Abstract
Introduction
Pseudomonads constitute critical agents of opportunistic infections in hospital settings particularly in immunocompromised patients and Pseudomonas aeruginosa is a major flagship member of these infectious agents. This study assessed the distribution of Pseudomonas spp. associated with infections in patients and their antibiotic resistance patterns as part of an antibiotic stewardship intervention program and resistance surveillance.
Methods
One hundred and fifty Pseudomonas spp. from different clinical specimens were obtained from the Obafemi Awolowo University Teaching Hospitals Complex Ile-Ife. Culture was carried out on MacConkey and blood agar while phenotypic characterization was done by Gram staining, oxidase, and catalase test. Species identification was done using MICROBACTTM 24E bacterial identification kit and confirmed by 16S rDNA polymerase chain reaction (PCR) assay. Antibiotic susceptibility testing to eight antibiotics in four classes was done.
Results
Pseudomonas aeruginosa was the most frequently occurring species (96.0%); P. putida (2.67%) and P. fluorescens (0.67%) were also identified as well as an isolate of Burkholderia pseudomallei (0.67%). The highest resistance rate among isolates was observed towards gentamicin (35.4%); piperacillin/tazobactam was the most active antibiotic. Multidrug-resistant (MDR) strains constituted 12.8% of the isolates and most MDR strains also displayed a high multiple antibiotic resistance index (MAR).
Conclusions
Pseudomonas aeruginosa is emerging as a highly MDR pathogen in our hospital setting. This calls for the establishment of a surveillance system and antimicrobial stewardship programme in place. Furthermore, we propose a review of the current antibiotics prescription policy, and infection control programmes (ICPs) if we must control the spread of MDR-P. aeruginosa in this environment.
Keywords: Pseudomonas spp., multiple antibiotic resistance index (MAR), multidrug resistance (MDR), antibiotics stewardship
Introduction
Pseudomonas species represent a metabolically versatile and ubiquitous class of organisms naturally found in soil, water, and vegetation. They are not considered as part of the normal flora of humans and are often implicated in opportunistic infections.1 Pseudomonas aeruginosa, a flagship member of Pseudomonas spp. is one of the main opportunistic pathogens involved in nosocomial infections globally, particularly in immunocompromised patients due to a combination of intrinsic and acquired mechanisms used to develop resistance to all effective antibiotics.2
Pseudomonas spp. exhibits an ever-growing multidrug-resistance that cuts across fluoroquinolones, aminoglycosides, third and fourth generation cephalosporins and advanced beta-lactams and was assigned to a serious level of threat as a result of this observation.3,4 Emergence of multidrug resistant (MDR) Pseudomonas aeruginosa is a serious healthcare challenge with significant morbidity and mortality worldwide.5 Infections caused by MDR pathogens require timely and apposite therapy to improve patients' survival.6
Pseudomonas aeruginosa infection is usually severe, with high mortality rates in hospitalized patients with cancer, cystic fibrosis, and burns.7 It is accountable for about 10% of all nosocomial infections and considered as one of the most critical agents of Gram-negative bacterial infections with reports of increasing antibiotic resistance worldwide.8
There is a dearth of data on the magnitude of this problem for developing countries; hence, this study was done to assess the distribution of Pseudomonas spp. associated with infections in patients and their antibiotic resistance surveillance patterns with a view on antibiotic stewardship intervention to control the spread of MDR pseudomonads in our hospital setting.
Methods
Study area, sample size and ethical approval
This cross-sectional study was undertaken at Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC) Ile-Ife and Department of Medical Microbiology and Parasitology, Obafemi Awolowo University (OAU), Ile-Ife, Osun State, Nigeria. Ethical approval was sought and obtained from the Ethics and Research Committee of the OAUTHC (IRB/IEC/0004553). A total of 150 non-repetitive Pseudomonas spp. isolated from different clinical specimens from patients at the hospitals between March-September 2018 were employed as a non-patient contact for this study. Relevant demographic and clinical data of the patients were collected using a predesigned questionnaire.
Isolates collection and characterization
The isolates were collected on nutrient agar slants and transported to the Research Laboratory of the Department of Medical Microbiology and Parasitology of OAU, Ile-Ife for further analysis. Pure colonies of isolates were obtained by culture on MacConkey agar, blood agar and Trypticase soy agar and incubating at 35°C for 24 h. Morphological and biochemical characteristics of the isolates were determined by, Gram staining, catalase test, oxidase test and growth at 42°C in line with standard procedures.9 Definitive species of isolates was confirmed using MICROBACTTM 24E identification kit and Polymerase Chain Reaction (PCR).
Antibiotic susceptibility testing
Commercially available antibiotic discs (Mast, United Kingdom) were used to determine the susceptibility profile of the isolates. The Modified Kirby Bauer technique was employed and inhibition zones were interpreted according to the guidelines of Clinical and Laboratory Standard Institute (CLSI).10 The antibiotics tested include piperacillin (75 µg), piperacillin/tazobactam (100/10 µg), ciprofloxacin (5 µg), levofloxacin (5 µg), amikacin (30 µg), gentamicin (30 µg), imipenem (10 µg), and meropenem (10 µg). P. aeruginosa ATCC 27853 was used as a reference strain for the test. Multidrug-resistance was defined as resistance to at least one antibiotic in three or more classes of antibiotics and the multiple antibiotic resistance (MAR) index of the isolates was recorded as defined by Krumperman.4,11
DNA extraction
DNA was extracted from isolates using the boiling method. Three colonies of each isolate were emulsified in 100 µL of sterile distilled water in an Eppendorf tube, boiled for 15 minutes and centrifuged at 10,000 rpm for five minutes in a microcentrifuge. The supernatant was transferred to a new Eppendorf tube after centrifugation and was used as template DNA for polymerase chain reaction (PCR).
Identification of Pseudomonas spp. and Pseudomonas aeruginosa by PCR
PCR was used to confirm the identities of the isolates. A 25 µL PCR mixture (12.5 µL one Taq Quick-Load 2X master mix with standard buffer, 0.5 µL of 10 µM each of forward primer and reverse primer, 3 µL template DNA and 8.5 µL of nuclease-free water) was set up to amplify the genes of Pseudomonas spp. and Pseudomonas aeruginosa using the primers PAGS 618 bp (F: GGGGGATCTTCGGACCTCA, R: TCCTTAGAGTGCCCACCCG) and PASS 956 bp (F: GGGGGATCTTCGGACCTCA, R: TCCTTAGAGTGCCCACCCG) respectively.12 PCR conditions were observed according to Ghosh et al.13 Each amplicon (10 μL) was electrophoresed on a 1.5% agarose gel pre-stained with 0.5 μg/mL ethidium bromide in 1X Tris-Acetate-EDTA buffer and viewed with a transilluminator (Avebury, UK). The positions of the PCR products were determined by the positions of the 100 bp molecular weight marker (Biolabs, UK).
Data analysis
Data analysis was done using the IBM Statistical Product and Service Solutions (SPSS) version 20 (IMB Corp, USA). Descriptive statistics (frequencies, percentages, etc.) of data were presented.
Results
During the study period from March to September 2018, 150 isolates of Pseudomonas spp. from diverse clinical specimens were obtained from our hospital and employed as a non-patient contact for this study. The majority of the isolates (n=90; 60%) were recovered from patients with no recent (>4 weeks) hospital admission and the rest (n=60; 40%) were from those with recent (<4 weeks) hospital admission. There were slightly more isolates recovered from female patients n=76 (51%) compared to males n=74 (49%). The highest frequency of the isolates (n=43; 28.7%) was recovered from patients in the age range of 30-39 years, while the least (n=11; 7.3%) was from the age group less than 10 years old (Table 1).
Table 1. Demographic profile of patients with Pseudomonas spp. infections.
| Parameter | No of Pseudomonas spp. isolated (n=150), n (%) |
|---|---|
| Age groups | |
| <10 years | 11 (7.3) |
| 10-19 years | 13 (8.7) |
| 20-29 years | 24 (16) |
| 30-39 years | 43 (28.7) |
| 40-49 years | 18 (12) |
| 50-59 years | 11 (7.3) |
| ≥60 years | 30 (20) |
| Gender | |
| Male | 74 (49.3) |
| Female | 76 (50.7) |
| Hospital stay | |
| Recent admission (<4 weeks) | 60 (40) |
| No recent admission (>4 weeks) | 90 (60) |
| Invasive medical device | |
| IV/catheter use | 54 (36) |
| No IV/catheter use | 96 (64) |
| Drug use | |
| No antibiotic use | 123 (82) |
| Antibiotic use | 27 (18) |
| Comorbidity | |
| No comorbidity | 102 (68) |
| Comorbidity | 48 (32) |
| Type of clinical specimen | |
| Wound swab | 66 (44.0) |
| Urine | 45 (30.0) |
| Blood | 10 (6.7) |
| Ear swab | 12 (8.0) |
| Pus | 3 (2.0) |
| Eye swab | 2 (1.3) |
| Aspirate | 3 (2.0) |
| Urethral swab | 3 (2.0) |
| Pleural fluid | 2 (1.3) |
| Catheter tip | 2 (1.3) |
| Sputum | 1 (0.67) |
| HVS | 1 (0.67) |
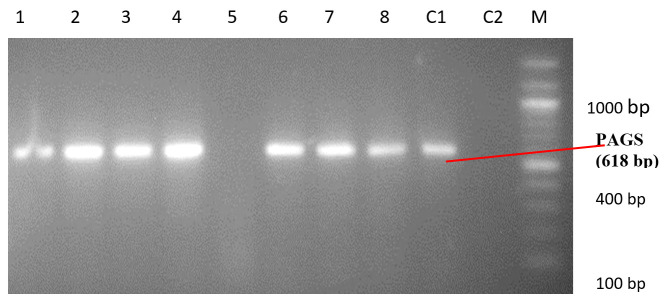
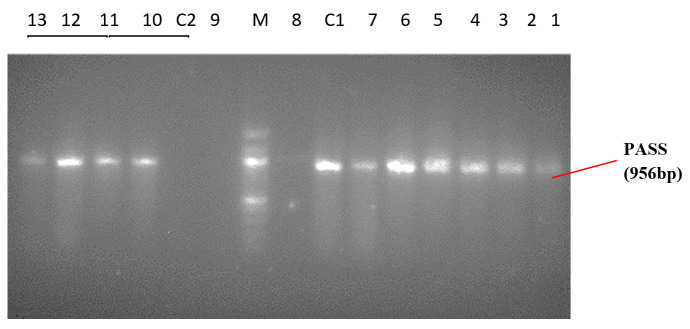
As shown in Table 1, wound swab was the predominant specimen source (n=66; 44%) followed by urine. Forty-five (30%) isolates were recovered from urine samples out of which 40% were catheter-stream and 60% mid-stream urine. Ear swabs ranked next in frequency giving 8% of the isolates recovered predominantly from chronic otitis media in adult patients, while blood was 6.7%. Sputum and high vaginal swab yielded the lowest frequency of isolates (n=1; 0.67%). A uniplex PCR was used to identify Pseudomonas spp. and Pseudomonas aeruginosa. PA-GS, a genus-specific primer pair used to amplify the 16Sr RNA gene of Pseudomonas spp. yielded 618 bp while PA-SS a species-specific primer pair used to amplify the 16SrRNA gene of Pseudomonas aeruginosa yielded 956 bp (Figures 1 and 2). Hence, all the isolates identified as Pseudomonas spp. and Pseudomonas aeruginosa by the phenotypic method were confirmed by PCR.
Figure 1. PCR amplicons of Pseudomonas spp. PAGS positive isolates- 1:205IK, 2:729, 3:720, 4:721, 6:725WG, 7:912, 8: 609. PAGS negative isolate - 5:101IK. C2: negative control E. coli ATCC 25922. C1: positive control P. aeruginosa ATCC 27853. M: 100 bp ladder N0551S (New England Biolabs Inc.).
Figure 2. PCR amplicons of Pseudomonas aeruginosa. PASS positive isolates: 1-803, 2-S16, 3-720, 4-721, 5-S84K, 6-725WG, 7-912, 10-609, 11-718, 12- 604, 13-144K. PASS negative isolates- 8:101IK, 9:416WG C2- negative control E. coli ATCC 25922. C1- positive control P. aeruginosa ATCC27853. M - 100bp ladder N0551S (New England Biolabs Inc.).
Of the isolates studied, 144 (96%) P. aeruginosa strains were recovered representing the highest frequency. Most of the isolates of P. aeruginosa were recovered from wound swabs (44%), P. fluorescens n=4 (2.67%) were from blood, ranking next in frequency, while an isolate each (0.67%) of P. putida (from catheter tip) and Burkholderia (Pseudomonas) pseudomallei (from sputum) were also recovered.
As shown in Table 2, the isolates exhibited the highest resistance to gentamicin (35%); imipenem and piperacillin-tazobactam were the most effective antibiotics with resistance rates of 7% and 6% respectively.
Table 2. Antibiotic susceptibility patterns of Pseudomonas spp. (n=150).
| Antibiotic class | Antibiotics | Susceptible isolates, n (%) | Resistant isolates, n (%) | Total | |||
|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Pseudomonas putida | Pseudomonas fluorescens | Burkholderia pseudomallei | ||||
| Aminoglycosides | Gentamicin | 98 (65) | 47 (31.3) | 1 (0.7) | 3 (2) | 1 (0.7) | 52 (35) |
| Amikacin | 130 (87) | 17 (11.3) | 1 (0.7) | 1 (0.7) | 1 (0.7) | 20 (13) | |
| Quinolone | Ciprofloxacin | 106 (71) | 40 (26.7) | 1 (0.7) | 2 (1.3) | 1 (0.7) | 44 (29) |
| Levofloxacin | 108 (72) | 39 (26) | 1 (0.7) | 1 (0.7) | 1 (0.7) | 42 (28) | |
| Beta-lactam | Piperacillin | 124 (83) | 25 (16.7) | 1 (0.7) | 1 (0.7) | 1 (0.7) | 28 (17) |
| Piperacillin-tazobactam | 141 (94) | 8 (5.3) | 1 (0.7) | 0 (0) | 0 (0) | 9 (6) | |
| Carbapenem | Imipenem | 139 (93) | 11 (7.3) | 0 (0) | 0 (0) | 0 (0) | 11 (7) |
| Meropenem | 134 (89) | 13 (8.6) | 1 (0.7) | 1 (0.7) | 1 (0.7) | 16 (11) | |
MDR strains constituted 12.7% of the isolates, out of which 26.3% were from outpatients and 73.7% from inpatients. All the MDR strains (n=19; 12.7%) had a high multiple antibiotic resistance (MAR) index (Table 3).
Table 3. Distribution of multidrug resistant strains of Pseudomonas spp. isolated.
| Pseudomonas spp. | No (%) of isolates | Multidrug resistant strains, n (%) | Multiple antibiotic resistance (MAR) index |
|---|---|---|---|
| Pseudomonas aeruginosa | 144 (96.0) | 16 (84.2) | 0.5-1.0 |
| Pseudomonas fluorescens | 4 (2.7) | 1 (5.6) | 0.75 |
| Pseudomonas putida | 1 (0.67) | 1 (5.6) | 0.875 |
| Burkholderia pseudomallei | 1 (0.67) | 1 (5.6) | 0.75 |
| Total | 150 (100) | 19 (12.7) | 0.5-1.0 |
Discussion
Although Pseudomonas aeruginosa was the predominant species isolated in this setting, other species of pseudomonads were infrequently encountered or isolated. In certain chronic health facilities, Stenotrophomonas maltophilia accounted for 80 percent of opportunistic infections by pseudomonads in immunocompromised clinical situations underscoring the need to identify pseudomonads from various clinical specimens to species level for epidemiological and surveillance purposes.14
We observed also that S. maltophilia was not represented in this study, however, the predominance of P. aeruginosa reported by us aligns with the findings of Gad et al.15 that reported P. aeruginosa (75.7%) as the predominant species in a series of 107 Pseudomonas strains recovered from 445 clinical specimens. In our study, the majority of the P. aeruginosa isolates were recovered from wound swab (44%) while in the study reported by Gad et al.,15 urine (22.5%) gave the highest yield of Pseudomonas aeruginosa, but in another series of 102 Pseudomonas aeruginosa reported by Shrestha et al.,16 both urine and sputum specimens accounted for the highest number of isolates (n=37; 36.3%) each, which highlights the major regular specimen source for the recovery of pseudomonads, particularly in immunocompromised situations. However, the differences can be explained due to the different types of clinical specimens in the different studies and the inclusion of environmental pseudomonas isolates in the study reported by Gad et al.15
The highest frequency of the isolates (28.7%) was recovered from patients in the age range of 30-39 years, contrary to the observation of Okon et al.17 who reported the highest occurrence in the 20-29 years age range. Although there were slightly more isolates of pseudomonads recovered from female patients (51%) compared to males (49%) in this study, the anatomical structure of the female reproductive system makes the invasiveness of Pseudomonas spp. easier when immunity is compromised. Ear swabs ranked next in frequency giving 8% of the isolates recovered predominantly from chronic otitis media in adult patients. This finding is in tandem with reports by studies in and outside Nigeria.19,20 As an opportunistic pathogen, P. aeruginosa often requires a breach in the first-line defense of the skin to initiate infection, this breach in immunity was found to be of significant influence in the high frequencies of the patients’ cases in trauma, burn wounds and surgery wounds from which the isolates were recovered.
Increased resistance of P. aeruginosa to antibiotics continues to pose a major threat to patient care due to limited treatment options.16 In this study, we observed resistant strains of pseudomonads across the different classes of antibiotics tested. However, the highest resistance rates among isolates were observed towards gentamicin (35.4%) while piperacillin/tazobactam was the most active antibiotic with a low resistance rate (6%), and Peshattiwar20 reported similar findings in their study. The observed resistance rate in our study reflects the current antibiotic prescription pattern and the selective pressure that followed is that gentamicin and ciprofloxacin have been much longer in circulation which will explain their relatively higher rates of resistance compared to piperacillin/tazobactam and imipenem with lower resistance rate because they are relatively recent in hospital practice in this country. Our observation is slightly different from what Shrestha et al.16 reported from Kathmandu, Nepal. In their study, P. aeruginosa exhibited high rates of resistance to piperacillin (57.1%) and ciprofloxacin (36.7%) among others, while only 6.5% of the isolates were resistant to imipenem in agreement with our reported resistance rate of 7% for imipenem. Gad et al.15 from Egypt reported a higher rate for gentamicin (59%) and meropenem (22%) in an earlier study. Gentamicin was introduced to the market in the ’60s and this suggests a higher chance of exposure within the study population in comparison to the carbapenems. The high probability of exposure to the drug is also a driver for resistance. The level of resistance to fluoroquinolones detected in this study is low when compared to a previous study.21 The isolation of an MDR B. pseudomallei from an end-stage renal disease patient in this study is of particular interest since a misdiagnosis of the disease is highly probable given the fact that it requires a high index of suspicion and the clinical context of isolation for the clinical significance of the pathogen to be determined and not discarded as a contaminant or colonizer.
Previous studies from Nigeria have indicated high rates of multidrug resistance in P. aeruginosa especially to fluoroquinolones, aminoglycosides and third-generation cephalosporins. In a study by Adejuyigbe et al.22 on septicemia in high-risk newborns at a teaching hospital in Ile-Ife, Nigeria, Pseudomonas aeruginosa accounted for 18.8% of the causative organisms with a high degree of in-vitro antimicrobial resistance. Oluranti et al.23 reported an incidence of 19.6% MDR P. aeruginosa. Igbalajobi et al.24 reported 18-31% resistance to penicillins, aminoglycosides and fluoroquinolones. P. aeruginosa has also been implicated as a prominent cause of post-operative wound infection in Nigeria.25 Pseudomonas aeruginosa is one of the most important opportunistic pathogens responsible for 10-15% of nosocomial infections worldwide.8
From the foregoing, it can be unequivocally stated that P. aeruginosa has now emerged as a highly multidrug-resistant pathogen with concomitant high multiple antibiotic resistance index in this environment. This is also in addition to the intrinsic nature of Pseudomonas being inertly impervious to most antibiotics due to the cell wall structure as well as its ease of spread in nosocomial settings. All the MDR strains (19; 100%) had a high MAR index suggesting a high-risk source where antibiotics are regularly and inappropriately used leading to high selective pressure. We can, therefore, safely speculate that the widespread, easy access and unrestrained antibiotic use have accelerated the incidence of antibiotic resistance and MDR strains in this environment. MDR strains constituted 12.8% of the isolates out of which 26.3% were from outpatients and 73.7% from inpatients suggesting the hospital as an important reservoir of multidrug-resistant strains. MDR isolates of 12.8% were observed with a high MAR index that may just be a tip of the iceberg phenomenon since this study was hospital-based and not widespread community-based research.
Piperacillin/tazobactam and imipenem were the most active antibiotics observed in this study. Our study underscores the importance of antibiotic susceptibility testing of clinical isolates in this environment.
The study was conceptualized more as a laboratory based study with less patient contact and not being a funded research the scale was limited. Larger studies are needed to further investigate the magnitude of antimicrobial resistance in this environment.
Conclusions
Pseudomonas aeruginosa has now emerged as a highly multidrug-resistant MDR pathogen with concomitant high multiple antibiotic resistance index in our hospital setting. This calls for the establishment of a surveillance system and antimicrobial stewardship programme in place. We propose a review of the current antibiotics prescription policy, and infection control programmes if we must control the spread of MDR-P. aeruginosa in this environment.
Footnotes
Authors’ contributions statement: AO and OO designed and supervised the study. AA and OA collected and analyzed the data and performed the background literature review for the manuscript. AA and BO carried out the laboratory work and conducted the statistical analysis. AO, OO and BO drafted the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
Acknowledgment: The authors thank the resident doctors and medical laboratory scientists of the Department of Medical Microbiology and Parasitology, OAU, Ile-Ife for their support.
References
- 1.Wisplinghoff H. Pseudomonas spp., Acinetobacter spp. and miscellaneous Gram-negative bacilli. In: Cohen J, Powderly WG, Opal SM, et al., editors. Infectious Diseases. 4th edition. Elsevier; 2017. pp. 1579–1599.e2. [DOI] [Google Scholar]
- 2.Pachori P, Gothalwal R, Gandhi P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019;6:109–19. doi: 10.1016/j.gendis.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal G, Kapil A, Kabra SK, Das BK, Dwivedi SN. Characterization of Pseudomonas aeruginosa isolated from chronically infected children with cystic fibrosis in India. BMC Microbiol. 2005;5:43. doi: 10.1186/1471-2180-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect Dis. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed MA, Hassan AAI, Jarir S, et al. Emergence of multidrug- and pandrug- resistant Pseudomonas aeruginosa from five hospitals in Qatar. Infect Prev Pract. 2019;1:100027. doi: 10.1016/j.infpip.2019.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peter S, Oberhettinger P, Schuele L, et al. Genomic characterisation of clinical and environmental Pseudomonas putida group strains and determination of their role in the transfer of antimicrobial resistance genes to Pseudomonas aeruginosa. BMC Genomics. 2017;18:859. doi: 10.1186/s12864-017-4216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan HA, Ahmad A, Mehboob R. Nosocomial infections and their control strategies. Asian Pac J Trop Biomed. 2015;5:509–14. doi: 10.1016/j.apjtb.2015.05.001. [DOI] [Google Scholar]
- 8.Al-Dahmoshi H, Al-Obaidi RD, Al-Khafaji N. Pseudomonas aeruginosa: diseases, biofilm and antibiotic resistance. In: Das T, editor. Pseudomonas aeruginosa. Biofilm Formation, Infections and Treatments. IntechOpen; 2020. [DOI] [Google Scholar]
- 9.Cheesbrough M. District laboratory practice in tropical countries. Part 2. 2nd edition. Cambridge: Cambridge University Press; 2006. pp. 319–29. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 27th edition. CLSI supplement M100. Wayne, Pennsylvania, USA: CLSI; 2016. [Google Scholar]
- 11.Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol. 1983;46:165–70. doi: 10.1128/aem.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spilker T, Coenye T, Vandamme P, LiPuma JJ. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol. 2004;42:2074–9. doi: 10.1128/JCM.42.5.2074-2079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh N, Goel AK, Lukka H, Bhattacharya P, Kamboj DV. Prevalence of drug resistant and exotoxin A producing Pseudomonas aeruginosa in cutaneous infections in a tribal area in South India. Afr J Microbiol Res. 2011;5:5512–7. doi: 10.5897/AJMR11.940. [DOI] [Google Scholar]
- 14.Iglewski BH. Pseudomonas. Medical Microbiology. 4th Edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 15.Gad GF, El-Domany RA, Zaki S, Ashour HM. Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: prevalence, antibiogram and resistance mechanisms. J Antimicrob Chemother. 2007;60:1010–7. doi: 10.1093/jac/dkm348. [DOI] [PubMed] [Google Scholar]
- 16.Shrestha S, Amatya R, Adhikari RP. Prevalence and antibiogram of Pseudomonas aeruginosa isolated from clinical specimens in a Teaching Hospital, Kathmandu. Int J Infect Dis. 2016;45:115–6. doi: 10.1016/j.ijid.2016.02.292. [DOI] [Google Scholar]
- 17.Okon K, Agukwe P, Oladosu W, Balogun S, Uba A. Antibiotic resistance pattern of Pseudomonas aeruginosa isolated from clinical specimens in a tertiary hospital in northeastern Nigeria. Internet J Microbiol. 2009;8:1–6. [Google Scholar]
- 18.Appiah-Korang L, Asare-Gyasi S, Yawson AE, Searyoh K. Aetiological agents of ear discharge: a two year review in a teaching hospital in Ghana. Ghana Med J. 2014;48:91–5. doi: 10.4314/gmj.v48i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umar AI, Garba I, Jidda ML, et al. Multidrug-resistant Pseudomonas aeruginosa isolated from ear and wound swabs in some selected hospital laboratories in Sokoto Metropolis, Nigeria. Calabar J Health Sci. 2020;3:31–35. doi: 10.25259/CJHS_8_2019. [DOI] [Google Scholar]
- 20.Peshattiwar PD, Peerapur BV. ESBL and MBL mediated resistance in Pseudomonas aeruginosa: an emerging threat to clinical therapeutics. J Clin Diagn Res. 2011;5:1552–4. doi: 10.20546/ijcmas.2020.904.088. [DOI] [Google Scholar]
- 21.Yusuf I, Arzai AH, Haruna M, Sharif AA, Mi G. Detection of multi drug resistant bacteria in major hospitals in Kano, North-West, Nigeria. Braz J Microbiol. 2014;45:791–8. doi: 10.1590/S1517-83822014000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adejuyigbe EA, Adeodu OO, Ako-Nai KA, Taiwo O, Owa JA. Septiceamia in high risk neonates at a teaching hospital in Ile-Ife, Nigeria. East Afr Med J. 2001;78:540–3. doi: 10.4314/eamj.v78i10.8965. [DOI] [PubMed] [Google Scholar]
- 23.Oluranti OO, Ubanagu CI, Oluwasemowo OO, Akinola OT, Nejo YT, Motayo BO. Prevalence and antibiotic resistance profile of Pseudomonas aeruginosa from hospital sinks in South Western Nigeria. J Adv Med Med Res. 2019;29:1–7. doi: 10.9734/jammr/2019/v29i230067. [DOI] [Google Scholar]
- 24.Igbalajobi OA, Oluyege AO, Oladeji AC, Babalola JA. Antibiotic resistance pattern of Pseudomonas aeruginosa isolated from clinical samples in Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State of Nigeria. Br Microbiol Res J. 2016;12:1–6. doi: 10.9734/BMRJ/2016/22515. [DOI] [Google Scholar]
- 25.Iduh UM, Chollom CS, Nuhu A, et al. Nosocomial infections in post-operative wounds due to Staphylococcus aureus and Pseudomonas aeruginosa in Benue State Nigeria. Afr J Microbiol Res. 2015;9:1989–96. doi: 10.5897/AJMR2014.6809. [DOI] [Google Scholar]