Purpose of review
The COVID-19 pandemic is a global public health crisis with considerable mortality and morbidity. A role for cytokine storm and therapeutic immunomodulation in a subgroup of patients with severe COVID-19 was proposed early in the pandemic. The concept of cytokine storm in COVID-19 has been criticised, given the lack of clear definition and relatively modest cytokinaemia (which may be necessary for viral clearance) compared with acute respiratory distress syndrome and bacterial sepsis. Here we consider the arguments for and against the concept of cytokine storm in COVID-19.
Recent findings
Several criteria have been proposed to identify the subgroup of COVID-19 patients exhibiting a cytokine storm. The beneficial effects of corticosteroids and interleukin-6 inhibition suggest that inflammation is a modifiable pathogenic component of severe COVID-19. The presence of genetic polymorphisms and pathogenic auto-autoantibodies in severe COVID-19 also suggests a significant contribution of immune dysregulation to poor outcomes.
Summary
Hyperinflammation is a key component of severe COVID-19, residing underneath the cytokine storm umbrella term, associated with poor outcomes. Better understanding of the aetiopathogenesis, with identification of biomarkers to predict treatment responses and prognosis, will hopefully enable a stratified and ultimately precision medicine approach.
Keywords: COVID-19, cytokine storm syndromes, hyperinflammation
INTRODUCTION
The COVID-19 pandemic is a major global public health crisis with considerable mortality and morbidity that has exposed complex clinical, scientific and philosophical challenges. The clinical spectrum of COVID-19 ranges from mild, self-limiting symptoms in the majority of cases, to its most severe form manifesting as acute respiratory distress syndrome (ARDS) with multiorgan system failure, the requirement for mechanical ventilation and high risk of death. Initial reports from Wuhan, China demonstrated that some patients with COVID-19 exhibited clinical deterioration at approximately day 10 following symptom-onset, in association with a declining viral load [1], leading to the hypothesis that pathology is driven by an overexuberant inflammatory response, rather than direct viral injury [2▪▪]. A biphasic model of COVID-19 was proposed [3], with an initial viraemic phase, followed by a host hyperinflammatory phase in a subgroup of patients with a self-amplifying, dysregulated immune response associated with high mortality. Despite paucity of data, at an early stage in the pandemic, we recommended evaluating for virally driven hyperinflammation, ‘cytokine storm syndrome’, in patients with severe COVID-19 and proposed that immunomodulation in this subgroup of patients might reduce the high mortality [2▪▪]. This was prompted by observations that predictors of fatality in COVID-19 included ferritin and interleukin (IL)-6 [4] and early reports of IL-6 inhibition with tocilizumab (off-label) demonstrating an efficacy signal. Despite global recommendations against the routine use of corticosteroids early in the COVID-19 pandemic due to worsening outcomes with previous pandemics (severe acute respiratory distress syndrome [SARS] and middle east respiratory syndrome) we found corticosteroids were being used at very high numbers with anecdotal benefits [5▪]. The urgent need for effective treatments to address the rising mortality and anecdotal benefits with immunosuppressive therapies provided impetus to accelerate clinical trials of immunomodulatory agents to target hyperinflammation at a remarkable pace. Following a series of randomized controlled trials demonstrating efficacy, dexamethasone (not formally approved for COVID-19) and tocilizumab (IL-6 blockade; not formally approved for COVID-19) are now standard of care in the treatment of severe COVID-19 and JAK1/2 inhibition with baricitinib (not formally approved for COVID-19) has emergency use authorization from the US FDA. Despite these results and correlative studies revealing immune activation, the concept of cytokine storm in COVID-19 continues to spur significant debate. Critics have questioned the definition of cytokine storm including the threshold levels of cytokines and raised concerns that the term may have distracted focus (e.g. from antiviral strategies or immune stimulants). Concerns were raised regarding the risks of immunosuppression with potential viral resurgence from a reservoir (albeit at low level) as well as super-infections in a heterogeneous patient population and that hypercytokinaemia may be a necessary physiological response required for antiviral immunity and viral clearance [6▪▪].
Here, we consider the features for and against the concept of COVID-19-cytokine storm and potential future directions that may help personalise disease management.
Box 1.
no caption available
OVERVIEW OF CYTOKINE STORM
Terminology of hyperinflammatory disorders has been the subject of much debate [7–9] even prior to the advent of COVID-19. Cytokine storm is an umbrella term encompassing several hyperinflammatory disorders of immune dysregulation characterized by constitutional symptoms, systemic inflammation, and multiorgan dysfunction that can lead to multiorgan failure and death if inadequately treated. These hyperinflammatory disorders include pathogen-induced, neoplasia-induced, monogenic, and iatrogenic causes. Two representative cytokine storm disease groups are haemophagocytic lymphohistiocytosis (HLH) and multicentric Castleman disease (MCD). HLH, usually manifesting with cytopenia and organ dysfunction, can occur due to genetic defects (primary HLH) or be triggered by infection, rheumatic disorders, and malignant disease (secondary HLH). The cytokine and chemokine storm often including IL-6 in MCD can occur due to uncontrolled infection with human herpesvirus-8 (HHV-8-associated MCD), a monoclonal plasma cell population (POEMS-associated MCD), or for an idiopathic cause (iMCD) [10]. Though these and other cytokine storm disorders share common clinical, immunological, and pathological abnormalities, treatments differ substantially. For example, extremely high IL-6 levels are found in CRS post-CAR-T cell therapy and IL-6 receptor blockade (tocilizumab) is highly effective in treating CART-CRS and iMCD, whereas anti-IL-1 is often preferred in HLH/MAS secondary to underlying rheumatic disease [8].
The aetiopathogenesis of cytokine storms is not fully understood but is thought to occur as a result of inappropriate recognition (e.g., autoinflammatory disorders) or ineffective recognition with immune evasion (e.g., EBV-associated HLH), an inappropriate response with an exaggerated effector response and cytokine production (e.g., chimeric antigen receptor [CAR] T cell therapy) or an ineffective response due to immune evasion (e.g., sepsis), or failure to return to homeostasis (e.g., primary HLH). With each of these triggers, there is a failure of negative feedback mechanisms (e.g., regulatory cell types, decoy receptors, anti-inflammatory cytokines) that are supposed to prevent hyperinflammation and the overproduction of inflammatory cytokines and soluble mediators, leading to multiorgan failure. Specific pathological cell types differ between cytokine storm disorders, but often include T cells, neutrophils, macrophages, and NK cells. Although a number of cytokines are elevated and signalling pathways are activated in these hyperinflammatory states, the effectiveness of Interferon-γ, IL-1, IL-6, TNF, IL-18, JAK, mTOR, and MAPK inhibitors suggest that they are central to pathogenesis [11,12,13▪▪].
Given the lack of a single formal definition of cytokine storm [6▪▪], and disagreement about how these disorders with an excessive, harmful immune response differ from an evolutionarily acceptable, appropriate inflammatory response in response to a pathogen, e.g. the severe acute respiratory coronavirus 2 (SARS-CoV-2), we recently proposed the following three criteria [13▪▪] for identifying a cytokine storm:
-
(1)
Elevated circulating cytokine levels
-
(2)
Acute systemic inflammatory symptoms
-
(3)
Either secondary organ dysfunction (often renal, hepatic, or pulmonary) due to inflammation beyond that which could be attributed to a normal response to a pathogen (if a pathogen is present), or any cytokine-driven organ dysfunction (if no pathogen is present).
Given the dearth of available evidence, this definition deliberately does not propose a specific threshold for elevations in cytokine levels above the normal range, and we do not recommend specific cytokine panels or mandate individual cytokines that are essential for diagnosis [13▪▪].
COVID-19 CLINICAL COURSE
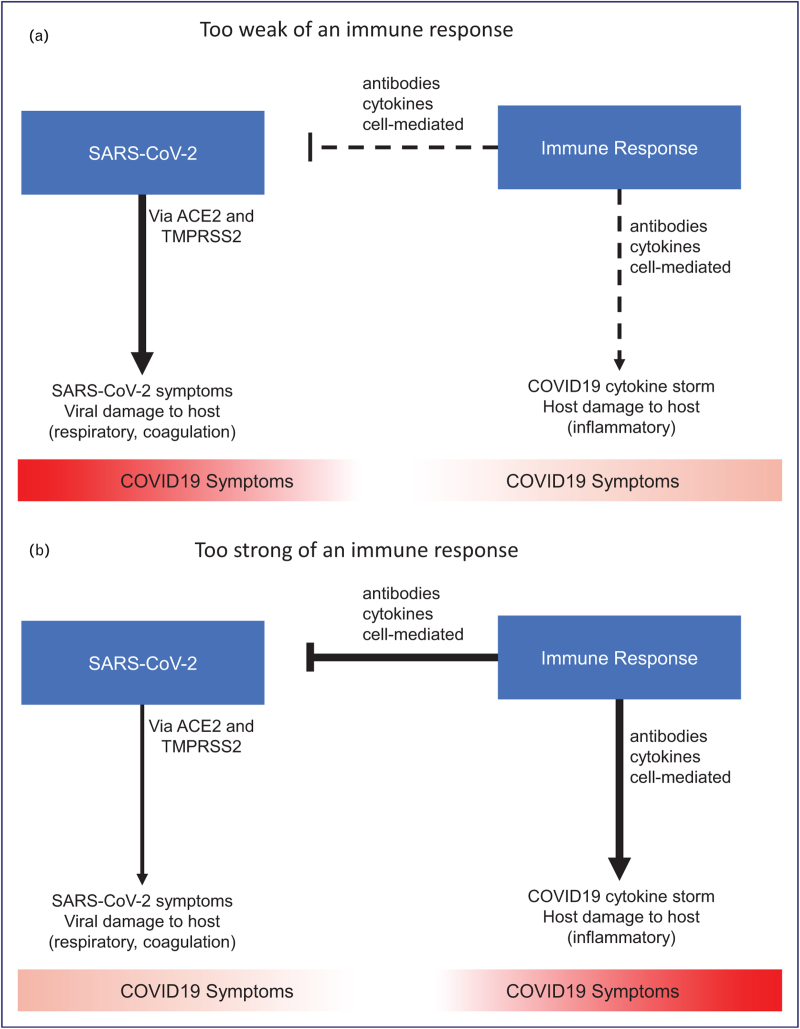
Infection with SARS-CoV-2 can result in a range of clinical courses from asymptomatic infection to a more classic respiratory infection to multiorgan system dysfunction that would meet our definition of cytokine storm. In Fig. 1 , we propose a framework for this heterogeneous host immune response vs virus interaction that is supported by data from randomised controlled trials.
FIGURE 1.
A framework for the heterogeneous host immune response in COVID-19. (A) In patients with too weak of an immune response, poorly controlled viral infection leads to direct SARS-CoV-2 related symptoms. (B) In patients with too strong of an immune response, viral damage is mitigated but antibodies, cytokines, and cell-mediated factors contribute to inflammatory symptoms. (C, D) The optimal response can require antivirals, neutralizing antibodies, and immune stimulants early in the disease course when patients may be mounting too weak of an immune response due to genetic factors or auto-antibodies against interferons. Alternatively, the optimal response may require antithrombotics and immunosuppressants late in the disease course when patients are mounting too strong of an immune response involving hyperinflammation and hypercoagulation. SARS-CoV-2, severe acute respiratory coronavirus 2.
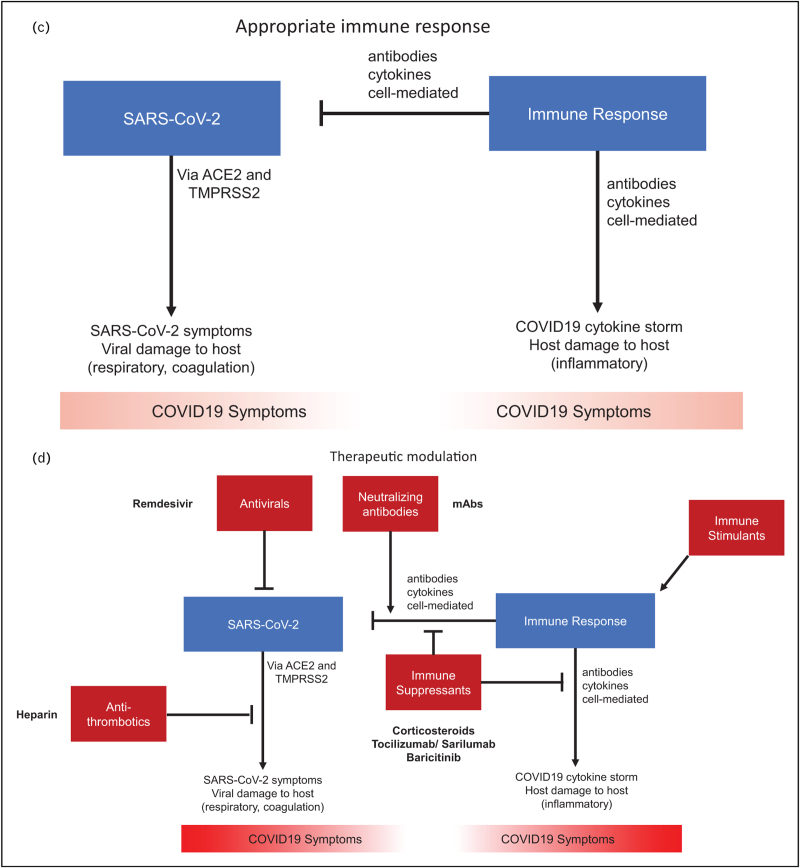
FIGURE 1 (Continued).
A framework for the heterogeneous host immune response in COVID-19. (A) In patients with too weak of an immune response, poorly controlled viral infection leads to direct SARS-CoV-2 related symptoms. (B) In patients with too strong of an immune response, viral damage is mitigated but antibodies, cytokines, and cell-mediated factors contribute to inflammatory symptoms. (C, D) The optimal response can require antivirals, neutralizing antibodies, and immune stimulants early in the disease course when patients may be mounting too weak of an immune response due to genetic factors or auto-antibodies against interferons. Alternatively, the optimal response may require antithrombotics and immunosuppressants late in the disease course when patients are mounting too strong of an immune response involving hyperinflammation and hypercoagulation. SARS-CoV-2, severe acute respiratory coronavirus 2.
CYTOKINE LEVELS IN COVID-19
One of the strongest sources of dissent regarding the concept of COVID-19 cytokine storm, relates to the only modest elevation in circulation of one specific cytokine out of hundreds: IL-6. A systematic review and meta-analysis of studies in patients with severe or critical disease, where IL-6 levels were recorded included 25 COVID-19 studies (n = 1245 patients) with comparator groups including four trials each in sepsis (n = 5320), CRS (n = 72) and ARDS unrelated to COVID-19 (n = 2767) [14]. In patients with severe or critical COVID-19, the pooled mean serum IL-6 concentration was 36.7 pg/mL (95% CI 21.6–62.3 pg/mL; I2 = 57.7%). Mean IL-6 concentrations were nearly 100 times higher in patients with cytokine release syndrome (3110.5pg/mL, 632.3–15,302.9 pg/mL; P < 0.0001), 27 times higher in patients with sepsis (983.6 pg/mL, 550.1–1758.4 pg/mL; P < 0.0001), and 12 times higher in patients with ARDS unrelated to COVID-19 (460 pg/mL, 216.3–978.7 pg/mL; P < 0.0001). Furthermore, a recent study demonstrated that only a small proportion of COVID-19 patients exhibited a cytokine profile considered by the authors to be consistent with cytokine storm and though several cytokines (including IL-6) were associated with mortality, the levels of these cytokines were in a similar range as patients with seasonal influenza [15]. However, data from iMCD demonstrate that the level of circulating IL-6 is not a reliable predictor of response to IL-6 inhibition with some patients with low IL-6 levels benefitting from siltuximab and others with very elevated IL-6 levels not responding [16,17].
Several studies have shown that patients with severe COVID-19 do indeed have elevated cytokine levels, meeting the proposed definition of cytokine storm syndromes. Longitudinal immunological correlates of disease outcomes demonstrated distinct signatures of ‘immunological misfiring’ in COVID-19 [18]. The immune profiles of patients with moderate disease (admitted to hospital who survived and did not require intensive care admission) were enriched with tissue reparative growth factors, such as epidermal growth factor, platelet-derived growth factor and vascular endothelial growth factor, with low expression of inflammatory cytokines, whereas patients with severe disease (those who died or required ICU admission) had highly elevated pro-inflammatory cytokines including IL-1α, IL-1β, IL-6, IL-18 and TNF [18]. Analysis from 471 hospitalised patients and 39 outpatients with mild disease demonstrated IL-6, granulocyte colony stimulating factor (GM-CSF) and CXCL10 was associated with severity and accompanied by elevated markers of endothelial injury and thrombosis [19]. Principal component network analysis demonstrated central roles for IL-6 and GM-CSF in COVID-19 pathogenesis. Interestingly comparison with historical influenza samples, showed that IL-6 was equally elevated in both conditions, whereas GM-CSF was prominent only in COVID-19 [19]. Surprisingly, investigational cytokine removal with CytoSorb led to worsening outcomes in critically ill patients on extracorporeal membrane oxygenation (ECMO) in a small randomized controlled trial, though the timing of initiation and removal of anti-inflammatory and other regulatory factors may have contributed to worsening outcomes [20].
It is important to remember that circulating cytokine levels can be difficult to measure because cytokines have short half-lives, systemic levels may not accurately reflect local microenvironment, tissue concentrations (e.g. the pulmonary compartment), and measurements may not be easily obtained worldwide in real-time, due to the high costs and slow turn-around time for results [21▪].
HYPERINFLAMMATION IN COVID-19
Critically ill patients with COVID-19 often demonstrate features suggestive of cytokine storm, including fever, raised inflammatory markers and ARDS. However, the hyperinflammatory response in severe COVID-19 appears to be a unique and distinct entity and typically does not meet the classification criteria developed for MAS or HLH. The HScore (which generates a probability for the presence of HLH [22]) has poor diagnostic utility in COVID-19-cytokine storm [23–25]. Although ferritin levels predict mortality in COVID-19 [4], ranges are lower than those reported in patients with secondary HLH, and the clinical syndrome in severe COVID-19 is lung-dominant without splenomegaly, typically without hypofibrinogenaemia, substantial derangements in liver function or cytopenias [26]. Of note, lymphopenia is almost universal in patients with severe COVID-19 [27], but the lymphocyte lineage is not classically affected in secondary HLH. In the context of COVID-19, therefore, lymphopenia might be the outcome of a viral driver or due to lung infiltration. Bone marrow haemophagocytosis, which is often reported in HLH, has also been seen in nonsurvivors of COVID-19 but it is unclear as to whether the haemophagocytosis should be expected in the context of critical illness or directly attributable to a hyperinflammatory state.
Several observational studies have aimed to develop criteria to identify a hyperinflammatory endotype associated with poor outcome in COVID-19. The currently published scoring systems include the validated Temple [28▪], COVID-19-associated hyperinflammatory syndrome (cHIS) [29▪], and COVID-19-associated hyperinflammation (COV-HI) criteria [30▪]. They differ in complexity and the number and thresholds of laboratory indices employed (Table 1). The Temple criteria used univariate logistic regression to identify variables associated with COVID-CS and then principal components analysis to find predictors that clustered together, followed by an iterative computational algorithm to define optimal cut-off values [28▪]. Ferritin and CRP did not add predictive power but were included in the final criteria per expert preference. The final model included essential entry criteria of confirmed COVID-19, ground glass opacities on chest imaging (computed tomography [CT] or radiograph), ferritin > 250ng/mL and CRP > 4.6 mg/dL; and [3] one feature from each cluster: cluster I (low albumin, low lymphocytes, high neutrophils), and cluster II (elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), D-dimer, LDH, troponin I), and cluster 3 (low anion gap, high chloride, high potassium, high blood urea nitrogen: creatinine ratio). Of 513 inpatients, 173 met these criteria (34%) and demonstrated far less favourable outcomes – a greater length of hospital stay (15.1 vs 5.7 days) and higher mortality (28.8% vs 6.6%). Differences were even more marked in the validation cohort and may have been higher without the use of steroids.
Table 1.
Classification criteria for COVID-cytokine storm
| Temple [28▪] | cHIS [29▪] | COV-HI [30▪] | |
| Sample size (n) | |||
| Derivation cohort | 513 | 299 | 269 |
| Validation cohort | Yes (258) | Yes | No |
| Clinical/Imaging | |||
| Fever | – | >38 °C | – |
| Ground-glass opacities chest imaging | CT (or X-ray)∗ | – | – |
| Laboratory | |||
| Ferritin (ng/mL) | >250∗ | 700 | >1500 |
| CRP (mg/L) | >46∗ | ≥150 | >150 |
| IL-6 (pg/mL) | – | ≥15 | – |
| Triglyceride (mg/dL) | – | ≥150 | – |
| Neutrophil: lymphocyte ratio | – | ≥10 | – |
| Hb (g/dL) | – | ≤9.2 | – |
| Platelets (×109 cells/L) | – | ≤110 | – |
| d-dimer (μg/ml) | >4.9 | ≥1.5 | – |
| LDH (U/L) | >416 | ≥400 | – |
| Aspartate transaminase (AST) (U/L) | >87 | ≥100 | – |
| Alanine transaminase (ALT) (U/L) | >60 | – | |
| Troponin I (ng/mL) | >1.09 | – | – |
| Albumin (g/dl) | <2.8 | – | – |
| Lymphocytes (%) | <10.2 | – | – |
| Neutrophil Abs (K/mm3) | >11.4 | – | – |
| Anion gap (nmol/L) | <6.8 | – | – |
| Chloride (nmol/L) | >106 | – | – |
| Potassium (nmol/L) | >4.9 | – | – |
| Blood urea nitrogen: creatinine ratio | >29 | – | – |
| Fulfilment of criteria | |||
| Interpretation: | ∗Entry criteria (orange) ground glass opacities on CT chest (or radiograph) AND elevated ferritin and CRP) with ≥1 variable from each of 3 clusters: cluster 1 (low albumin, low lymphocytes, high neutrophils); cluster 2 (elevated alanine aminotransferase, aspartate aminotransferase, D-dimer, LDH, troponin I); cluster 3 (low anion gap, high chloride, high potassium, high blood urea nitrogen:creatinine ratio). | ≥2 from 6 criteria encompassing fever, macrophage activation, haematological dysfunction, coagulopathy, hepatic injury and cytokinaemia (including CRP concentration) | ≥1 of CRP > 150 mg/L (or daily doubling from > 50 mg/L) or Ferritin > 1500 μg/L |
cHIS, COVID-19-associated hyperinflammatory syndrome; CT, computed tomography.
Table showing three studies aiming to define subgroups of COVID-19 patients with associated cytokine storm/hyperinflammation and worsening outcomes, including essential (asterix) and possible (bold) criteria. The Temple criteria includes essential criteria (ground glass opacities on chest imaging, elevated ferritin and CRP) with one or more of the following criteria from three clusters. Cluster 1 (low albumin, low lymphocytes, high neutrophils); cluster 2 (elevated alanine aminotransferase, aspartate aminotransferase, D-dimer, LDH, troponin I); cluster 3 (low anion gap, high chloride, high potassium, high blood urea nitrogen:creatinine ratio). The cHIS criteria requires at least two of 6 criteria encompassing fever, macrophage activation, haematological dysfunction, coagulopathy, hepatic injury and cytokinaemia (including CRP concentration). The COV-HI criteria requires either an elevated CRP or ferritin.
Clinical criteria for cHIS includes a six criterion additive scale of fever, macrophage activation (hyperferritinaemia), haematological dysfunction (neutrophil to lymphocyte ratio), hepatic injury (lactate dehydrogenase or AST), coagulopathy (D-dimer), and cytokinaemia (CRP, IL-6, or triglycerides) [29▪]. In total, 161 (54%) of 299 patients met > 2 cHIS criteria during their hospital admission; these patients had an increased risk of mortality (odds ratio 1.6 [95% CI 1.2–2.1], P = 0.0020) and requirement of invasive mechanical ventilation (odds ratio 4.3 [3.0–6.0], P < 0.0001). The cHIS score also correlated with severity of oxygen requirement and risk for clinical deterioration. The COVID-19-associated hyperinflammation (COV-HI) criteria is a more simple score, based on measurement of C-reactive protein (≥150 mg/L, or a doubling in 24 h from > 50 mg/L) and ferritin (>1500 μg/L) [30▪]. In total, 90 of 269 (33%) patients met the COV-HI criteria at admission. Despite having a younger median age and fewer comorbidities, patients with this phenotype had higher mortality (36 [40%] of 90 patients) than patients without the phenotype (46 [26%] of 179) during the 28-day follow-up period, and meeting the criteria was associated with an increased next-day risk of death or need for escalated respiratory support (combined endpoint; hazard ratio 2.24 [95% CI 1.62–2.87]), after adjustment for age, sex and comorbidity.
Taken together these studies demonstrate existence of subgroups of COVID-19 patients exhibiting hyperinflammation that are associated with poor outcomes ([28▪,29▪,30▪], however the criteria used to define hyperinflammation are widely variable, e.g., the ferritin thresholds range from 250 to 1500 ng/mL (Table 1) and the Temple criteria includes markers of inflammation, systemic cell death, multiorgan tissue damage and electrolyte imbalance, whereas the COV-HI criteria focus only on inflammatory markers. The definitions of hyperinflammation were somewhat arbitrarily defined by either expert consensus [28▪,30▪], or literature review [29▪], which was limited by the paucity of data available at the time, potentially introducing confirmation bias. The vast majority of patients in the Temple cohort received steroids, which makes indirect comparisons of prevalence and outcomes difficult. Independent validation of these criteria in other cohorts is necessary, however will be challenging given that standard of care definitions have changed over time, by virtue of the accrual of clinical experience, improved supportive care and advances in background therapies (widespread use of dexamethasone and IL-6 blockade). Interestingly a recent report demonstrated that patients with systemic rheumatic disease hospitalised with COVID-19 had increased risk for hyperinflammation compared with matched comparators [31]. The cHIS criteria identified patients with hyperinflammation associated with poor outcomes in both patients with systemic rheumatic disease and comparators [31].
IMMUNOMODULATORY TREATMENT IN COVID-19
Improvement in COVID-19 outcomes with selective cytokine blockade and immunosuppressive agents further supports the pathologic role of excessive cytokines and the existence of a cytokine storm in a portion of patients. However, lack of treatment response does not always refute a cytokine storm, because the efficacy of treatments is likely to depend on a number of factors including patient selection, dosing, and timing of intervention, as illustrated by the swinging pendulum of ‘positive’ and ‘negative’ efficacy trials of IL-6 blockade in COVID-19 [32]. Further support for this concept comes from trends towards worsening outcomes with administration of dexamethasone too early in the disease course [33] as well as administration of intravenous interferon beta-1a late in the disease course [34].
Corticosteroids and IL-6 blockade (tocilizumab or sarilumab; not formally approved for use in COVID-19) are now standard of care in patients with severe COVID-19 and JAK1/2 inhibition with baricitinib has emergency use authorization in the United States based on randomized controlled trials, supporting the concept of COVID-19 cytokine storm. However, the therapeutic implications of elevated inflammatory markers are unknown and posthoc subgroup analyses of these large trials are eagerly anticipated. It is tempting to speculate prediction of treatment response may be possible using criteria for COVID-hyperinflammation. An observational study suggested that patients with COVID-19 have a good response to glucocorticoids when the CRP level is high but a poor response when the level is low [35]. However, the prespecified CRP subgroup analysis in the REMAP-CAP trial, suggested a similar effect across all CRP terciles and did not support a differential treatment effect of IL-6 blockade by baseline CRP levels, compared with the placebo arm [36].
Whilst corticosteroids undoubtedly reduce mortality in severe COVID-19, it is intriguing that the doses required (6 mg daily dexamethasone dosage equivalent to approximately 40 mg oral prednisolone) are far lower than the doses usually required for cytokine storm, suggesting that the hyperinflammatory component of severe COVID-19 is different from other cytokine storms or that further benefit could be gained with increased dosing. An 86-patient randomized controlled trial found that a weight-adjusted course of methylprednisolone (2 mg/kg/day) was superior to the relatively lower 6 mg/day of dexamethasone [37]. The suggestion of potential harm with corticosteroids in patients not requiring supplemental oxygen [38] and an optimal ‘window of opportunity’ for inhibiting IL-6 [32] (in addition to corticosteroids) suggests that combination immunomodulation may be of benefit at a late stage in patients with severe disease. This is consistent with the widely accepted biphasic model of COVID-19 with an initial viraemic phase followed by hyperinflammatory phase, in which immunostimulation that enhances antiviral activity is helpful early (and probably harmful late) in the disease course, whereas immunosuppression is helpful late and harmful early [13▪▪]. In keeping with this theory, publications related to nebulised interferon-beta [39] and not yet published reports about inhaled or recombinant GM-CSF suggest they may be of benefit, especially early in the disease, whereas blockade with monoclonal antibodies directed against GM-CSF (targeting the ligand or its receptor) may be beneficial late in the disease course [40▪].
OTHER MECHANISMS INFLUENCING SEVERITY IN COVID-19
Although SARS-CoV-2 infections in children are generally mild and nonfatal, a paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), also known as multisystem inflammatory syndrome in children (MIS-C) or paediatric multisystem inflammatory syndrome can lead to serious illness and long-term side-effects. Pathophysiology of MIS-C/PIMS-TS is still unclear and possible mechanisms include antibody or T-cell recognition of self-antigens (viral molecular mimicry of the host) resulting in autoantibodies, antibody or T-cell recognition of viral antigens expressed on infected cells, formation of immune complexes which induce inflammation, and viral superantigen sequences which activate host immune cells [41]. Patients with MIS-C/PIMS-TS are effectively treated with immunomodulatory therapies, such as intravenous immunoglobulin, glucocorticoids and therapeutic blockade of IL-1 and IL-6. Patients with MIS-C/PIMS-TS meet the criteria of cytokine storm, however this is a very distinct hyperinflammatory disorder from severe COVID-19.
ARDS is a leading cause of mortality in COVID-19. In patients with ARDS from any cause, two distinct phenotypes have already been defined that can be identified using a model involving clinical and biomarker parameters [42]: a hyperinflammatory phenotype (characterised by elevated proinflammatory cytokines, increased incidence of shock, and higher mortality) [43] and a hypoinflammatory phenotype [44]. Using this model, the hyperinflammatory subphenotype of ARDS was less prevalent in patients with COVID-19 ARDS, compared with non-COVID ARDS [45]. In patients with COVID-19 ARDS, the mortality was higher in patients with the hyperinflammatory subphenotype (63%) compared with the hypoinflammatory subphenotype (39%) [45]. Although this pattern was expected, the mortality in both groups overall was higher than expected when compared with non-COVID ARDS data, suggesting that there may be additional factors in COVID-19 accounting for high mortality, other than a cytokine storm alone, but also suggesting that a cytokine storm is unlikely to be specific only to COVID-19, and the host hyperinflammatory response is influential in ARDS from other causes as well. However, it is important to remember that the vast majority of cytokine-directed or immunosuppressive agents have failed to demonstrate an effect in non-COVID ARDS, so this may not be a clinically relevant comparison or may demonstrate the need for stratified and precision medicine approaches in non-COVID ARDS in order to target the subphenotype most likely to benefit [46]
Coexisting conditions such as hypertension, diabetes, and obesity are associated with more severe cases of COVID-19, possibly because of the preexisting chronic inflammatory state or a lower threshold for the development of organ dysfunction from the immune response [13▪▪]. Other host factors, including genetic variation may also impact COVID-19 severity. IFN down-regulation may increase vulnerability to viral infections and autoantibodies against IFN may dampen the host antiviral response to prevent damage from pathogen-induced inflammation. Genome-wide association in 2,444 patients with COVID-19 identified polymorphisms associated with critical illness, including interferon (IFN) pathway genes IFNAR2 and OAS1/2/3, suggesting increased susceptibly to viral infections and impaired host defence [47]. Mutations in genes involved in the regulation of type I and III IFN immunity were enriched in patients with severe COVID-19, using a candidate gene approach [48]. The importance of interferons in the immune response against COVID-19 is also reflected in the striking finding of autoantibodies against type I interferons (mostly against IFN-α2 and IFN-ω, largely showing neutralising capacity in vitro) in 135 (13.7%) of 987 patients with life-threatening COVID-19 [49▪]. These antibodies were only detected in 4 (0.3%) of 1227 unexposed, healthy individuals [49▪]. Additionally, single-cell transcriptional profiling revealed profound suppression of interferon signalling among patients with COVID-19 compared with seasonal influenza [15]. Finally, a recent study found that interferon-stimulated gene expressing cells were systemically absent in patients with severe COVID-19 compared to mild disease [50]. Paradoxically, patients with severe COVID-19 produced very high levels of anti-SARS-CoV-2 antibodies and had a lower viral load, but they also produced antibodies that functionally blocked the production of the ISG-expressing cells associated with mild disease. Another study that screened 194 patients with COVID-19 for autoantibodies against 2,770 extracellular and secreted proteins (members of the exoproteome) found that COVID-19 patients exhibited marked increases in autoantibody reactivities, particularly immunomodulatory proteins (including cytokines, chemokines, complement components and cell-surface proteins) as compared to uninfected individuals [51]. Interestingly murine surrogates of these autoantibodies increased disease severity in a mouse model of SARS-CoV-2 infection [51]. These autoantibodies likely contribute to pathogenesis through a variety of mechanisms including impairing virological control by inhibiting immunoreceptor signalling and stimulating antibody-mediated inflammation. Thus, efforts to eliminate pathological autoantibodies may be a promising approach to prevent severe COVID-19
CONCLUSION AND FUTURE DIRECTIONS
The pathomechanistic hypothesis of COVID-19 involving a cytokine storm has stimulated an extraordinary degree of thinking, discussion and research. The term ‘cytokine storm’ has provoked some controversy, given the lack of clear definition until recently. It is now widely accepted that in a subgroup of patients with severe COVID-19 there is an exuberant inflammatory response, triggered by an initial viral insult, resulting in significant secondary organ dysfunction that can be averted in a portion of patients with targeted anti-cytokine or immunosuppressive therapies. Severe COVID-19 is likely to reside under the cytokine storm umbrella, possibly as a distinct entity. It is now clear that the host inflammatory responses contributing to lung injury in COVID-19 are complex and that conventional criteria (e.g. the H-score) for classical, established cytokine storm syndromes like HLH/MAS perform poorly to identify COVID-19-associated hyperinflammation. In Table 2, we present the evidence for and against severe COVID-19 involving a cytokine storm.
Table 2.
Evidence for and against severe COVID-19 involving a cytokine storm
| FOR | AGAINST | |
| Cytokine levels | Cytokine levels (e.g IL-6, GM-CSF) are elevated in severe COVID-19 and increasing levels are strongly associated with worsening outcomes There are increased frequencies of circulating activated CD4+ and CD8+ T cells and plasmablasts in severe COVID-19 | The elevated cytokines and activated immune cells in severe COVID-19 may be necessary for controlling SARS-CoV-2 infection The levels of several cytokines are only modestly elevated in COVID-19, relative to ARDS, sepsis, CART-CRS, and influenza |
| Clinical and Laboratory features | Clinical and lab abnormalities, such as elevated CRP and d-dimer levels, hypoalbuminemia, renal dysfunction, and effusions, are observed in COVID-19, as they are in other cytokine storms | These clinical and laboratory abnormalities can appear in an appropriate robust immune response to a pathogen Lymphopenia is not often found in cytokine storm disorders, but it is a hallmark of severe COVID-19 |
| Classification criteria | Severe COVID-19 patients demonstrate all three features of cytokine storm (13): elevated circulating cytokines, acute inflammatory symptoms, and organ dysfunction secondary to hyperinflammation New classification criteria have been proposed that are associated with hyperinflammation and worsening outcomes: Temple (28), COVID-19-associated hyperinflammatory syndrome (cHIS)(29), and COVID-19-associated hyperinflammation (COV-HI) (30) | Conventional criteria for cytokine storm observed in HLH perform poorly in COVID-19 (e.g. H score) |
| Treatment | Immunomodulation (Corticosteroids and IL-6 inhibition) can reduce mortality in severe COVID-19, suggesting that excess inflammation is a modifiable pathogenic component of severe COVID-19 Additional immunomodulators including JAK1/2 inhibitors have demonstrated a potential role in severe COVID-19 | Cytokine removal with CytoSorb led to worsening outcomes in critically ill patients on extracorporeal membrane oxygenation (ECMO) |
| Other host factors | Increased SARS-CoV-2 specific antibodies and decreased viral loads are found in patients with severe COVID-19 Longitudinal immunological correlates of disease outcomes have demonstrated distinct signatures of ‘immunological misfiring’ in COVID-19 | Other host factors also have significant contribution to poor outcomes in severe COVID-19, including chronic illness comorbidities, thromboembolic events, genetic polymorphisms and auto-autoantibodies directed against interferons and other proteins. |
SARS-CoV-2, severe acute respiratory coronavirus 2.
Patients with severe COVID-19 have a distinct immunopathology that resembles hyperinflammation. Using single parameter thresholds (e.g. IL-6 or CRP alone) or response to immunomodulation to confirm or refute the presence of cytokine storm is perhaps too reductionistic. Accumulating data will hopefully enable the generation of multivariate, composite prognostic models, incorporating routinely available biomarkers (e.g. CRP) with clinical variables (e.g. oxygen saturations) and cytokine/chemokine panels (e.g. IL-6, CXCL-9) to enable prognostic scoring and identification of optimal treatment approaches with strong predictions of response [52]. There has been some progress in prognostic biomarker discovery from the first wave of the pandemic, but attention now needs to turn to predictive biomarkers, with the advent of more immunomodulatory treatment options on the horizon (e.g. janus kinase inhibition). Soluble urokinase plasminogen activator receptor (suPAR) is emerging as a potential candidate companion biomarker to predict responses to IL-1 receptor antagonism with anakinra (not formally approved for COVID-19, but used in HLH [53▪]) in COVID-19 [54].
The beneficial effect of corticosteroids [33,55], IL-6 receptor antagonists [36], and recent reports with JAK inhibition (tofacitinib, JAK1/3 inhibitor, not formally approved for COVID-19 [56]) suggest that inflammation is a modifiable component of COVID-19 pathogenesis. However, there is spectrum of clinical phenotypes with differential responses to immunomodulatory therapy. The pattern, severity and mechanism of the hyperinflammatory state in COVID-19 and influence on endothelial activation and the hypercoagulable state and thrombotic outcomes is still unclear [57], as is the potential impact of hyperinflammation in the acute phase on complications in the convalescent phase, during which patients may have persistent symptoms (‘long-COVID’ or ‘post-COVID syndrome’). It is unclear if the widescale use of immunomodulation in the acute phase and roll-out of COVID-19 vaccination will modulate the occurrence or presentation of hyperinflammation in COVID-19.
During a rapidly evolving global pandemic, it is important to maintain measured clinical and scientific equipoise. Hypotheses and research questions are intended to stimulate and refine thinking and research. The concept of COVID-19 cytokine storms or hyperinflammation is now widely accepted, a hypothesis proposed during the early data-deprived stage of the pandemic. However, despite the considerable progress at rapid pace, there still remain multiple unknowns and continuing data collection and bioregistries are imperative.
COVID-19 has brought global attention to the concept of cytokine storms. Although there has been progress in understanding the mechanistic basis for the imitation and propagation of cytokine storm syndromes, there remains a considerable unmet need for effective therapies. We are continuing to advance the CORONA Project (www.CDCN.org/CORONA) to identify and advance the most promising treatments for COVID-19. Better understanding of the aetiopathogenesis, and identification of biomarkers to predict treatment response and prognosis, will hopefully enable a stratified and ultimately precision medicine treatment approach.
Acknowledgements
None.
Author Contribution: P.M. and D.C.F. co-wrote the manuscript. Both authors contributed to discussions, revised and approved the manuscript.
Financial support and sponsorship
The Parker Institute for Cancer Immunotherapy (D.C.F.).
Conflicts of interest
P.M. is a Medical Research Council (MRC) – GlaxoSmithKline (GSK) Experimental Medicine Initiative to Explore New Therapies (EMINENT) clinical training fellow with project funding outside the submitted work. P.M. receives co-funding by the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre (UCLH BRC). P.M. reports consultancy fees from Lily, SOBI, Pfizer and Abbvie, outside the submitted work. D.C.F. has received research funding from Janssen Pharmaceuticals and EUSA Pharma as well as study drug from Pfizer for a clinical trial in Castleman disease. D.C.F. has received research funding from the Parker Institute for Cancer Immunotherapy for COVID-19 therapeutic identification. D.C.F. holds two provisional pending patents for the diagnosis and treatment of Castleman disease.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis 2020; 20:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪▪.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; To our knowledge the first proposals for considering cytokine storm in COVID-19.
- 3.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪.Fajgenbaum DC, Khor JS, Gorzewski A, et al. Treatments administered to the first 9152 reported cases of COVID-19: a systematic review. Infect Dis Ther 2020; 9:435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]; A good summary of treatments administered early in the pandemic.
- 6▪▪.Sinha P, Matthay MA, Calfee CS. Is a ‘cytokine storm’ relevant to COVID-19? JAMA Intern Med 2020; 180:1152–1154. [DOI] [PubMed] [Google Scholar]; A narrative regarding the arguments against cytokine storm in COVID-19.
- 7.Nikiforow S, Berliner N. To ‘lump’ or to ‘split’ in macrophage activation syndrome and hemophagocytic lymphohistiocytosis. Arthritis Rheumatol 2019. [DOI] [PubMed] [Google Scholar]
- 8.Mehta P, Cron RQ, Hartwell J, et al. Intravenous anakinra for cytokine storm syndromes - authors’ reply. Lancet Rheumatol 2020; 2:e522–e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigrovic PA. COVID-19 cytokine storm: what is in a name? Ann Rheum Dis 2021; 80:3. [DOI] [PubMed] [Google Scholar]
- 10.Pierson SK, Stonestrom AJ, Shilling D, et al. Plasma proteomics identifies a ’chemokine storm’ in idiopathic multicentric Castleman disease. Am J Hematol 2018; 93:902–912. [DOI] [PubMed] [Google Scholar]
- 11.Pai RL, Japp AS, Gonzalez M, et al. Type I IFN response associated with mTOR activation in the TAFRO subtype of idiopathic multicentric Castleman disease. JCI Insight 2020; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajgenbaum DC, Langan RA, Japp AS, et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6-blockade-refractory idiopathic multicentric Castleman disease. J Clin Invest 2019; 129:4451–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪▪.Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 2020; 383:2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of cytokine storms.
- 14.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020; 8:1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mudd PA, Crawford JC, Turner JS, et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv 2020; 6:eabe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casper C, Chaturvedi S, Munshi N, et al. Analysis of inflammatory and anemia-related biomarkers in a randomized, double-blind, placebo-controlled study of siltuximab (Anti-IL6 Monoclonal Antibody) in patients with multicentric castleman disease. Clin Cancer Res 2015; 21:4294–4304. [DOI] [PubMed] [Google Scholar]
- 17.Morra DE, Pierson SK, Shilling D, et al. Predictors of response to anti-IL6 monoclonal antibody therapy (siltuximab) in idiopathic multicentric Castleman disease: secondary analyses of phase II clinical trial data. Br J Haematol 2019; 184:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol 2021; 6:eabg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Supady A, Weber E, Rieder M, et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med 2021; 9:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; An overview of cytokine storms in COVID-19.
- 22.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014; 66:2613–2620. [DOI] [PubMed] [Google Scholar]
- 23.Leverenz DL, Tarrant TK. Is the HScore useful in COVID-19? Lancet 2020; 395:e83–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood H, Jones F, Hui K, et al. Secondary HLH is uncommon in severe COVID-19. Br J Haematol 2020; 190:e283–e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenz G, Moog P, Bachmann Q, et al. Title: Cytokine release syndrome is not usually caused by secondary hemophagocytic lymphohistiocytosis in a cohort of 19 critically ill COVID-19 patients. Sci Rep 2020; 10:18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cron RQ, Chatham WW. The rheumatologist's role in Covid-19. Br J Haematol 2020. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.Caricchio R, Gallucci M, Dass C, et al. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis 2021; 80:88. [DOI] [PubMed] [Google Scholar]; Scoring criteria for defining COVID-19-associated hyperinflammation.
- 29▪.Webb BJ, Peltan ID, Jensen P, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol 2020; 2:e754–e763. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scoring criteria for defining COVID-19-associated hyperinflammation.
- 30▪.Manson JJ, Crooks C, Naja M, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scoring criteria for defining COVID-19-associated hyperinflammation.
- 31.Hsu TY, D'Silva KM, Patel NJ, et al. Laboratory trends, hyperinflammation, and clinical outcomes for patients with a systemic rheumatic disease admitted to hospital for COVID-19: a retrospective, comparative cohort study. Lancet Rheumatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angriman F, Ferreyro BL, Burry L, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir Med 2021; 9:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2020; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. N Engl J Med 2020; 384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med 2020; 15:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis 2021; 21:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Mehta P, Chambers RC, Dagna L. Granulocyte-macrophage colony stimulating factor in COVID-19: friend or foe? Lancet Rheumatol 2021; 3:e394–e395. [DOI] [PMC free article] [PubMed] [Google Scholar]; A summary of the challenges encountered during clinical trials in COVID-19, focusing on the complexity of blocking or administering GM-CSF.
- 41.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020; 20:e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha P, Delucchi KL, McAuley DF, et al. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med 2020; 8:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy K, Sinha P, O’Kane CM, et al. Subphenotypes in critical care: translation into clinical practice. Lancet Respir Med 2020; 8:631–643. [DOI] [PubMed] [Google Scholar]
- 44.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha P, Calfee CS, Cherian S, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med 2020; 8:1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care 2020; 24:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pairo-Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021; 591:92–98. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020; 370:eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study demonstrating anti-interferon antibodies in COVID-19.
- 50.Combes AJ, Courau T, Kuhn NF, et al. Global absence and targeting of protective immune states in severe COVID-19. Nature 2021; 591:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021. [DOI] [PubMed] [Google Scholar]
- 52.Chen LYC, Hoiland RL, Stukas S, et al. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J 2020; 56:2003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Mehta P, Cron RQ, Hartwell J, et al. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol 2020; 2:e358–e367. [DOI] [PMC free article] [PubMed] [Google Scholar]; A viewpoint with a comprehensive background on the terminology of cytokine storms.
- 54.Kyriazopoulou E, Panagopoulos P, Metallidis S, et al. Anakinra to prevent respiratory failure in COVID-19. medRxiv 2020; 2020.10.28.20217455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta P, Haskard DO, Laffan MA, et al. Thromboses and COVID-19: reducing inflammation in addition to thromboprophylaxis. Lancet Rheumatol 2021; 3:e171–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]