Purpose of review
Predicting treatment response and optimizing treatment regimen in patients with neovascular age-related macular degeneration (nAMD) remains challenging. Artificial intelligence-based tools have the potential to increase confidence in clinical development of new therapeutics, facilitate individual prognostic predictions, and ultimately inform treatment decisions in clinical practice.
Recent findings
To date, most advances in applying artificial intelligence to nAMD have focused on facilitating image analysis, particularly for automated segmentation, extraction, and quantification of imaging-based features from optical coherence tomography (OCT) images. No studies in our literature search evaluated whether artificial intelligence could predict the treatment regimen required for an optimal visual response for an individual patient. Challenges identified for developing artificial intelligence-based models for nAMD include the limited number of large datasets with high-quality OCT data, limiting the patient populations included in model development; lack of counterfactual data to inform how individual patients may have fared with an alternative treatment strategy; and absence of OCT data standards, impairing the development of models usable across devices.
Summary
Artificial intelligence has the potential to enable powerful prognostic tools for a complex nAMD treatment landscape; however, additional work remains before these tools are applicable to informing treatment decisions for nAMD in clinical practice.
Keywords: artificial intelligence, deep learning, machine learning, neovascular age-related macular degeneration, treatment prediction
INTRODUCTION
Although anti-vascular endothelial growth factor (anti-VEGF) therapy has been the gold standard for treating neovascular age-related macular degeneration (nAMD) for over a decade [1], predicting treatment response and optimizing treatment regimen remain challenging. Newer and emerging therapies are expected to provide additional treatment options for patients [2], increasing complexity of treatment decisions.
Artificial intelligence-based models have the potential to increase confidence in clinical development of new therapeutics, facilitate individual prognostic predictions, and ultimately inform treatment decisions in clinical practice. However, although much progress has been made in applying artificial intelligence to nAMD, significant barriers remain to bringing artificial intelligence-based models to individual patients.
Box 1.
no caption available
BACKGROUND
Anti-VEGF therapy, notably aflibercept (Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA), ranibizumab (Genentech, Inc., South San Francisco, California, USA), and off-label use of bevacizumab (Genentech, Inc.), became standard-of-care treatments for nAMD [3], following demonstration that dosing on a fixed treatment regimen provided significant vision gains, on average, from baseline in pivotal phase 3 clinical trials [1,4,5]. However, achieving these optimal outcomes involved frequent intravitreal injections; in clinical trials, mean vision gains from baseline of approximately seven to 11 letters at 1 year were achieved with approximately 7.5–12 total anti-VEGF injections [4–8].
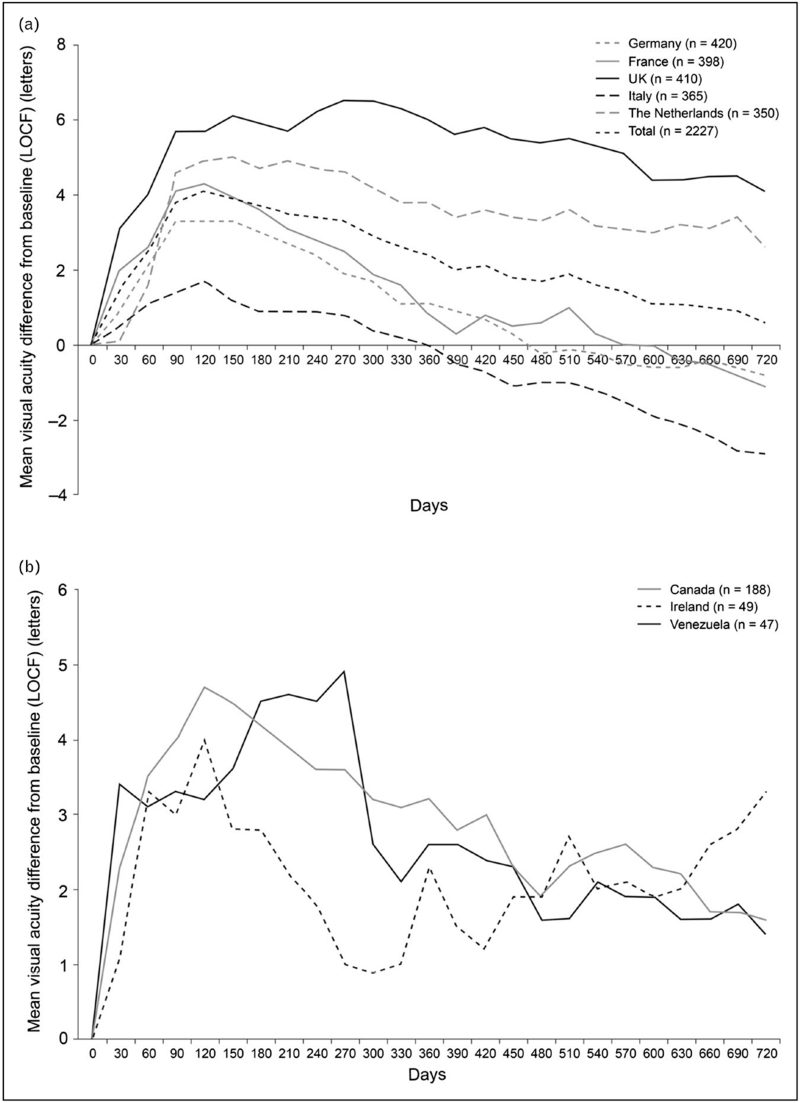
In contrast, patients in real-world clinical practice are not, on average, achieving these vision outcomes (Fig. 1) [9,10]. This has been attributed to several factors, particularly differences between real-world and clinical trial patient populations and differences in treatment frequency [11▪]. Notably, several studies, including large real-world studies [9,10,11▪,12,13▪,14] and a recent systematic review [15▪], found that dose frequency is a consistent indicator of vision outcomes, with real-world studies reporting average vision gains of zero to three letters with approximately five to seven injections in the first year of treatment.
FIGURE 1.
Mean change in visual acuity score from baseline over time for all patients by country: (a) Germany, France, United Kingdom, Italy, and the Netherlands, and (b) Canada, Ireland, and Venezuela in the AURA retrospective, observational, multicenter study of patients with neovascular age-related macular degeneration who started treatment with ranibizumab between January 1, 2009 and August 31, 2009 [10]. Data based on effectiveness analysis set using a last observation carried forward (LOCF) approach. The mean number of injections received in 2 full years was: United Kingdom, 9.0; the Netherlands, 8.7; France, 6.3; Germany, 5.6; Italy, 5.2; Ireland, 11.0; Canada, 9.9; Venezuela, 3.2. Figure reprinted from Ref [10].
This gap highlights the overall unmet need to balance anti-VEGF injection frequency and burden in clinical practice. To date, efforts to address this have focused on understanding which baseline characteristics are associated with treatment response and exploring different regimens, particularly as-needed (PRN) and treat-and-extend.
Several studies have identified baseline visual acuity as a consistent predictor of long-term visual outcomes [16–19]; however, it is thought to correlate indirectly with disease severity and anatomical changes of the neurosensory retina. Baseline anatomical features, including larger choroidal neovascularization (CNV) lesion size, ellipsoid zone disruption, external limiting membrane interruption, intraretinal fluid (IRF) presence, subretinal fluid (SRF) absence, and increased choroidal thickness have been associated with worse vision outcomes [16,19]. In a study of treatment frequency, patients with occult CNV, presence of retinal fluid, and fluorescein leakage after 1 year of fixed monthly/bimonthly dosing were less likely to achieve every-12-week dosing in year 2 [20].
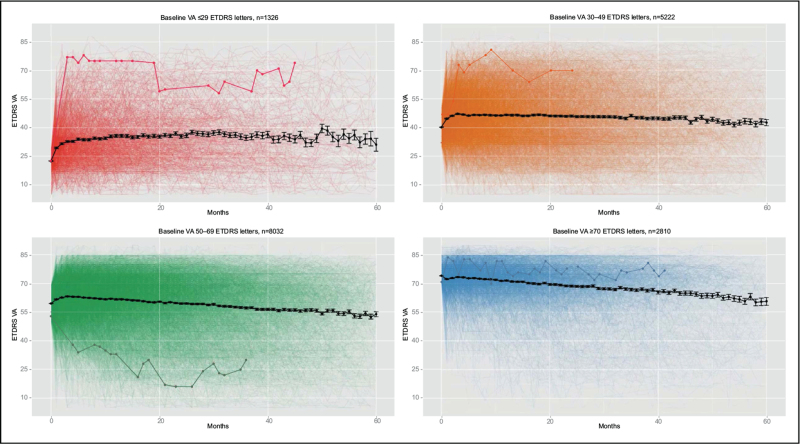
In the clinic, physicians make treatment decisions for individual patients, while treatment paradigms are traditionally based on average treatment response of a cohort. A key unsolved challenge is identifying the optimal treatment regimen for each individual, with the least burden and maximum visual gains, particularly because need for frequent treatment [20–22] and anti-VEGF treatment response under real-world conditions (Fig. 2; Supplemental Movie) vary greatly. For example, in the HARBOR clinical trial, treatment required by patients on a PRN regimen ranged from 3 to 24 injections over 2 years, with a nearly flat distribution [22]. In year 2 of the VIEW clinical trial, about half of patients had PRN treatment intervals of at least every 12 weeks, with similar vision outcomes as patients requiring more frequent treatment [20]. Although informative on a population level, these traditional analyses based on standard imaging evaluations have not greatly influenced individual treatment decisions.
FIGURE 2.
Illustration of individual responses to anti-vascular endothelial growth factor therapy for patients with neovascular age-related macular degeneration, stratified by baseline VA from a real-world large electronic medical records-extracted database [47]. Each faint line represents the VA (Early Treatment Diabetic Retinopathy Study [ETDRS] letter score) from one patient (one eye) over 5 years of time, with one line, representing one patient, bolded for illustration in each panel. Black lines represent the mean. See also Supplementary Movie. VA, visual acuity.
DEVELOPMENT AND APPLICATION OF ARTIFICIAL INTELLIGENCE MODELS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION
Artificial intelligence, and particularly the subfield of deep learning, has the potential to identify features prognostic for individual patient outcomes. However, most advances in applying artificial intelligence to nAMD have focused on development and application of models to facilitate image analysis, particularly for automated segmentation, extraction, and quantification of imaging-based features from optical coherence tomography (OCT). Key artificial intelligence-based models for OCT image analysis and recent applications of artificial intelligence to nAMD are discussed herein.
Training, tuning, and testing of artificial intelligence-based algorithms typically require large, high-quality datasets. Nonetheless, in nAMD, multiple research groups have developed artificial intelligence-based algorithms using relatively few datasets, of which HARBOR and the Moorfields Eye Hospital real-world age-related macular degeneration (AMD) database stand out in the literature.
The phase 3 HARBOR trial (NCT00891735) assessed ranibizumab for 1097 patients with treatment-naïve nAMD, comparing two dosages and monthly and PRN treatment regimens [8,22]. Notably, HARBOR was the first major clinical trial for nAMD to use spectral-domain OCT, which allows for high-sensitivity feature extraction.
Moorfields Eye Hospital, a tertiary referral retinal center in the United Kingdom, maintains a real-world database of electronic medical records and associated OCT images from patients with AMD treated with at least one ranibizumab or aflibercept injection from 2008 to 2018 and with at least 1 year of follow-up [23]. Altogether, the Moorfields AMD dataset includes 8174 eyes of 6664 patients; a de-identified version of the segmentation results is openly available to the research community [23,24▪].
A key model for OCT image segmentation and disease classification, developed by De Fauw and colleagues [25], uses a deep learning-based framework with two independent networks to perform automated diagnosis of retinal diseases on OCT scans. This methodology has been applied to investigating imaging biomarkers and visual outcomes [24▪,26▪▪]. Following this, another group developed a novel automated segmentation model using a convolutional neural network [27▪]. This model was built using a large, real-world electronic medical records-based dataset from the United Kingdom, annotated by clinical experts with 13 of the most common AMD biomarkers on OCT, including IRF, SRF, and pigment epithelial detachment (PED) [27▪].
The Notal OCT Analyzer [28] and Medical University of Vienna artificial intelligence-based Fluid Monitor [29] are fully automated tools for fluid detection and quantification on OCT images. These have facilitated quantitative measurements across multiple large datasets [30▪] and been applied to questions investigating retinal fluid measurements and visual outcomes [17,30▪,31,32▪,33▪,34▪,35], particularly to more precisely quantify and map changes in IRF and SRF over time.
As an illustration, application of the Notal OCT Analyzer to a real-world dataset demonstrated that, by quartile, larger fluctuations in IRF, SRF, PED, central subfield thickness, and total fluid during the anti-VEGF maintenance phase were associated with worse visual acuity at 2 years [34▪]. Other exploratory studies applying the artificial intelligence-based Fluid Monitor supported differential impact of IRF and SRF on vision outcomes. In both the HARBOR and FLUID trials, increased IRF, but not SRF, volumes in the central 1 mm were negatively associated with visual acuity [letters per 100 nl fluid, IRF: –4.00 and –2.84; SRF: +1.10 and +1.43 (not significant), respectively] [32▪,33▪]. Similarly, a stronger association of IRF than SRF with visual acuity was found by applying the De Fauw et al.[25] methodology to the Moorfields AMD database [24▪].
Other studies have developed and applied artificial intelligence-based models to OCT image analysis for a diverse set of research questions, including determining whether visual acuity can be predicted from OCT [36]; extracting higher-order features, such as ellipsoid zone integrity and subretinal hyperreflective material volume [37]; facilitating correlational analysis among multiple features on OCT [35]; comparing ‘typical’ nAMD with polypoidal choroidal vasculopathy [38,39]; and clustering patients based on CNV features using unsupervised machine learning [39].
PREDICTING NEOVASCULAR AGE-RELATED MACULAR DEGENERATION TREATMENT RESPONSE WITH ARTIFICIAL INTELLIGENCE
Several groups have begun to develop algorithms for predicting treatment response and treatment frequency needs. Within the parameters of our literature search, three studies to date specifically examined treatment response using artificial intelligence, with each approaching and defining response differently.
Two studies focused on the anatomic response to anti-VEGF treatment on OCT. The first developed a novel method with convolutional neural networks, using data from a real-world cohort at Second Affiliated Hospital of Xi’an Jiaotong University. The study found that effectiveness of anti-VEGF treatment on CNV or cystoid macular edema could be predicted with area under the curve of 0.81 using baseline OCT images [40]. However, ‘effective’ appeared to be a binary treatment response that was not clearly defined. In the other study, a conditional generative adversarial network was used to develop a deep learning model capable of generating posttreatment OCT images [41▪▪]. This model, trained on a real-world retrospective dataset from Konkuk University Medical Center, was designed to generate OCT images representing 1 month after completion of three monthly anti-VEGF loading doses. A model including baseline OCT, fluorescein angiography, and indocyanine green angiography images, rather than OCT images alone, performed best in its prediction of each of IRF, SRF, PED, and subretinal hyperreflective material [41▪▪].
To explore predictive ability of quantitative OCT parameters for posttreatment visual outcomes, Fu et al.[26▪▪] applied De Fauw et al.'s[25] deep learning method to the Moorfields AMD database. Together, baseline visual acuity and OCT parameters had a predictive accuracy for 3 months post injection and 12 months post baseline of R2 = 0.49 and 0.38, respectively, which improved to R2 = 0.79 and 0.63 by incorporating previous treatment response (incremental visual acuity and OCT changes).
Finally, one group developed an end-to-end deep learning model for predicting treatment requirements for patients receiving anti-VEGF on a PRN regimen per investigator discretion; the specific patient population was not identified [42▪▪]. OCT images were analyzed based on previous models [29] for fluid quantification to exclude patients for whom model and investigator decisions disagreed on more than three noninjection events over 2 years. Based on longitudinal images, the model categorized patients as having ‘low,’ ‘intermediate,’ and ‘high’ treatment requirements (up to five, five to 15, and ≥16 injections, respectively). Although the model did not perform well classifying patients in the intermediate group, area under the curve of 0.85 and 0.81 was achieved in binary classifications of low versus all or high versus all treatment requirements [42▪▪]. However, this study did not ultimately correlate these treatment requirements with vision outcomes [42▪▪].
CHALLENGES FOR ARTIFICIAL INTELLIGENCE-BASED MODEL DEVELOPMENT IN NEOVASCULAR AGE-RELATED MACULAR DEGENERATION
Highlighting challenges in the field to date, no studies were identified in our literature search that evaluated whether artificial intelligence-based models could predict the treatment regimen required for an ‘optimal’ visual response for an individual patient. Thus far, studies have largely explored anti-VEGF treatment response, either indirectly, by studying association between OCT parameters and vision outcomes, or directly, by approaching the question of whether treatment response could be predicted based on retinal images.
A well-known issue in machine learning is that artificial intelligence-based models reflect the biases inherent to the datasets used to develop them. Unfortunately, in nAMD, few large datasets with high-quality spectral-domain OCT data are available, and these same datasets have been utilized by multiple groups for training, tuning, and testing of artificial intelligence-based models. This has also limited the nAMD population characteristics included in the models to date. Clinical trial populations, defined by specific inclusion and exclusion criteria, are generally more homogenous and less demographically diverse than real-world populations. In contrast, real-world patient populations, such as the Moorfields AMD database [23], have larger variability in demographics, disease state and severity, treatment approaches, and OCT imaging schedule and protocols.
Lack of counterfactual data is another significant limitation for both model development and judging a model's ability to predict treatment needs. In the context of nAMD, each patient is unique in their disease, baseline clinical presentation, and treatment response; it may be argued that a specific pretreatment state cannot be recreated. Therefore, it may not be possible to assess how that patient may have fared with an alternative treatment strategy or to ascertain their best-achievable vision outcomes. A potential strategy to mitigate this limitation is to ensure that large, diverse patient populations with accurate, thorough data are used for model development. Absenting that, artificial intelligence-based models may not accurately apply to individual patients and will carry forward biases of the datasets used to build them.
Finally, absence of OCT data standards [43] impacts both availability of high-quality datasets for artificial intelligence model development and generalizability of these models. As a result, models created to date are generally device specific, impairing their broader application to clinical practice where different OCT devices are in use. To be useful in clinical practice, artificial intelligence-based models will need to be designed for functionality and interpretability outside of controlled research settings.
APPLICATIONS OF ARTIFICIAL INTELLIGENCE-BASED NEOVASCULAR AGE-RELATED MACULAR DEGENERATION TREATMENT PREDICTIONS
Artificial intelligence-based nAMD treatment predictions have potential applications for both clinical research and clinical practice, with the goal of achieving the best visual outcome for each individual patient.
In clinical research, artificial intelligence-based models could improve clinical trial design, including patient identification, selection, and randomization, as well as adjustments in trial analysis. Artificial intelligence can also improve efficiency and standardization of image grading, enabling analysis on a larger and more detailed scale than possible with current practices and standard technologies. For smaller, early-stage studies, or those with heterogeneous populations, application of artificial intelligence-based models could improve understanding of treatment responses and increase confidence in decision making. Artificial intelligence can also create ‘synthetic’ treatment arms, that is, hypothetical, simulated comparator arms that could be used to model additional patient populations or alternative treatments for clinical trials, including sham arms [44,45▪].
In clinical practice, considering treatment options for nAMD currently available, physician decisions are limited by the optimal treatment regimens for maximum visual gains and least treatment burden. As an extreme example, physicians following a monthly treatment regimen would not have a compelling motivation to use an artificial intelligence-based prediction model. However, in the near future, complexity of treatment decisions is expected to increase with the expansion of the nAMD treatment landscape to potentially include new mechanisms of action, long-acting delivery options, and gene therapy [2].
Within the field of retina, and particularly OCT image analysis, artificial intelligence has the potential to assist physicians in elucidating individual needs as quickly and accurately as possible, thereby improving patient care, in several ways. First, artificial intelligence can equip physicians with better models for efficient image analysis, which could expand the information readily available for making treatment decisions. Also, given the variety of clinical expertise, artificial intelligence could raise the bar of standard of care by providing insights into pathology that may fall outside a particular physician's day-to-day experience. Finally, artificial intelligence can extract features beyond what an expert can discern on individual images; for example, a deep learning model was developed to create OCT angiography-like images from structural OCT [46].
CONCLUSION
Artificial intelligence-based models can potentially improve clinical research and clinical practice in nAMD, enabling best visual outcomes with least treatment burden for each individual patient. Artificial intelligence has facilitated analysis of OCT and multimodal image datasets on a scale not previously possible, furthering knowledge of the disease and response to treatment. Furthermore, artificial intelligence has a number of applications to clinical trial design, implementation, and analysis, which could improve the process of clinical development at all stages and improve confidence in decision making, particularly for early-stage clinical trials. Artificial intelligence also has the potential to create powerful tools to inform point-of-care treatment decisions in a treatment landscape for nAMD of increasing complexity. However, a large gap remains between application of artificial intelligence to research and application to treatment decisions in clinical practice. A key limitation toward this goal is the shortage of large, robust datasets that represent the heterogeneity of the patients, their disease, and treatment response.
Acknowledgements
Kathryn Condon, for scientific and medical writing expertise.
Financial support and sponsorship
Funding was provided by Genentech, Inc., a member of the Roche Group, for the study and third-party writing assistance, which was provided by Kathryn H. Condon, PhD, CMPP, of Envision Pharma Group.
Conflicts of interest
D.F. and E.M.N. are employees of Genentech, Inc. and report stock/stock options in F. Hoffmann-La Roche. A.Y.L. reports support from the US Food and Drug Administration; grants from Carl Zeiss Meditec, Novartis, Regeneron, and Santen; personal fees from Genentech, Inc., Gyroscope, Johnson & Johnson, Topcon, and Verana Health, outside of the submitted work. This article does not reflect the opinions of the US Food and Drug Administration.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov 2016; 15:385–403. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khersan H, Hussain RM, Ciulla TA, Dugel PU. Innovative therapies for neovascular age-related macular degeneration. Expert Opin Pharmacother 2019; 20:1879–1891. [DOI] [PubMed] [Google Scholar]
- 3.Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration Preferred Practice Pattern®. Ophthalmology 2020; 127:1–65. [DOI] [PubMed] [Google Scholar]
- 4.Heier JS, Brown DM, Chong V, et al. VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology 2012; 119:2537–2548. [DOI] [PubMed] [Google Scholar]
- 5.Martin DF, Maguire MG, Ying G-S, et al. CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355:1432–1444. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355:1419–1431. [DOI] [PubMed] [Google Scholar]
- 8.Busbee BG, Ho AC, Brown DM, et al. HARBOR Study Group. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013; 120:1046–1056. [DOI] [PubMed] [Google Scholar]
- 9.Ciulla TA, Huang F, Westby K, et al. Real-world outcomes of anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol Retina 2018; 2:645–653. [DOI] [PubMed] [Google Scholar]
- 10.Holz FG, Tadayoni R, Beatty S, et al. Multicountry real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015; 99:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Holz FG, Figueroa MS, Bandello F, et al. LUMINOUS study investigators. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from LUMINOUS, a global real-world study. Retina 2020; 40:1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large, 5-year, multinational, prospective, real-world, observational study of 6241 patients with treatment-naïve nAMD. Examined relationship between mean visual acuity at 1 year and anti-VEGF injection frequency, loading doses, and baseline visual acuity.
- 12.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014; 121:1092–1101. [DOI] [PubMed] [Google Scholar]
- 13▪.Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti–vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina 2020; 4:19–30. [DOI] [PubMed] [Google Scholar]; Retrospective US-based electronic medical records study including 49 485 eyes of treatment-naïve patients with nAMD who received anti-VEGF injections. Showed correlation between the number of injections in the first year and mean visual acuity change.
- 14.Rao P, Lum F, Wood K, et al. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS Registry. Ophthalmology 2018; 125:522–528. [DOI] [PubMed] [Google Scholar]
- 15▪.Spaide RF. Antivascular endothelial growth factor dosing and expected acuity outcome at 1 year. Retina 2021; 41:1153–1163. [DOI] [PubMed] [Google Scholar]; Systematic review and analysis of 96 published randomized clinical trials and observational studies; defined relationship between anti-VEGF dose frequency and visual acuity change at 1 year in patients with nAMD.
- 16.Regillo CD, Busbee BG, Ho AC, et al. Baseline predictors of 12-month treatment response to ranibizumab in patients with wet age-related macular degeneration. Am J Ophthalmol 2015; 160:1014–1023.e2. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Erfurth U, Bogunovic H, Sadeghipour A, et al. Machine learning to analyze the prognostic value of current imaging biomarkers in neovascular age-related macular degeneration. Ophthalmol Retina 2018; 2:24–30. [DOI] [PubMed] [Google Scholar]
- 18.Gill CR, Hewitt CE, Lightfoot T, Gale RP. Demographic and clinical factors that influence the visual response to anti-vascular endothelial growth factor therapy in patients with neovascular age-related macular degeneration: a systematic review. Ophthalmol Ther 2020; 9:725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Lai TYY. Baseline predictors of visual acuity outcome in patients with wet age-related macular degeneration. Biomed Res Int 2018; 2018:9640131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khurana RN, Rahimy E, Joseph WA, et al. Extended (every 12 weeks or longer) dosing interval with intravitreal aflibercept and ranibizumab in neovascular age-related macular degeneration: post hoc analysis of VIEW trials. Am J Ophthalmol 2019; 200:161–168. [DOI] [PubMed] [Google Scholar]
- 21.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020; 127:72–84. [DOI] [PubMed] [Google Scholar]
- 22.Ho AC, Busbee BG, Regillo CD, et al. HARBOR Study Group. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014; 121:2181–2192. [DOI] [PubMed] [Google Scholar]
- 23.Fasler K, Moraes G, Wagner S, et al. One- and two-year visual outcomes from the Moorfields age-related macular degeneration database: a retrospective cohort study and an open science resource. BMJ Open 2019; 9:e027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.Moraes G, Fu DJ, Wilson M, et al. Quantitative analysis of OCT for neovascular age-related macular degeneration using deep learning. Ophthalmology 2021; 128:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]; Application of the artificial intelligence-based methodology developed by De Fauw et al. 2018 to the Moorfields Eye Hospital AMD database, enabling large-scale quantitative analysis of nine segmented features on OCT, and their correlation with visual acuity, in over 2500 patients.
- 25.De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med 2018; 24:1342–1350. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Fu DJ, Faes L, Wagner SK, et al. Predicting incremental and future visual change in neovascular age-related macular degeneration using deep learning. Ophthalmol Retina 2021; doi:10.1016/j.oret.2021.01.009. [DOI] [PubMed] [Google Scholar]; Building from the Moraes et al. 2021 artificial intelligence-enabled quantitative OCT analysis, Fu et al. 2021 evaluated whether future visual outcomes could be predicted based on baseline visual acuity and quantitative OCT parameters for patients with nAMD receiving anti-VEGF therapy.
- 27▪.Liefers B, Taylor P, Alsaedi A, et al. Quantification of key retinal features in early and late age-related macular degeneration using deep learning. Am J Ophthalmol 2021; 226:1–12. [DOI] [PubMed] [Google Scholar]; Novel deep learning model for the segmentation of 13 features on OCT commonly associated with neovascular or atrophic AMD; includes features beyond the fluid-based parameters included in previous models. Demonstrated equal or superior performance to human annotation for several features.
- 28.Chakravarthy U, Goldenberg D, Young G, et al. Automated identification of lesion activity in neovascular age-related macular degeneration. Ophthalmology 2016; 123:1731–1736. [DOI] [PubMed] [Google Scholar]
- 29.Schlegl T, Waldstein SM, Bogunovic H, et al. Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology 2018; 125:549–558. [DOI] [PubMed] [Google Scholar]
- 30▪.Keenan TDL, Chakravarthy U, Loewenstein A, et al. Automated quantitative assessment of retinal fluid volumes as important biomarkers in neovascular age-related macular degeneration. Am J Ophthalmol 2021; 224:267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]; Application of artificial intelligence-based quantitative OCT analysis to three real-world studies and one clinical trial (HARBOR) of nAMD, demonstrating how artificial intelligence facilitates large-scale longitudinal analyses.
- 31.Michl M, Fabianska M, Seeböck P, et al. Automated quantification of macular fluid in retinal diseases and their response to anti-VEGF therapy. Br J Ophthalmol 2020; doi:10.1136/bjophthalmol-2020-317416. [DOI] [PubMed] [Google Scholar]
- 32▪.Reiter GS, Grechenig C, Vogl W-D, et al. Analysis of fluid volume and its impact on visual acuity in the FLUID study as quantified with deep learning. Retina 2021; 41:1318–1328. [DOI] [PubMed] [Google Scholar]; Application of artificial intelligence-based quantitative OCT analysis to the FLUID study; evaluated association of IRF/SRF with vision outcomes.
- 33▪.Schmidt-Erfurth U, Vogl WD, Jampol LM, Bogunović H. Application of automated quantification of fluid volumes to anti-VEGF therapy of neovascular age-related macular degeneration. Ophthalmology 2020; 127:1211–1219. [DOI] [PubMed] [Google Scholar]; Application of artificial intelligence-based quantitative OCT analysis to the HARBOR trial; evaluated association of IRF/SRF with vision outcomes.
- 34▪.Chakravarthy U, Havilio M, Syntosi A, et al. Impact of macular fluid volume fluctuations on visual acuity during anti-VEGF therapy in eyes with nAMD. Eye (Lond) 2021; doi:10.1038/s41433-020-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Application of artificial intelligence-based quantitative OCT analysis to data extracted from electronic medical records; evaluated association of fluid fluctuations with vision outcomes.
- 35.Riedl S, Cooney L, Grechenig C, et al. Topographic analysis of photoreceptor loss correlated with disease morphology in neovascular age-related macular degeneration. Retina 2020; 40:2148–2157. [DOI] [PubMed] [Google Scholar]
- 36.Kawczynski MG, Bengtsson T, Dai J, et al. Development of deep learning models to predict best-corrected visual acuity from optical coherence tomography. Transl Vis Sci Technol 2020; 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehlers JP, Zahid R, Kaiser PK, et al. Longitudinal assessment of ellipsoid zone integrity, subretinal hyperreflective material, and sub-RPE disease in neovascular. Ophthalmol Retina 2021; doi:10.1016/j.oret.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H, Jang M, Kim HC, Chung H. Association of imaging factors derived from convolutional neural network with visual outcomes in age-related macular degeneration and polypoidal choroidal vasculopathy. Sci Rep 2019; 9:19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosoda Y, Miyake M, Yamashiro K, et al. Deep phenotype unsupervised machine learning revealed the significance of pachychoroid features in etiology and visual prognosis of age-related macular degeneration. Sci Rep 2020; 10:18423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng D, Chen X, Zhou Z, et al. A preliminary study of predicting effectiveness of anti-VEGF injection using OCT images based on deep learning. Annu Int Conf IEEE Eng Med Biol Soc 2020; 2020:5428–5431. [DOI] [PubMed] [Google Scholar]
- 41▪▪.Lee H, Kim S, Kim MA, et al. Post-treatment prediction of optical coherence tomography using a conditional generative adversarial network in age-related macular degeneration. Retina 2021; 41:572–580. [DOI] [PubMed] [Google Scholar]; Developed a deep learning model that generates OCT images for 1 month after three anti-VEGF loading doses for nAMD using baseline OCT, fluorescein angiography, and indocyanine green angiography images. Evaluates where the model did and did not accurately predict posttreatment OCT features.
- 42▪▪.Romo-Bucheli D, Erfurth US, Bogunovic H. End-to-end deep learning model for predicting treatment requirements in neovascular AMD from longitudinal retinal OCT imaging. IEEE J Biomed Health Inform 2020; 24:3456–3465. [DOI] [PubMed] [Google Scholar]; Developed and evaluated an end-to-end model for predicting patients with nAMD requiring ‘low’ or ‘high’ treatment anti-VEGF frequency on a PRN regimen over 2 years.
- 43.Lee AY, Campbell JP, Hwang TS, et al. American Academy of Ophthalmology. Recommendations for standardization of images in ophthalmology. Ophthalmology 2021; 128:969–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas DS, Lee AY, Müller PL, et al. UK AMD EMR Users Group. Contextualizing single-arm trials with real-world data: an emulated target trial comparing therapies for neovascular age-related macular degeneration. Clin Transl Sci 2021; 14:1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Lee CS, Lee AY. How artificial intelligence can transform randomized controlled trials. Transl Vis Sci Technol 2020; 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reviews the potential applications of artificial intelligence to clinical trial design and analysis.
- 46.Lee CS, Tyring AJ, Wu Y, et al. Generating retinal flow maps from structural optical coherence tomography with artificial intelligence. Sci Rep 2019; 9:5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz R, Warwick A, Olvera-Barrios A, et al. Evolving treatment patterns and outcomes of neovascular age-related macular degeneration over a decade. Ophthalmol Retina 2021; doi:10.1016/j.oret.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.