-19 Global Rheumatology Alliance. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020; 79:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examines outcomes and risk factors in 600 patients with rheumatic disease.
- 30.Gianfrancesco MA, Hyrich KL, Gossec L, et al. COVID-19 Global Rheumatology Alliance Steering Committee. COVID-19 Global Rheumatology Alliance Steering Committee Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol 2020; 2:e250–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winthrop KL, Brunton AE, Beekmann S, et al. COVID-19 Study Team. SARS CoV-2 infection among patients using immunomodulatory therapies. Ann Rheum Dis 2021; 80:269–271. [DOI] [PubMed] [Google Scholar]
- 32.Haberman RH, Castillo R, Chen A, et al. NYU WARCOV Investigators. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheumatol 2020; 72:1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. COVID-19 Global Rheumatology Alliance. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021; 80:930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article examines outcomes and risk factors in almost 4000 patients with rheumatic disease.
- 34.Schäfer M, Strangfeld A, Hyrich KL, et al. Response to: ‘Correspondence on ‘Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician reported registry” by Mulhearn et al. Ann Rheum Dis 2021; doi: 10.1136/annrheumdis-2021-220134 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Avouac J, Drumez E, Hachulla E, et al. FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021; 3:e419–e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi B, Choudhary MC, Regan J, et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med 2020; 383:2291–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gianfrancesco MA, Leykina LA, Izadi Z, et al. Race/ethnicity association with COVID-19 outcomes in rheumatic disease: data from the COVID-19 Global Rheumatology Alliance Physician Registry. Arthritis Rheumatol 2020; 73:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludvigsson JF, Axelrad J, Halfvarson J, et al. Inflammatory bowel disease and risk of severe COVID-19: A nationwide population-based cohort study in Sweden. United European Gastroenterol J 2021; 9:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attauabi M, Poulsen A, Theede K, et al. Prevalence and outcomes of COVID-19 among patients with inflammatory bowel disease-a Danish Prospective Population-based Cohort Study. J Crohns Colitis 2021; 15:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh AK, Jena A, Sharma Kumar-MP, et al. Risk and outcomes of coronavirus disease (COVID-19) in patients with inflammatory bowel disease: a systematic review and meta-analysis. United European Gastroenterol J 2020; 9:159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson PC, Richards D, Tanner HL, Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol 2020; 2:e653–e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson PC, Liew DFL, Liew JW, et al. The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med (N Y) 2020; 1:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan N, Mahmud N, Trivedi C, et al. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut 2021; doi: 10.1136/gutjnl-2021-324356 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baniandrés-Rodríguez O, Vilar-Alejo J, Rivera R, et al. BIOBADADERM Study Group. Incidence of severe COVID-19 outcomes in psoriatic patients treated with systemic therapies during the pandemic: a Biobadaderm cohort analysis. J Am Acad Dermatol 2021; 84:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a northern Italy experience. J Allergy Clin Immunol 2021; 147:558.e1–560.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Mahil SK, Dand N, Mason KJ, et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis - insights from a global registry-based study. J Allergy Clin Immunol 2021; 147:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]; COVID-19 outcomes in skin psoriasis.
- 47.Mahil SK, Yates M, Langan SM, et al. PsoProtect, CORE-UK study groups. Risk-mitigating behaviours in people with inflammatory skin and joint disease during the COVID-19 pandemic differ by treatment type: a cross-sectional patient survey. Br J Dermatol 2020; doi: 10.1111/bjd.19755 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology 2021; 96:e575–e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes R, Whitley L, Fitovski K, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord 2020; 49:102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camelo-Filho AE, Silva AMS, Estephan EP, et al. Myasthenia gravis and COVID-19: clinical characteristics and outcomes. Front Neurol 2020; 11:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Day AL, Winthrop KL, Curtis JR. The effect of disease-modifying antirheumatic drugs on vaccine immunogenicity in adults. Cleve Clin J Med 2020; 87:695–703. [DOI] [PubMed] [Google Scholar]
- 53.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021; doi: 10.1136/annrheumdis-2021-220289 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Deepak P, Kim W, Paley MA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv 2021. [Google Scholar]; This article describes the response to mRNA vaccines in patients with immune-mediated disease. [Preprint]
- 55.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021; 325:1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]