Supplemental Digital Content is available in the text.
Keywords: echocardiography, rheumatic heart disease, screening
Abstract
Background:
Echocardiographic screening can detect asymptomatic cases of rheumatic heart disease (RHD), facilitating access to treatment. Barriers to implementation of echocardiographic screening include the requirement for expensive equipment and expert practitioners. We aimed to evaluate the diagnostic accuracy of an abbreviated echocardiographic screening protocol (single parasternal-long-axis view with a sweep of the heart) performed by briefly trained, nonexpert practitioners using handheld ultrasound devices.
Methods:
Participants aged 5 to 20 years in Timor-Leste and the Northern Territory of Australia had 2 echocardiograms: one performed by an expert echocardiographer using a GE Vivid I or Vivid Q portable ultrasound device (reference test), and one performed by a nonexpert practitioner using a GE Vscan handheld ultrasound device (index test). The accuracy of the index test, compared with the reference test, for identifying cases with definite or borderline RHD was determined.
Results:
There were 3111 enrolled participants; 2573 had both an index test and reference test. Median age was 12 years (interquartile range, 10–15); 58.2% were female. Proportion with definite or borderline RHD was 5.52% (95% CI, 4.70–6.47); proportion with definite RHD was 3.23% (95% CI, 2.61–3.98).
Compared with the reference test, sensitivity of the index test for definite or borderline RHD was 70.4% (95% CI, 62.2–77.8), specificity was 78.1% (95% CI, 76.4–79.8).
Conclusions:
Nonexpert practitioners can be trained to perform single parasternal-long-axis view with a sweep of the heart echocardiography. However, the specificity and sensitivity are inadequate for echocardiographic screening. Improved training for nonexpert practitioners should be investigated.
Clinical Perspective.
Echocardiographic screening can be used for the early diagnosis of rheumatic heart disease, but implementation has been limited because of resource implications and uncertainty regarding long-term benefits. This study demonstrates that nonexpert practitioners can be trained with to do echocardiographic screening for rheumatic heart disease, achieving moderate sensitivity and specificity. Improvements in training could lead to a feasible nonexpert practitioner led model for rheumatic heart disease screening.
See Editorial by Strasserking and Davila-Roman
Rheumatic heart disease (RHD) is a complication of group A streptococcal infection, associated with household crowding and poverty, which affects more than 30 million people worldwide.1,2 Clinical presentations are frequently late and associated with complications including heart failure, arrhythmias, cerebrovascular accidents and, infective endocarditis, even in well-resourced settings like Australia.3,4 Young people in Timor-Leste, and Aboriginal and Torres Strait Islander people in the Northern Territory (NT) of Australia have high rates of RHD, and there are opportunities to improve diagnosis.5–7 In Timor-Leste, the prevalence of definite RHD in school-aged children is estimated to be 1.8%.5 Prevalence estimates for definite RHD in the Top End region of the NT range between 1.5% and 5.2%.6,7
Echocardiographic screening facilitates early detection of RHD, before onset of symptoms with superior sensitivity and specificity compared with auscultation.8–10 In 2012, World Heart Federation criteria (Table I in the Data Supplement) were published to guide a consistent and reproducible approach to the echocardiographic diagnosis of RHD.11,12 If RHD is detected on echocardiography, secondary prophylaxis using regular benzyl-benzathine penicillin G injections can be instituted according to guidelines,13 which may halt progression of RHD by preventing further episodes of group A streptococcal infection and associated acute rheumatic fever.4 Treatment of moderate and severe RHD detected on screening is recommended.13 The impact of screening and treatment on clinical outcomes for borderline or mild definite RHD is not yet established.14 Previous economic analyses support echocardiographic screening as a cost-effective approach to detecting RHD in some settings.15,16
Echocardiographic screening meets some of the principles of screening for diseases: high burden of disease, latent phase which can be detected with a screening test, and treatments available.9,17 However, implementation has been controversial, because of the lack of data showing efficacy of early treatment of screening-detected borderline and mild RHD and concerns about resource implications of screening and the impact on health systems that are already burdened by RHD and other competing health priorities.9
Strategies to address barriers to implementation have been investigated, including training nonexpert practitioners to perform echocardiography,18,19 using small handheld devices,20–22 and abbreviating echocardiography screening protocols.23
Our previous work has demonstrated that an abbreviated echocardiography protocol, based on a single parasternal-long-axis view with a sweep of the heart (SPLASH), was sensitive and specific for the detection of RHD, when performed by experienced cardiologists, using portable ultrasound devices (Vivid I or Vivid Q, GE Healthcare).24 In this study, we combined this abbreviated screening protocol with the use of handheld devices and brief training for nonexpert practitioners.
We hypothesized that briefly trained, nonexpert practitioners performing SPLASH echocardiography using handheld devices would accurately detect definite and borderline RHD. We designed a prospective diagnostic study (named the Pedrino project) to test this hypothesis.
Methods
Transparency and Openness Promotion Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Design
We conducted a prospective, cross-sectional, echocardiography screening study, which included a comparison of a new approach to the detection of RHD (SPLASH performed by briefly trained nonexpert practitioners using a handheld ultrasound device) against the reference test (complete screening echocardiogram performed by an expert echocardiographer).
Setting and Community Involvement in Codesign
The study was conducted in communities in Dili (urban) and Bobonaro (rural) municipalities of Timor-Leste, and in Maningrida, NT, Australia, between March 2018 and November 2018. Timor-Leste has a population of 1.2 million people, and limited access to specialist cardiac services.25 Maningrida (which is identified at the request of community leaders and Traditional Owners) has a population of 2366, 91% of whom are Aboriginal people.26 Specialist pediatric and cardiology services are accessible through visiting clinics and transfer-in to the regional center of Darwin when needed. Following a cluster of acute rheumatic fever cases in 2014,27 community members contributed to the conception and design of the Pedrino project. There was a strong preference for local staff participation in training and delivery of echocardiography. Similar consultations in Timor-Leste highlighted the importance of equipping Timorese clinicians with skills to perform echocardiography, given limited access to specialist cardiology services. In Timor-Leste, screening occurred in a combination of public and church schools. In Maningrida, screening occurred in the public school and in remote outstations.7
Inclusion Criteria
All people aged between 5 and 20 years who were present at the screening site on the day of screening were eligible. Ethical approval to enroll participants without written consent, using an opt-out approach, was obtained in Timor-Leste, consistent with previous recommendations from community leaders.5 In Australia, all participants required written consent from the parent or guardian for those aged <18 years. Information about the study was provided in local languages using short videos. A history of cardiac disease was not sought before recruitment, and in cases where this was known (or identified on later review of clinical records), participants were still included.
Exclusion Criteria
Children aged under 5 years, and adults aged over 20 years were excluded, as were those who did not have an age or date of birth recorded at the time of screening. Those who chose to opt-out in Timor-Leste, and those who did not provide written consent in Australia, were also excluded.
Echocardiography Training
Echocardiography training was delivered in English as a combination of online modules,28 lectures, and face-to-face practical training delivered over 6 days by cardiac sonographers and cardiologists with expertise in the diagnosis of RHD. Nonexpert practitioners were identified from Timor-Leste and the NT, with emphasis on selecting people from the communities that would be involved in the study. Nonspecialist doctors, nurses, and health workers without tertiary qualifications were included. Most participants spoke English as a second language. They were taught SPLASH echocardiography, and to identify any mitral regurgitation (MR) and/or aortic regurgitation (AR) as being abnormal. They were not taught to identify other pathological valvular changes, associated with RHD. To successfully complete training, nonexpert practitioners had to perform a minimum of 50 supervised SPLASH studies, which included volunteers with normal hearts and with RHD, and pass written and practical assessments (Methods in the Data Supplement).
Index Test
The index test was a SPLASH echocardiogram, using 2-dimensional and color Doppler to identify the presence or absence of any MR or AR, performed by a briefly trained nonexpert practitioner using a Vscan handheld ultrasound device (GE Healthcare), who was blinded to clinical information and reference test result. The Vscan is a phased array 1.7 to 3.8 MHz probe, with color Doppler Nyquist limits fixed at 64 cm/second. The nonexpert practitioner scanned in the parasternal-long-axis plane only, storing at least 4 image loops (4 seconds), including 2-dimensional and color Doppler images of mitral and aortic valves, incorporating a sweeping technique to visualize anterior and posterior aspects of each valve. They determined the presence of valvular regurgitation, measured jet length for MR. A positive index test was defined as any MR and/or AR, to maximize sensitivity for a screening test. Identification of morphological features of RHD was not included as part of the index test.
Reference Test
The reference test was a standard screening echocardiogram, including 2-dimensional, color and continuous wave Doppler of parasternal-long-axis, parasternal-short-axis, apical-4-chamber and apical-5-chamber views performed by a cardiologist or cardiac sonographer with expertise in the echocardiographic diagnosis of RHD, using a Vivid I or Vivid Q portable ultrasound machine (GE Healthcare), who was blinded to the index test result. Reporting was conducted in real time. A positive reference test was defined as a diagnosis of definite or borderline RHD using World Heart Federation 2012 echocardiographic criteria (Table I in the Data Supplement).11 Any cases with an abnormal screening study underwent a full anatomic scan in the field to investigate for congenital heart disease. All abnormal cases were reviewed in real time by a panel of 3 expert echocardiographers including at least one cardiologist to determine a consensus diagnosis.29 The SPLASH test was conducted before the reference test, and the result of the SPLASH test was not provided to the participant.
External Review of Images
Images obtained during the initial SPLASH echocardiogram were stored on Synapse Cardiovascular server (Fujifilm, Japan) and reviewed by an expert echocardiographer who was blinded to the assessment of the nonexpert practitioner and the final diagnosis for each case. Images were assessed for image quality and presence or absence of MR (including jet length measurement) and/or AR.
Sample Size Calculation
Sample size was calculated assuming a combined prevalence of definite and borderline RHD of 3%.5,6 To demonstrate 95% sensitivity of the index test for detection of definite or borderline RHD, with a precision of 0.05, a sample size of 2434 was required.30
Data Management and Analysis
Data were collected using a REDCap 8.7.4 (Vanderbilt University) database hosted at Menzies School of Health Research (Darwin, Australia).31 Statistical analysis was conducted using STATA 15.1 (StataCorp). Descriptive analyses were conducted, and proportion of cases with RHD was calculated with 95% CI. Differences in disease burden between study sites were assessed using Fisher exact test.
Primary analysis included sensitivity and specificity of the index test for diagnosis of definite or borderline RHD, compared with the reference standard. Participants who did not complete both tests were excluded from the primary analysis. Negative predictive value, positive predictive value, and diagnostic odds ratio were also calculated. Secondary analyses were conducted to determine the accuracy of the index test for different severity of definite RHD, and to identify changes in sensitivity and specificity using different cutoff values for MR jet length. Borderline and definite RHD cases were classified as low-, intermediate-, or high-risk of disease progression based on echocardiographic features,32,33 and diagnostic accuracy calculated for these categories (Table II in the Data Supplement).
Findings from external review of SPLASH echocardiography images were compared with findings recorded by nonexpert practitioners at the time of screening, and inter-rater reliability was measured using Cohen’s kappa coefficient and reported with 95% CI.
Follow-Up of Cases
Individuals with newly diagnosed definite and borderline RHD (based on reference test) received information and education about RHD in local languages. Secondary prophylaxis was commenced for new definite RHD with a dose of benzathine penicillin G administered within 24 hours, and families were referred to local clinical services for follow-up. Those with congenital or other cardiac abnormalities were referred through usual pathways. Other coincidental clinical findings requiring treatment were addressed in collaboration with local clinics.
Ethics
Ethics approval was obtained from the Human Research Ethics Committee of the NT Department of Health and Menzies School of Health Research and from the Ethics Committee of the Instituto Nacional de Saude in Timor-Leste.
Role of the Funding Source
Funders had no role in study design, implementation, analysis or reporting.
Results
Echocardiography Training
Of 18 nonexpert practitioners who undertook the training, 15 successfully completed training and passed the assessments, including 6 nonspecialist doctors, 4 nurses, and 5 health workers, allowing them to participate in screening (Methods in the Data Supplement).
Echocardiography Screening
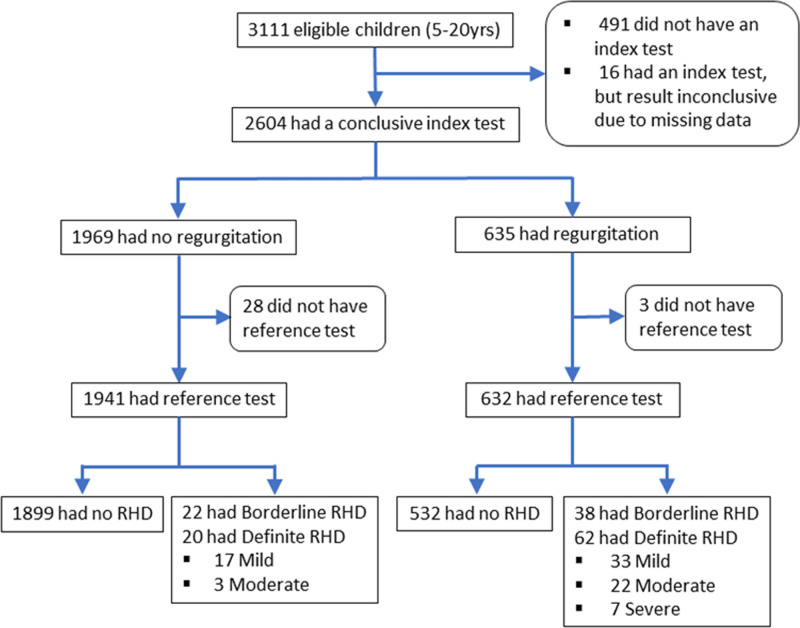
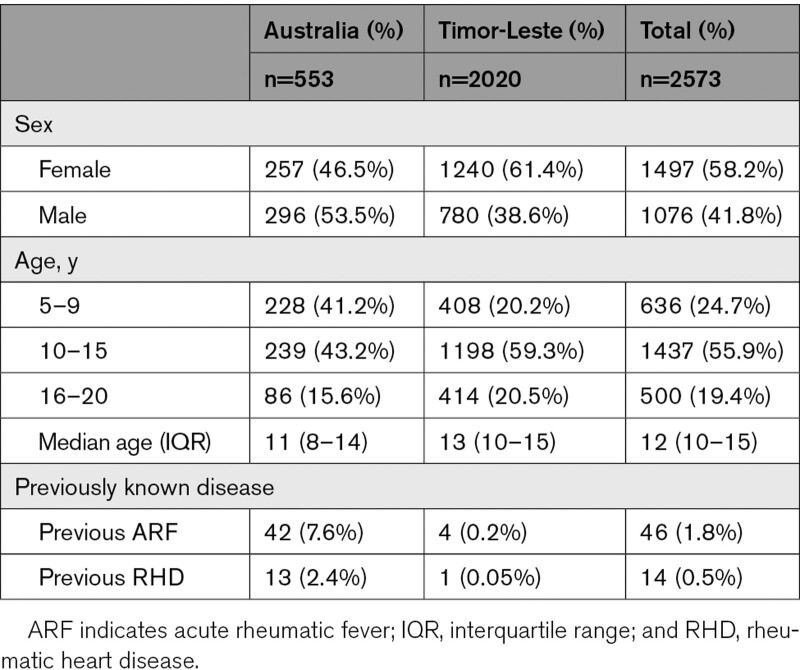
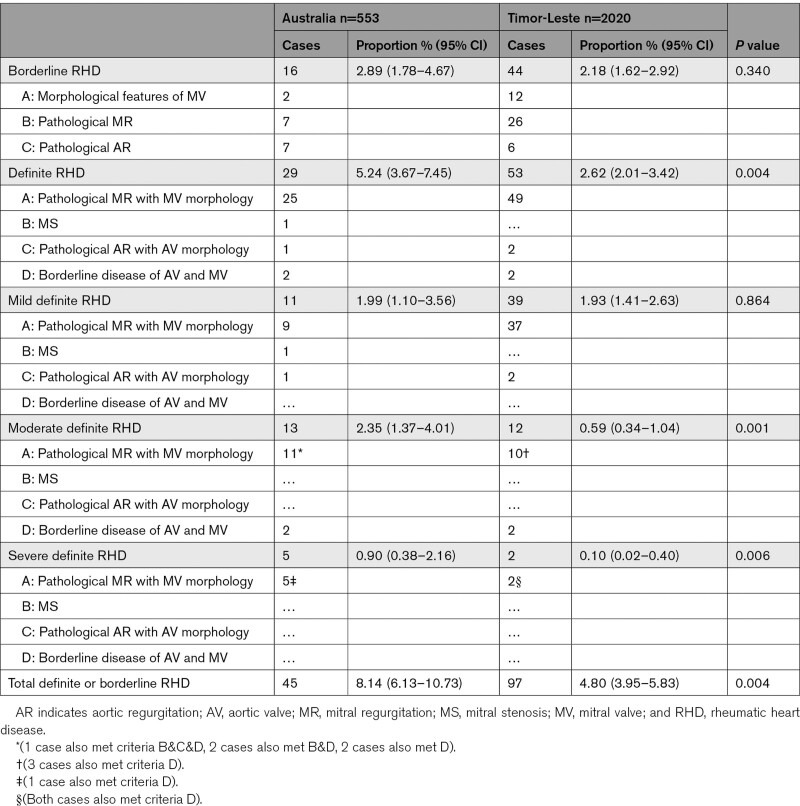
Of 3111 people enrolled, 2573 had both index and reference tests and were included in the analysis (Figure 1). Most (78.5%) were from Timor-Leste. Of 553 from Australia, 534 (96.6%) were Indigenous. Median age was 12 years (interquartile range, 10–15); 1497 (58.2%) were female (Table 1). The proportion with definite or borderline RHD was 5.52% (95% CI, 4.70–6.47), and the proportion with definite RHD was 3.19% (95% CI, 2.57–3.94; Table 2). Most cases had not been diagnosed previously (128/142, 90.1%). Three severe cases were referred and successfully underwent urgent cardiac surgery. Congenital heart disease was found in 57/2573 (2.2%); 3 (from Timor-Leste) were referred for surgical management in Australia.
Figure 1.
Study enrollment flow chart. RHD indicates rheumatic heart disease.
Table 1.
Participant Characteristics
Table 2.
Proportion of Cases With Borderline and Definite Rheumatic Heart Disease in Australia and Timor-Leste Based on Findings From Reference Test
Index and reference tests were mostly performed on the same day (99.2%) or within the same week of screening (0.7%).
Mitral regurgitation alone was involved in 78.2% of cases, while 10.5% had both MR and AR, and 11.3% had isolated AR.
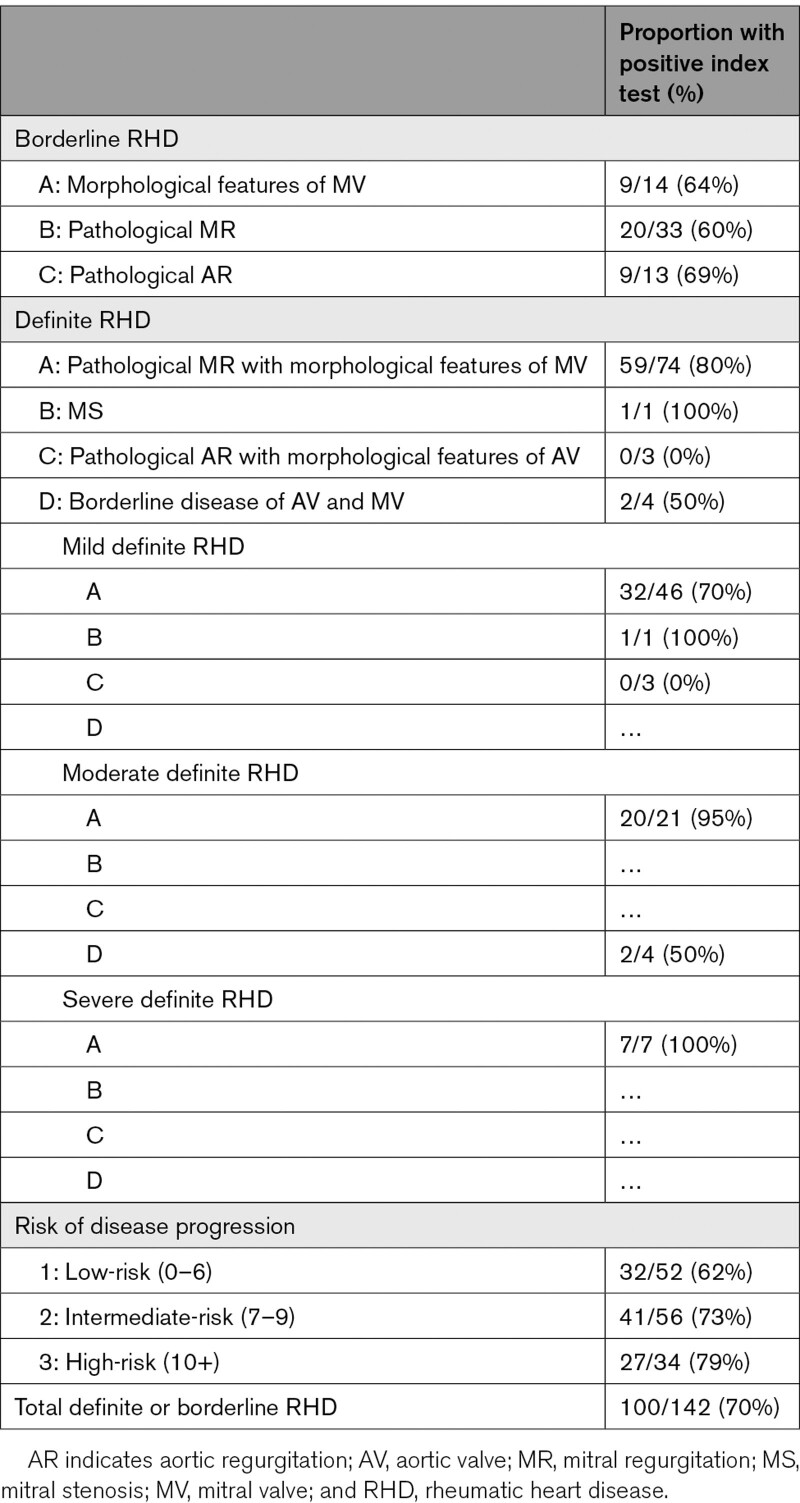
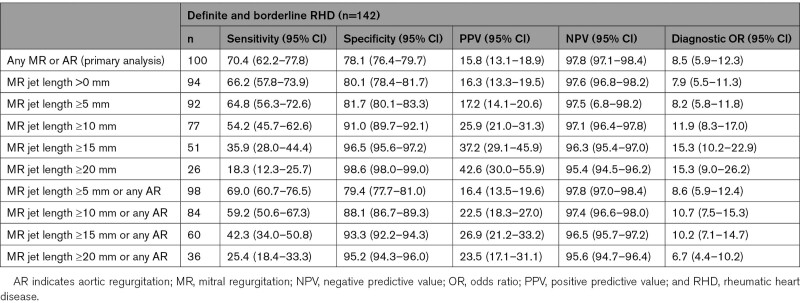
Compared with the reference test, the sensitivity of the index test for detection of definite or borderline RHD was 70.4% (95% CI, 62.2–77.8) and specificity 78.1% (95% CI, 76.4–79.8). Specificity was similar for the detection of moderate or severe RHD compared with the detection of all definite or borderline RHD, but sensitivity for detection of moderate or severe RHD was 90.6% (95% CI, 75.0–98.0; Table 3). Specificity improved, but sensitivity was worse if cutoff MR jet lengths of 5, 10, 15, or 20 mm were used, compared with 0 mm (Table 4). Example comparisons between reference and index test images can be seen in Figure 2.
Table 3.
Proportion of Cases With a Positive Index Test According to Classification of Disease
Table 4.
Diagnostic Accuracy of Index Test for Composite of Definite or Borderline RHD, at Varying MR Jet Length Cutoffs, As Measured by Nonexpert Practitioners
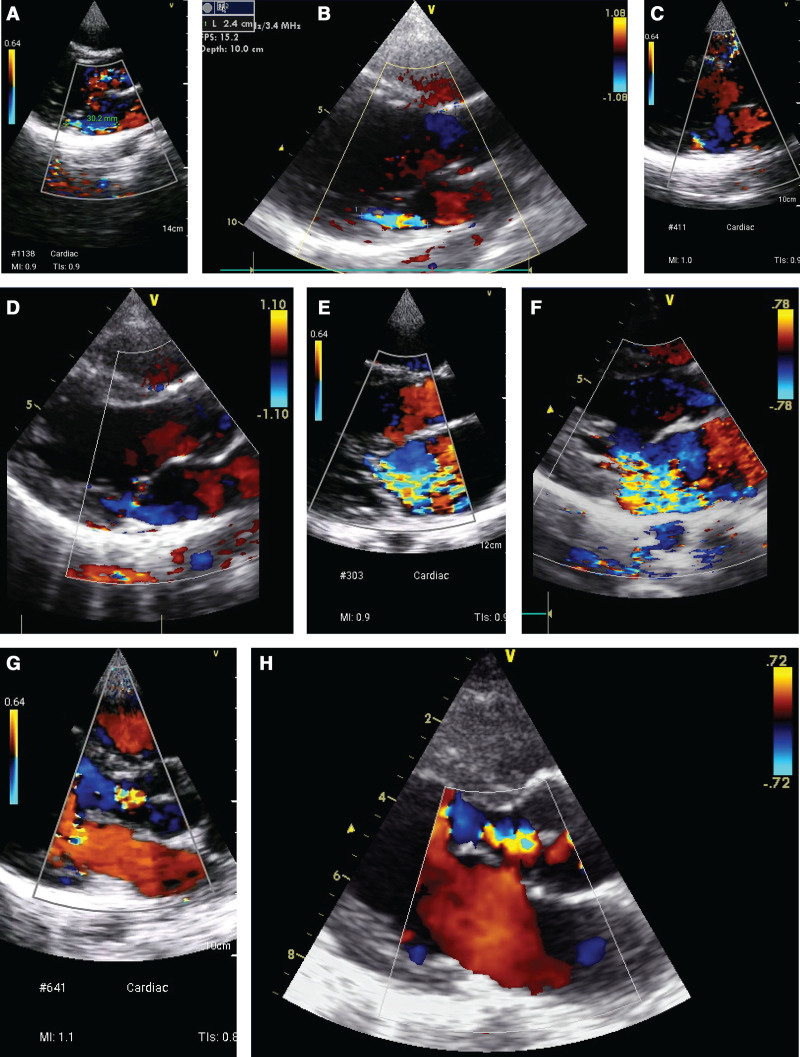
Figure 2.
Illustrative cases, in index and reference test pairs. Index test performed by nonexpert using GE Vscan handheld ultrasound system; reference test performed by expert using GE Vivid portable ultrasound system.A, Mild mitral regurgitation (MR), detected on index test, concordant with: (B) mild MR detected on reference test; (C) index test demonstrating good quality images for external review, but reported as no MR by nonexpert practitioner; (D) mild MR detected on reference test; (E) severe MR detected on index test concordant with: (F) severe MR detected on reference test; (G) index test demonstrating good quality images for external review, but reported as no aortic regurgitation (AR) by nonexpert practitioner; (H) mild AR detected on reference test.
When cases were reclassified according to risk of disease progression, the sensitivity of the index test was 75.6% (95% CI, 65.4–84.0) for cases deemed intermediate-risk and above, and 79.4% (95% CI, 62.1–91.3) for those at high-risk for further progression (Table 3).
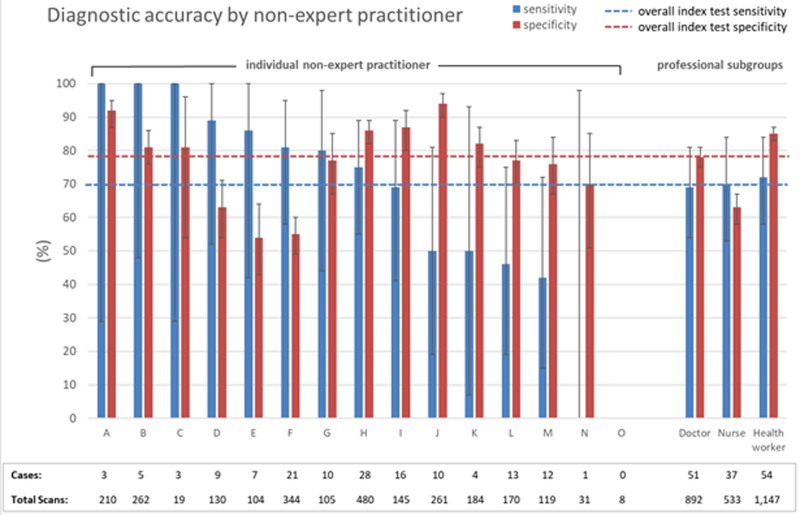
Nonexpert practitioners performed between 8 and 480 scans, with variable diagnostic accuracy (Figure 3). Those who performed >200 scans (n=5) appeared to have better sensitivity (76.1% [95% CI, 64.1–85.7]) and specificity (80.5% [95% CI, 78.4–82.5]) compared with those with fewer scans (sensitivity 65.3% [95% CI, 53.5–76.0]; specificity 74.4%; 71.4–77.1). There was no significant difference in sensitivity based on profession or country of origin of nonexpert practitioners.
Figure 3.
Diagnostic accuracy of screening by individual nonexpert practitioners and by professional categories (doctors, nurses, health workers).
External expert review of SPLASH images occurred for 2445/2573 (95.0%). Of these, 179/2445 (7.3%) could not be interpreted, and 620/2445 (25.3%) were deemed poor quality, although an assessment could be made. After removing the uninterpretable images, the kappa coefficient between nonexpert practitioner reported SPLASH and external review of images for presence of any MR and/or AR was 0.42 (95% CI, 0.38–0.47), with agreement in 78.2% of cases. There was no improvement in sensitivity nor specificity based on expert review of SPLASH images when considering borderline and definite RHD.
Discussion
This multicenter study provides important new evidence to inform strategies for population based RHD screening programs. As has been demonstrated previously,5–7 echocardiographic screening identified high rates of previously undiagnosed RHD in Australia and Timor-Leste, but the abbreviated screening protocol missed almost one-third of all cases, and one-fifth of those at high risk of progression. Briefly trained, nonexpert practitioners were able to identify >90% of moderate and severe RHD cases. This finding demonstrates the potential for brief training in SPLASH echocardiography for nonexpert practitioners, and a possible role in the identification of moderate or severe carditis during episodes of acute rheumatic fever. However, sensitivity for the detection of mild and borderline RHD was inadequate (and did not improve sufficiently with external review of images) to recommend this approach for screening, without changes to training. In addition, the low positive predictive value (15.8%) of an abnormal index test means that screening with this approach would result in a large number of normal subjects referred for confirmatory echocardiography.
Other studies have investigated diagnostic accuracy of echocardiography screening using briefly trained nonexpert practitioners. One study which involved 8 weeks of training, echocardiography incorporating multiple imaging windows and the use of standard portable ultrasound devices (rather than handheld devices), achieved sensitivity and specificity of ≈85%.19 Similar findings using handheld technology and training that involved 3 days of lectures followed by 30 hours of supervised practical echocardiography sessions have been described.34 Sensitivity and specificity were worse in another study using handheld devices, but only two-and-a-half days of training for practitioners with some background experience in echocardiography.35 Both studies using handheld devices included protocols using multiple planes for screening.
A retrospective analysis of parasternal-long-axis view images (obtained by expert sonographers using handheld devices) showed sensitivity of 81% and specificity of 76% for detection of any RHD.23 Most of the cases missed by the parasternal-long-axis-view images were borderline RHD. SPLASH echocardiography has distinct advantages over traditional multi-plane approaches to screening. It can be rapidly performed using portable technology, it does not require full removal of clothes, and it is much simpler to teach nonexperts.24
Since the release of the Vscan, several other handheld ultrasound machines (including Philips Lumify and Butterfly iQ+) have been released, which feature higher quality 2-dimensional and Doppler imaging, and improved functionality, image size, storage, and transfer. These newer devices may allow improved sensitivity for detection of RHD.
Changes to the training program are also needed. The fact that better accuracy was achieved by those who performed more scans suggests that a longer training course with a minimum of 100 supervised scans before final assessment could lead to improved performance. Training to improve image acquisition should be a priority. Recent advances suggest that in the future, adequate image acquisition may allow for machine learning to achieve automated identification of RHD.36 Major deficits in interpretation were in the identification of mild MR and any AR, and these should receive additional focus. Incorporation of language-based material for health workers with English as a second language would help with improved understanding.
Further research is needed to evaluate the impact of these suggested changes and determine whether or not they improve diagnostic accuracy sufficiently to warrant scale-up of abbreviated, nonexpert practitioner performed echocardiography, and incorporation of handheld echocardiography-specific diagnostic criteria for RHD into international guidelines. Consideration also needs to be given to the impact of additional work expectations on health care workers who are often already overloaded with clinical need.
This study had several limitations. Just over 500 eligible participants did not have an evaluable index test and could not be included in the analysis. Selection bias is possible, because of opportunistic recruitment and nonrandom study design. In addition, variable approaches to enrollment between sites in Timor-Leste and Australia, and inherent differences in context and health care access, resulted in some heterogeneity in patient population. Nevertheless, all participants came from places with a high burden of RHD. The number of scans performed by individual nonexpert practitioners varied considerably and was very low in some cases.
Training nonexpert practitioners to perform abbreviated echocardiography for RHD is feasible. Although accuracy results are below those desired for a screening program, further refinement of training programs and screening protocols are justified, followed by further studies of diagnostic accuracy of this novel approach to the detection of RHD.
Acknowledgments
We acknowledge the contributions of many volunteers, including staff from NT Cardiac, Take Heart (Moonshine Agency), Northern Territory Department of Health, West Arnhem Shire Council, Maningrida College (Lurra Language and Culture Unit), Maluk Timor, Bakhita Center, Bawinanga Aboriginal Corporation, Starlight Children’s Foundation, Malal’a Health Service, and the Timor-Leste Ministry of Health. We would particularly like to thank three legends: Eddie de Pina, Jo Killmister, and Trudy Francis.
Sources of Funding
The Pedrino Project is a Menzies School of Health Research project. It was supported by a Heart Foundation Vanguard Grant, a pilot grant from National Health and Medical Research Council (NHMRC) grant 1131932: improving Health Outcomes in the Tropical North: a multidisciplinary collaboration (HOT NORTH), Rotary Oceania Medical Aid for Children (ROMAC), Snow Foundation, Bawinanga Aboriginal Corporation, Humpty Dumpty Foundation, Starlight Children’s Foundation, and Malal’a Health Service. Drs Ralph and Engelman are supported by fellowships from the Australian NHMRC.
Disclosures
None.
Supplemental Materials
Data Supplement Methods
Data Supplement Tables I and II
Reference 37
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AR
- aortic regurgitation
- MR
- mitral regurgitation
- NT
- Northern Territory
- RHD
- rheumatic heart disease
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.120.011790.
For Sources of Funding and Disclosures, see page 809.
Contributor Information
Gillian A. Whalley, Email: gillianwhalleyphd@gmail.com.
Alex Kaethner, Email: a.kaethner@ntcardiac.com.
Helen Fairhurst, Email: Helen.Fairhurst@menzies.edu.au.
Hilary Hardefeldt, Email: hilary.hardefeldt@gmail.com.
Benjamin Reeves, Email: ben.auld@health.qld.gov.au.
Benjamin Auld, Email: ben.auld@health.qld.gov.au.
James Marangou, Email: jmarangou@gmail.com.
Ari Horton, Email: ari.horton@gmail.com.
Gavin Wheaton, Email: Gavin.Wheaton@sa.gov.au.
Terry Robertson, Email: Terry.Robertson@health.sa.gov.au.
Chelsea Ryan, Email: thefrancisfive@gmail.com.
Shannon Brown, Email: truejoyfrancis@gmail.com.
Greg Smith, Email: gpsmithy@gmail.com.
Januario dos Santos, Email: januario1987@hotmail.com.
Ricardo Flavio, Email: ricky_rhd@maluktimor.org.
Karolina Embaum, Email: Karolinaembun89@gmail.com.
Mario da Graca Noronha, Email: mariodagracan@gmail.com.
Sonia Lopes Belo, Email: belloisthebest@yahoo.es.
Maria Georginha dos Santos, Email: mariageorginha@gmail.com.
Jose Cabral, Email: aquintochepe@gmail.com.
Ivonia do Rosario, Email: ivoniam.dorosario@gmail.com.
Jessica Harries, Email: jess@maluktimor.org.
Laura A. Francis, Email: Laura.Francis@nt.gov.au.
Anthony D.K. Draper, Email: anthony.draper@live.com.au.
Christian L. James, Email: christianlukejames@gmail.com.
Kimberly Davis, Email: dr.kimberly.j.davis@gmail.com.
Jennifer Yan, Email: jennifer.yan@menzies.edu.au.
Alice Mitchell, Email: alicem404@gmail.com.
Ines da Silva Almeida, Email: theodorainez@yahoo.com.
Daniel Engelman, Email: drdanielengelman@gmail.com.
Kathryn V. Roberts, Email: kathryn.roberts@nt.gov.au.
Anna P. Ralph, Email: anna.ralph@menzies.edu.au.
Bo Remenyi, Email: Bo.Remenyi@menzies.edu.au.
References
- 1.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 2.Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group a streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis. 2018;12:e0006577. doi: 10.1371/journal.pntd.0006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zühlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36:1115–122a. doi: 10.1093/eurheartj/ehu449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. 2013;128:492–501. doi: 10.1161/CIRCULATIONAHA.113.001477 [DOI] [PubMed] [Google Scholar]
- 5.Davis K, Remenyi B, Draper AD, Dos Santos J, Bayley N, Paratz E, Reeves B, Appelbe A, Cochrane A, Johnson TD, et al. Rheumatic heart disease in Timor-Leste school students: an echocardiography-based prevalence study. Med J Aust. 2018;208:303–307. doi: 10.5694/mja17.00666 [DOI] [PubMed] [Google Scholar]
- 6.Roberts KV, Maguire GP, Brown A, Atkinson DN, Remenyi B, Wheaton G, Ilton M, Carapetis J. Rheumatic heart disease in Indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust. 2015;203:221.e1–221.e7. doi: 10.5694/mja15.00139 [DOI] [PubMed] [Google Scholar]
- 7.Francis JR, Fairhurst H, Hardefeldt H, Brown S, Ryan C, Brown K, Smith G, Baartz R, Horton A, Whalley G, et al. Hyperendemic rheumatic heart disease in a remote Australian town identified by echocardiographic screening. Med J Aust. 2020;213:118–123. doi: 10.5694/mja2.50682 [DOI] [PubMed] [Google Scholar]
- 8.Rothenbühler M, O’Sullivan CJ, Stortecky S, Stefanini GG, Spitzer E, Estill J, Shrestha NR, Keiser O, Jüni P, Pilgrim T. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta-analysis of prevalence among children and adolescents. Lancet Glob Health. 2014;2:e717–e726. doi: 10.1016/S2214-109X(14)70310-9 [DOI] [PubMed] [Google Scholar]
- 9.Roberts K, Colquhoun S, Steer A, Reményi B, Carapetis J. Screening for rheumatic heart disease: current approaches and controversies. Nat Rev Cardiol. 2013;10:49–58. doi: 10.1038/nrcardio.2012.157 [DOI] [PubMed] [Google Scholar]
- 10.Roberts KV, Brown AD, Maguire GP, Atkinson DN, Carapetis JR. Utility of auscultatory screening for detecting rheumatic heart disease in high-risk children in Australia’s Northern Territory. Med J Aust. 2013;199:196–199. doi: 10.5694/mja13.10520 [DOI] [PubMed] [Google Scholar]
- 11.Reményi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease–an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remenyi B, Carapetis J, Stirling JW, Ferreira B, Kumar K, Lawrenson J, Marijon E, Mirabel M, Mocumbi AO, Mota C, et al. Inter-rater and intra-rater reliability and agreement of echocardiographic diagnosis of rheumatic heart disease using the World Heart Federation evidence-based criteria. Heart Asia. 2019;11:e011233. doi: 10.1136/heartasia-2019-011233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralph Anna P., Noonan Sara, Wade Vicki, Currie Bart J; RHDAustralia (ARF/RHD Writing Group). The 2020 Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease. 20203rd edition [DOI] [PubMed] [Google Scholar]
- 14.Beaton A, Okello E, Engelman D, Grobler A, Scheel A, DeWyer A, Sarnacki R, Omara IO, Rwebembera J, Sable C, et al. Determining the impact of Benzathine penicillin G prophylaxis in children with latent rheumatic heart disease (GOAL trial): Study protocol for a randomized controlled trial. Am Heart J. 2019;215:95–105. doi: 10.1016/j.ahj.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 15.Zachariah JP, Samnaliev M. Echo-based screening of rheumatic heart disease in children: a cost-effectiveness Markov model. J Med Econ. 2015;18:410–419. doi: 10.3111/13696998.2015.1006366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts K, Cannon J, Atkinson D, Brown A, Maguire G, Remenyi B, Wheaton G, Geelhoed E, Carapetis JR. Echocardiographic screening for rheumatic heart disease in indigenous Australian children: a cost-utility analysis. J Am Heart Assoc. 2017;6:e004515. doi: 10.1161/JAHA.116.004515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson J, Junger G. Principles and Practice of Screening for Disease. 1968. World Health Organization. Accessed July 21, 2021. https://apps.who.int/iris/handle/10665/37650 [Google Scholar]
- 18.Engelman D, Kado JH, Reményi B, Colquhoun SM, Carapetis JR, Wilson NJ, Donath S, Steer AC. Screening for rheumatic heart disease: quality and agreement of focused cardiac ultrasound by briefly trained health workers. BMC Cardiovasc Disord. 2016;16:30. doi: 10.1186/s12872-016-0205-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelman D, Kado JH, Reményi B, Colquhoun SM, Carapetis JR, Donath S, Wilson NJ, Steer AC. Focused cardiac ultrasound screening for rheumatic heart disease by briefly trained health workers: a study of diagnostic accuracy. Lancet Glob Health. 2016;4:e386–e394. doi: 10.1016/S2214-109X(16)30065-1 [DOI] [PubMed] [Google Scholar]
- 20.Beaton A, Lu JC, Aliku T, Dean P, Gaur L, Weinberg J, Godown J, Lwabi P, Mirembe G, Okello E, et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: a field study. Eur Heart J Cardiovasc Imaging. 2015;16:475–482. doi: 10.1093/ehjci/jeu296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu JC, Sable C, Ensing GJ, Webb C, Scheel J, Aliku T, Lwabi P, Godown J, Beaton A. Simplified rheumatic heart disease screening criteria for handheld echocardiography. J Am Soc Echocardiogr. 2015;28:463–469. doi: 10.1016/j.echo.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 22.Nascimento BR, Nunes MC, Lopes EL, Rezende VM, Landay T, Ribeiro AL, Sable C, Beaton AZ. Rheumatic heart disease echocardiographic screening: approaching practical and affordable solutions. Heart. 2016;102:658–664. doi: 10.1136/heartjnl-2015-308635 [DOI] [PubMed] [Google Scholar]
- 23.Diamantino A, Beaton A, Aliku T, Oliveira K, Oliveira C, Xavier L, Perlman L, Okello E, Nascimento B, Ribeiro ALP, et al. A focussed single-view hand-held echocardiography protocol for the detection of rheumatic heart disease. Cardiol Young. 2018;28:108–117. doi: 10.1017/S1047951117001676 [DOI] [PubMed] [Google Scholar]
- 24.Remenyi B, Davis K, Draper A, Bayley N, Paratz E, Reeves B, Appelbe A, Wheaton G, da Silva Almeida IT, Dos Santos J, et al. Single parasternal-long-axis-view-sweep screening echocardiographic protocol to detect rheumatic heart disease: a prospective study of diagnostic accuracy. Heart Lung Circ. 2020;29:859–866. doi: 10.1016/j.hlc.2019.02.196 [DOI] [PubMed] [Google Scholar]
- 25.Paratz ED, Bayley N. Heart disease in East Timor: cross-sectional analysis of 474 patients attending Timor-Leste’s first cardiology service. Intern Med J. 2017;47:423–428. doi: 10.1111/imj.13381 [DOI] [PubMed] [Google Scholar]
- 26.Australian Bureau of Statistics. Maningrida (and outstations). 2016 Census QuickStats. Updated Oct 2017. Accessed September 1, 2019. https://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/IARE704003.
- 27.Francis JR, Gargan C, Remenyi B, Ralph AP, Draper A, Holt D, Krause V, Hardie K. A cluster of acute rheumatic fever cases among Aboriginal Australians in a remote community with high baseline incidence. Aust N Z J Public Health. 2019;43:288–293. doi: 10.1111/1753-6405.12893 [DOI] [PubMed] [Google Scholar]
- 28.Engelman D, Watson C, Remenyi B, Steer A. Echocardiographic Diagnosis of Rheumatic Heart Disease. Accessed September 1, 2019. https://www.wiredhealthresources.net/EchoProject/
- 29.Culliford-Semmens N, Nicholson R, Tilton E, Stirling J, Sidhu K, Webb R, Wilson N. The World Heart Federation criteria raise the threshold of diagnosis for mild rheumatic heart disease: Three reviewers are better than one. Int J Cardiol. 2019;291:112–118. doi: 10.1016/j.ijcard.2019.02.058 [DOI] [PubMed] [Google Scholar]
- 30.Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J. 2003;20:453–458. doi: 10.1136/emj.20.5.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunes MCP, Sable C, Nascimento BR, Lima EM, da Silva JLP, Diamantino AC, Oliveira KKB, Okello E, Aliku T, Lwabi P, et al. Simplified echocardiography screening criteria for diagnosing and predicting progression of latent rheumatic heart disease. Circ Cardiovasc Imaging. 2019;12:e007928. doi: 10.1161/CIRCIMAGING.118.007928 [DOI] [PubMed] [Google Scholar]
- 33.Bechtlufft BM, Nascimento BR, Sable C, Fraga CL, Barbosa MM, Reis SD, Diamantino AC, Meira ZMA, Castilho SRT, Arantes NF, et al. Validation of a simplified score for predicting latent rheumatic heart disease progression using a prospective cohort of Brazilian schoolchildren. BMJ Open. 2020;10:e036827. doi: 10.1136/bmjopen-2020-036827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirabel M, Bacquelin R, Tafflet M, Robillard C, Huon B, Corsenac P, de Frémicourt I, Narayanan K, Meunier JM, Noël B, et al. Screening for rheumatic heart disease: evaluation of a focused cardiac ultrasound approach. Circ Cardiovasc Imaging. 2015;8:e002324. doi: 10.1161/CIRCIMAGING.114.002324 [DOI] [PubMed] [Google Scholar]
- 35.Ploutz M, Lu JC, Scheel J, Webb C, Ensing GJ, Aliku T, Lwabi P, Sable C, Beaton A. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart. 2016;102:35–39. doi: 10.1136/heartjnl-2015-308236 [DOI] [PubMed] [Google Scholar]
- 36.Nascimento BR, Martins JFBS, Nascimento ER, Pappa GL, Sable CA, Beaton AZ, Gomes PR, Nunes MC, Oliveira KKB, Franco J, et al. Deep learning for automatic identification of rheumatic heart disease in echocardiographic screening images: data from the ATMOSPHERE-PROVAR study. J Am Coll Cardiol. 2020;75:3577. [Google Scholar]
- 37.IELTS. https://www.ielts.org/ (viewed November 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.