Objective:
Anal cancer precursor lesions high-grade anal intraepithelial neoplasia (HGAIN) are highly prevalent among HIV+ MSM. Treatment of HGAIN is frustrated by high recurrence rates. We investigated the efficacy of the quadrivalent human papillomavirus (qHPV) vaccine as posttreatment adjuvant in preventing HGAIN recurrence in HIV+ MSM.
Design:
Randomized, double-blind, placebo-controlled, multicentre trial.
Setting:
Three HIV outpatient clinics in Amsterdam, the Netherlands.
Subjects:
HIV+ MSM with CD4+ cell count more than 350 cells/μl, biopsy-proven intra-anal HGAIN successfully treated in the past year, and lesions still in remission at enrolment, as assessed by high-resolution anoscopy (HRA).
Intervention:
Participants were randomized to three doses of qHPV (Gardasil-4, MSD) or placebo with vaccinations at 0, 2, and 6 months. HRA was repeated at 6, 12, and 18 months.
Main outcome measure:
The primary outcome was cumulative, biopsy-proven HGAIN recurrence rate at 18 months, evaluated in an intention-to-treat (ITT) (received all vaccinations) and per-protocol analysis (all vaccinations and complete follow-up).
Results:
We randomized 126 participants of which 64 (50.8%) received qHPV and 62 (49.2%) placebo. All participants received three vaccinations, and in both groups for two participants follow-up was incomplete. We found no difference (P = 0.38) in cumulative HGAIN recurrence rates between the qHPV (44/64, 68.8%) and placebo group (38/62, 61.3%) in the ITT analysis [absolute risk reduction −7.5 (95% confidence interval (CI) −24.1 to 9.2)]. This was similar in the per-protocol analysis.
Conclusion:
Despite adequate serological responses to qHPV vaccination, short-term recurrence of HGAIN was not prevented. These findings do not support qHPV vaccination as a treatment adjuvant to prevent HGAIN recurrence in HIV+ MSM.
Keywords: anal intraepithelial neoplasia, anal squamous intraepithelial lesions, human papillomavirus, prophylactic quadrivalent HPV vaccination, treatment recurrence
Introduction
Since the introduction of combination antiretroviral therapy (cART) for treatment of HIV infection, new causes of morbidity and mortality have become evident. In people with HIV (PWH), particularly HIV-positive (HIV+) MSM, anal cancer is an increasing problem, with incidence rates up to 85 times higher than in the general population (85 cases per 100 000 person-years versus 1–2 per 100 000, respectively). However, also HIV-negative MSM have a substantially increased risk of developing anal cancer (19 per 100 000) [1]. Like cervical cancer, anal cancer is causally linked to infections with high-risk human papillomaviruses (HPV) [2]. Anal cancer is preceded by precursor lesions called anal intraepithelial neoplasia (AIN), also known as anal squamous intraepithelial lesions (aSIL). AIN can be subdivided into high-grade (HG)AIN (or HSIL) and low-grade (LG)AIN (or LSIL) [3]. Over 90% of HIV+ MSM have persistent anal HPV infections, and HGAIN (AIN2/3) is present in 29% of HIV+ MSM [4]. As in cervical intraepithelial neoplasia, early diagnosis and treatment of HGAIN have been advocated to prevent malignancy [5]. However, treatment of HGAIN in HIV+ MSM (using ablative techniques or topical creams) is frustrated by high recurrence rates, which can be over 50% after 12 months [6–8]. Moreover, treatment is costly and burdensome for patients [9].
The prophylactic quadrivalent HPV vaccine (qHPV) is highly efficacious as primary prevention against new persistent cervical infections with high-risk HPV (hrHPV) types 16 and 18 and low-risk HPV (lrHPV) types 6 and 11, and high-grade cervical intraepithelial neoplasia (HGCIN) [10]. Likewise, it is efficacious in young men (16–26 years) in preventing new anal HPV infections and anogenital condylomata acuminata, caused by lrHPV [11]. Vaccination with qHPV also reduced the risk for AIN by 54.2% in young HIV-negative MSM without a history of AIN and a maximum of five sex partners [12].
In recent years, several studies reported a secondary prevention role for the qHPV vaccine. In a nonconcurrent cohort study, qHPV vaccination significantly reduced (hazard ratio = 0.50), the HGAIN recurrence rate at 2 years after qHPV vaccination in HIV-negative MSM successfully treated for HGAIN [13]. A recent meta-analysis also indicated efficacy of qHPV vaccination in preventing recurrent HGCIN lesions in women who were vaccinated around treatment for HGCIN [14], although a recent randomized controlled trial (RCT) could not confirm this for HIV+ women [15]. The qHPV vaccine has proven to be immunogenic and well tolerated in PWH [16]. We, therefore, did a RCT to test the hypothesis that qHPV vaccination as a posttreatment adjuvant prevents recurrence of HGAIN in HIV+ MSM successfully treated for HGAIN in the year before vaccination, and assessed HPV type-specific antibody response and causative HPV types in recurrent HGAIN lesions.
Methods
Study design
We performed a randomized, parallel, placebo-controlled, double-blind, multicentre, phase IV trial (VACCAIN-P study) in three outpatient clinics in Amsterdam, the Netherlands (see Supplementary Methods). This study was investigator-initiated and government-granted. The study protocol (DOI: https://doi.org/10.21942/uva.12861044.v1) was approved by the ethics review board at the Academic Medical Center, Amsterdam, the Netherlands. This trial is registered with ClinicalTrials.gov, number NCT02087384.
Study participants
We recruited participants from the anal cancer screening program of three outpatient clinics for HIV and dermatology. We obtained written informed consent from all participants and screened for eligibility as described under ‘Procedures’.
Main eligibility criteria were: HIV+ MSM of at least 18 years of age, who had a CD4+ cell count greater than 350 cells/μl, had biopsy-proven intra-anal HGAIN, which was successfully treated in the past year [lesions with partial remission (from HGAIN to LGAIN (AIN1)] were also eligible, and had lesions still in remission (maximum LGAIN) at enrolment as established independently by two experienced high-resolution anoscopists at least 4 weeks after last treatment. For full eligibility criteria, see Supplementary Methods.
Randomization and masking
If eligible after screening, we randomly assigned participants to vaccination with qHPV or placebo in a 1 : 1 ratio using an independent web-based randomization tool. Randomization was stratified for treatment centre (academic versus nonacademic), timing of first vaccination after last treatment for intra-anal HGAIN (treatment ≤6 versus >6 months before vaccination), and result of last treatment for intra-anal HGAIN [complete versus partial (from HGAIN to LGAIN) remission]. For details on randomization and masking see Supplementary Methods. Participants, investigators, and those assessing outcomes were masked to treatment allocation throughout the entire study until database lock for analysis.
Procedures
At time of screening for eligibility, a medical record review and laboratory tests were performed. In addition, sociodemographic and sexual history characteristics, smoking status, and self-reported health status were recorded. Participants underwent digital anal--rectal examination (DARE), genital inspection and high-resolution anoscopy (HRA) by two independent experienced anoscopists, with biopsies if indicated as described previously [8], in a single or two separate screening HRA sessions (see Supplementary Methods), adhering to the International Anal Neoplasia Society Guidelines [17,18].
Participants were randomized to the qHPV L1 virus-like particle (VLP) vaccine (Gardasil-4; Merck Sharp & Dohme (MSD), Kenilworth, New Jersey, USA) or a placebo (0.9% saline). The first qHPV or placebo vaccine was administered within 3 months after the first screening HRA and 6 weeks after the second screening HRA, and subsequent vaccines 2 months (±1 week), and 6 months (±2 weeks) after first vaccination. Injections, 0.5 ml in the deltoid muscle, were generally given on the same side throughout the study.
DARE, genital inspection, and HRA were repeated at 6 months (FU6; combined with last vaccination), at 12 months (FU12), and at 18 months after first vaccination (FU18; 12 months after last vaccination), allowing ±2 weeks deviation from the interval. The focus of this trial was on HGAIN recurrence, therefore, in case of HGAIN recurrence during the study (primary endpoint reached), follow-up, also regarding secondary endpoints, was discontinued and treatment was started according to local procedures.
Biopsies were graded locally by board-certified pathologists, experienced in AIN histopathology. P16INK4Aimmunohistochemical staining was used, if necessary, to distinguish between LGAIN and HGAIN as recommended by the College of American Pathologists [19]. HGAIN recurrences were histopathologically confirmed by a second pathologist. Anal cytology was performed in participants that reached the last follow-up at 18 months as an additional check to rule out HGAIN recurrences not detected by HRA (see Supplementary Methods).
To determine whether recurrent HGAIN was vaccine type-induced, the causative HPV type in recurrent HGAIN lesions was assessed by HPV detection and genotyping at DDL Diagnostic Laboratory, Rijswijk, the Netherlands, using the SPF10 DEIA/LiPA 25 version 1 system (see Supplementary Methods). In case of multiple HPV types in the whole tissue section, including at least one vaccine type, laser-capture microdissection was used to determine the causative HPV type per biopsy region, as previously described [20]. Causative HPV types were assessed on a participant level. In case of multiple lesions per participant, a participant was considered having a vaccine-type recurrent HGAIN if at least one recurrent lesion was caused by a vaccine type.
HPV type-specific antibody response was assessed in venous blood samples, obtained before first vaccination (pre) and 3 months (±2 weeks) after last vaccination (post). Serum HPV-specific IgG antibodies against HPV L1 VLPs for vaccine HPV types 16, 18, 6, 11 were measured using a VLP-based multiplex immunoassay performed at the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands, as previously described (see Supplementary Methods) [21,22].
To assess vaccine safety, (serious) adverse event [(S)AE], including injection-site reactions (pain, erythema, swelling) and systemic adverse events (including fever and headache) were recorded by history taking and medical record review 1 week after the vaccinations and at all follow-up visits. Adverse events that were possibly, probably, or definitely related to vaccination, as determined by the investigator, were considered vaccine-related. (S)AEs were classified and graded according to the CTCAE version 4.0, 2010 (on a scale of 1 to 5, with higher scores indicating greater severity) [23].
Outcomes
The primary outcome was cumulative recurrence of biopsy-proven intra-anal or peri-anal HGAIN at 12 months after last vaccination (FU18). Secondary outcomes were: recurrence of intra-anal or peri-anal HGAIN at time of last vaccination (FU6) and at 6 months after last vaccination (FU12), cumulative occurrence of LGAIN at FU18, cumulative occurrence of anogenital condylomata at FU18, causative HPV genotype in recurrent HGAIN lesions, HPV type-specific antibody response after vaccination, and safety of the qHPV vaccine.
Statistical analyses
For sample size calculation see Supplementary Methods. Statistical analyses were performed as stated in the Statistical Analysis Plan (DOI: https://doi.org/10.21942/uva.12861026.v1) and using software stated in the Supplementary Methods. The primary outcome (cumulative recurrence of HGAIN at FU18) was evaluated for all randomized participants who received all three vaccinations [intention-to-treat (ITT) analysis), and for participants who received all three vaccinations and completed the follow-up (per-protocol analysis). Absolute risk reduction (ARR), and relative risks (RR), including 95% confidence intervals (CI), were estimated for the difference in recurrence rate between the qHPV vaccine and placebo group. We constructed Kaplan--Meier survival curves with corresponding log-rank tests to compare proportions free of recurrence between the qHPV and placebo group. Participants lost to follow-up were censored at their last visit. Incidence rates including 95% CIs were calculated per 100 person-years in the ITT analysis (see Supplementary Methods). Vaccine efficacy was calculated as 1 − (IRvaccine/IRplacebo).
Determinants of HGAIN recurrence at FU18 were assessed using a univariable and multivariable logistic regression model (see Supplementary Methods), forcing the vaccination group into the multivariable model, as well as the interactions between the vaccination group and the three stratification factors. We estimated odds ratios (OR) and adjusted OR (aOR) with their corresponding 95% CIs.
We performed sensitivity analyses using a worst-case scenario (i.e. participants who were lost to follow-up developed HGAIN if they were free of disease at their last attended visit) and a best-case scenario (i.e. all participants lost to follow-up did not develop the disease if they were free of disease at their last attended visit).
Secondary outcomes were only assessed by ITT analysis (for details see Supplementary Methods).
Results
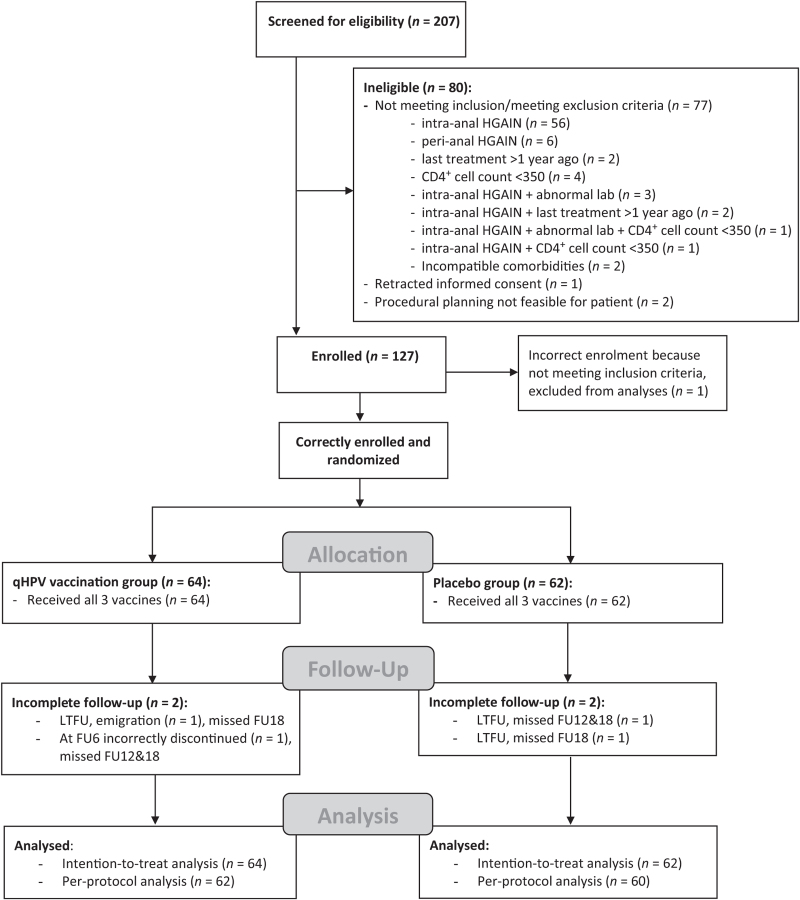
Enrolment started on 27 March 2014 and was completed on 1 June 2017. A total of 207 HIV+ MSM were screened for eligibility (Fig. 1). One hundred and twenty-seven (61.4%) men were enrolled and randomized. After randomization, one participant turned out to have had his previous HGAIN treatment more than 1 year before vaccination, thus incorrectly enrolled. His study participation was discontinued and he was excluded from the analyses. Of 126 correctly included and randomized participants, 64 (50.8%) were vaccinated with qHPV and 62 (49.2%) with placebo. All participants received all three vaccines and were eligible for the ITT analysis. All but four (3.2%) participants, two in each group, completed the planned follow-up (i.e. total follow-up to FU18 or earlier when reaching the endpoint HGAIN recurrence at FU6 or FU12). Therefore, 122 participants were eligible for the per-protocol analysis, of whom 62 participants received qHPV and 60 placebo. The study groups were well balanced, although the qHPV group had higher CD4+ cell counts at enrolment (Table 1). The trial ended on 20 February 2019 after completion of the planned follow-up for all participants and was conducted in accordance with the protocol.
Fig. 1.
Trial profile.
FU, follow-up; HGAIN, high-grade anal intraepithelial neoplasia; LTFU, lost to follow-up; qHPV, quadrivalent human papillomavirus vaccine.
Table 1.
Baseline characteristics of randomized participants.
| qHPV | Placebo | Total | |
| Participants | 64 (50.8%) | 62 (49.2%) | 126 (100%) |
| Age (years), mean ± SD | 48.3 (±8.0) | 50.3 (±10.8) | 49.3 (±9.5) |
| Smoking | |||
| Current smoker | 15 (23.4%) | 18 (29.0%) | 33 (26.2%) |
| Ex-smoker | 24 (37.5%) | 17 (27.4%) | 41 (32.5%) |
| Never smoked | 25 (39.1%) | 27 (43.6%) | 52 (41.3%) |
| Years living with HIV, median (IQR) | 12 [7–17] | 10.5 [6–19] | 12 [6–17] |
| On cART at enrolment | 63 (98.4%) | 61 (98.4%) | 124 (98.4%) |
| Time on cART (years), median [IQR] | 10 [4–15] | 8 [4–17] | 9.5 [4–15] |
| Nadir CD4+ cell count (cells/μl), median [IQR] | 235 [155–355] | 240 [150–330] | 240 [150–350] |
| Current CD4+ cell count (cells/μl), median [IQR] | 775 [605–890] | 615 [500–800] | 700 (560–880) |
| Recent plasma HIV-RNA load | |||
| Undetectable | 63 (98.4%) | 57 (91.9%) | 120 (95.2%) |
| Copies/ml if detectable, median [IQR] | 53.0 [0]a | 52.0 [44–151] | 52.5 [44–151] |
| History of any STI | 60 (93.8%) | 58 (93.6%) | 118 (93.7%) |
| Last intra-anal HGAIN treatment mode, n (%) | |||
| Cryotherapy | 8 (12.5%) | 4 (6.5%) | 12 (9.5%) |
| Electrocautery/coagulation | 49 (76.6%) | 52 (83.9%) | 101 (80.2%) |
| TCA | 3 (4.7%) | 1 (1.6%) | 4 (3.2%) |
| Imiquimod cream | 0 (0%) | 1 (1.6%) | 1 (0.8%) |
| Otherb | 4 (6.3%) | 4 (6.5%) | 8 (6.3%) |
| Treatment centre | |||
| Academic | 37 (57.8%) | 35 (56.5%) | 72 (57.1%) |
| Nonacademic | 27 (42.2%) | 27 (43.5%) | 54 (42.9%) |
| Timing first vaccination after last treatment for intra-anal HGAIN | |||
| <6 months | 44 (68.8%) | 45 (72.6%) | 89 (70.6%) |
| 6–12 months | 20 (31.3%) | 17 (27.4%) | 37 (29.4%) |
| Result of last treatment for intra-anal HGAIN | |||
| Complete remission | 47 (73.4%) | 42 (67.7%) | 89 (70.6%) |
| Partial remission (HGAIN → LGAIN) | 17 (26.6%) | 20 (32.3%) | 37 (29.4%) |
| Presence of intra/peri-anal LGAIN | |||
| Any | 36 (56.3%) | 35 (56.5%) | 71 (56.3%) |
| Intra-anal | 34 (53.1%) | 32 (51.6%) | 66 (52.4%) |
| Peri-anal | 4 (6.3%) | 3 (4.8%) | 7 (5.6%) |
| Presence of anogenital condylomata | 26 (40.6%) | 19 (30.6%) | 45 (35.7%) |
| Presence of genital condylomatac | 2 (3.3%) | 2 (3.2%) | 4 (3.2%) |
| Penile | 1 (1.6%) | 2 (3.3%) | 3 (2.4%) |
| Scrotal | 1 (1.6%) | 0 (0%) | 1 (0.8%) |
| Os pubis | 1 (1.6%) | 0 (0%) | 1 (0.8%) |
| Presence of anal condylomata | 25 (39.1%) | 18 (29.0%) | 43 (34.1%) |
| Intra-anal | 22 (34.4%) | 15 (24.2%) | 37 (29.4%) |
| Peri-anal | 6 (9.4%) | 4 (6.5%) | 10 (7.9%) |
Data are n (%), median [IQR], mean (±SD). μl, microliter; AIN, anal intraepithelial neoplasia; cART, combination antiretroviral therapy; CI, confidence interval; HGAIN, high-grade anal intraepithelial neoplasia; IQR, interquartile range; LGAIN, low-grade anal intraepithelial neoplasia; ml, mililiter; qHPV, quadrivalent human papillomavirus vaccine; SD, standard deviation; STI, sexually transmitted infection; TCA, trichloroacetic acid.
aOnly one participant.
bTherapeutic biopsy (n = 2); cryotherapy (six sessions) combined with 5-fluoro-uracil cream (34 weeks) (n = 1); electrocautery combined with 5-fluoro-uracil cream (n = 5).
c2 missing.
High-grade anal intraepithelial neoplasia recurrences
In total 61.9% (78/126) of participants had recurrent HGAIN within 18 months of follow-up since first vaccination. A total of 103 recurrent HGAIN lesions were detected in these 78 participants. Median number of recurrent HGAIN lesions per participant was 1 [IQR = 1–2], and one of 78 recurrences (1.3%) was peri-anal. Of the 78 participants with a recurrent HGAIN, 47% recurred at FU6, 24% at FU12 and 28% at FU18. No progression to anal cancer was found during follow-up.
Cumulative high-grade anal intraepithelial neoplasia recurrence at 12 months after last vaccination
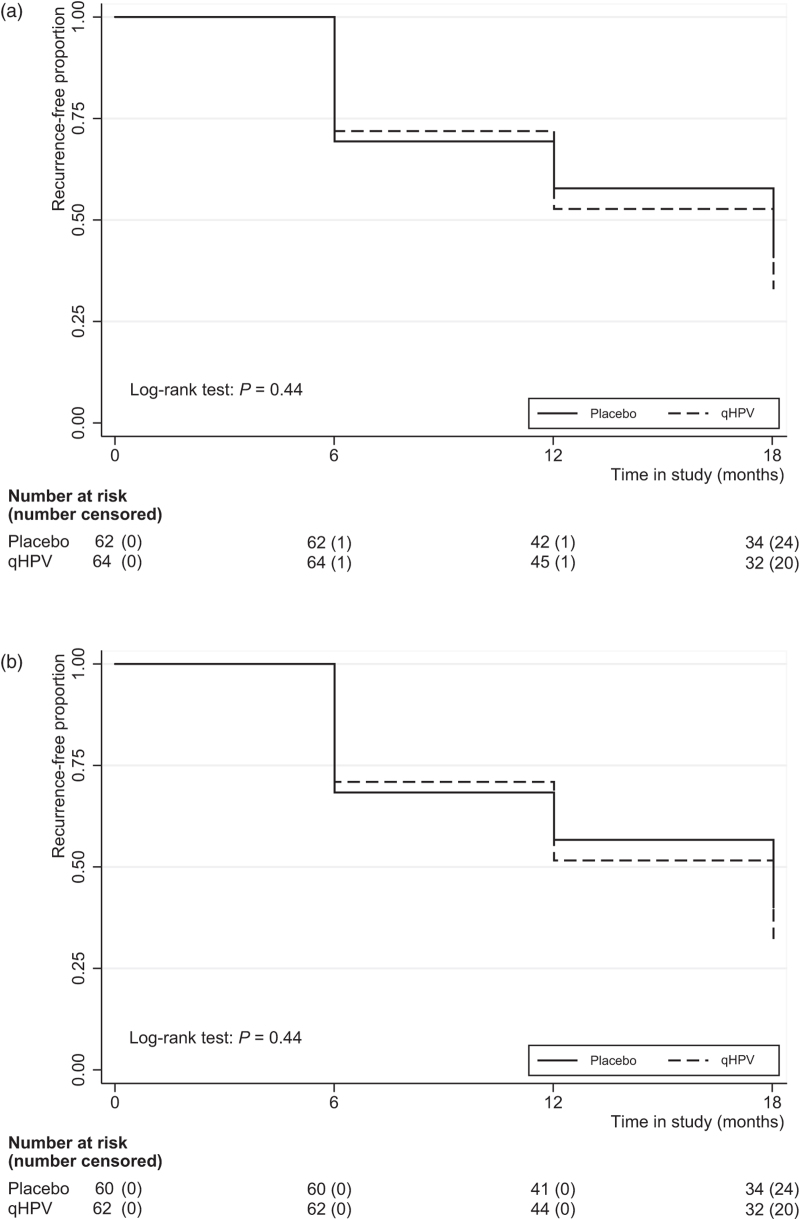
At FU18, cumulative HGAIN recurrence rates (primary outcome) in the ITT worst-case scenario analysis were 68.8% (44/64) for qHPV versus 61.3% (38/62) for placebo, which was not significantly different [P = 0.38; ARR = −7.5 percentage points (95% CI −24.1 to 9.2); RR = 1.12 (95% CI 0.87–1.45)]. The ITT best-case scenario and the per-protocol analyses yielded comparable outcomes. Table 2 summarizes all primary and secondary outcome data (see also Supplementary Results). Figure 2 shows Kaplan--Meier curves for the proportions free of HGAIN recurrence at FU6, FU12, and FU18 for the ITT and per-protocol analyses. In the ITT analysis, the incidence rate of recurrent HGAIN was 66.3 per 100 person-years (95% CI 49.0–89.7) for qHPV versus 56.5 (95% CI 40.7–78.3) for placebo (Supplementary Table 1). Vaccine efficacy against HGAIN recurrence was −17.4% (95% CI −20.3 to −14.6). The per-protociol analysis yielded similar results.
Table 2.
Summary of primary and secondary outcomes in the different analyses.
| Total | qHPV | Placebo | Absolute risk reduction | Relative risk | ||
| n (%) | n (%) | n (%) | P value | %-point (95% CI) | (95% CI) | |
| Cumulative HGAIN recurrence 12 months after last vaccination (FU18; ITT worst-case) | 0.38 | |||||
| No | 44 (34.9%) | 20 (31.3%) | 24 (38.7%) | −7.5% (−24.1 to 9.2%) | 1.12 (0.87–1.45) | |
| Yes | 82 (65.1%) | 44 (68.8%) | 38 (61.3%) | |||
| Cumulative HGAIN recurrence 12 months after last vaccination (FU18; ITT best-case) | 0.38 | |||||
| No | 48 (38.1%) | 22 (34.4%) | 26 (41.9%) | −7.6% (−24.5 to 9.4%) | 1.13 (0.86–1.49) | |
| Yes | 78 (61.9%) | 42 (65.6%) | 36 (58.1%) | |||
| Cumulative HGAIN recurrence 12 months after last vaccination (FU18; PP) | 0.37 | |||||
| No | 44 (36.1%) | 20 (32.3%) | 24 (40.0%) | −7.7% (−24.7 to 9.3%) | 1.13 (0.86–1.48) | |
| Yes | 78 (63.9%) | 42 (67.7%) | 36 (60.0%) | |||
| Cumulative occurrence of LGAIN 12 months after last vaccination (FU18; ITT worst-case)a | 0.50 | |||||
| No | 16 (29.1%) | 7 (25.0%) | 9 (33.3%) | −8.3 (−32.3 to 15.6%) | 1.13 (0.80–1.58) | |
| Yes | 39 (70.9%) | 21 (75.0%) | 18 (66.7%) | |||
| Cumulative occurrence of LGAIN 12 months after last vaccination (FU18; ITT best-case)a | 0.23 | |||||
| No | 33 (60.0%) | 19 (67.9%) | 14 (51.9%) | 16.0 (−9.6 to 41.6%) | 0.67 (0.34–1.30) | |
| Yes | 22 (40.0%) | 9 (32.1%) | 13 (48.2%) | |||
| Cumulative occurrence of anogenital condylomata 12 months after last vaccination (FU18; ITT worst-case)b | 0.32 | |||||
| No | 41 (50.6%) | 17 (44.7%) | 24 (55.8%) | −11.1 (−32.8 to 10.6%) | 1.25 (0.80–1.94) | |
| Yes | 40 (49.4%) | 21 (55.3%) | 19 (44.2%) | |||
| Cumulative occurrence of anogenital condylomata 12 months after last vaccination (FU18; ITT best-case)b | 0.18 | |||||
| No | 76 (93.8) | 34 (89.5%) | 42 (97.7%) | −8.2 (−19 to 2.6%) | 1.53 (0.53–38.76) | |
| Yes | 5 (6.2%) | 4 (10.5%) | 1 (2.3%) |
Data are n (%). CI, confidence interval; FU, follow-up; HGAIN, high-grade anal intraepithelial neoplasia; ITT, intention-to-treat; LGAIN, low-grade anal intraepithelial neoplasia; PP, per-protocol; qHPV, quadrivalent human papillomavirus vaccine.
Among participant without LGAIN at baseline (n = 55).
Among participants without anogenital condyloma at baseline (n = 81).
Fig. 2.
Proportion of participants free of recurrent high-grade anal intraepithelial neoplasia.
(a) Intention-to-treat analysis; (b) per-protocol analysis; HGAIN, high-grade anal intraepithelial neoplasia; qHPV, quadrivalent human papillomavirus vaccine.
In multivariable analysis, higher CD4+ cell count at enrolment was associated with recurrence [aOR = 1.30 per 100 cell increase (95% CI 1.05–1.61); P = 0.02; Supplementary Table 2]. Risk for cumulative HGAIN recurrence at FU18 for qHPV compared with placebo was 1.03 [(95%CI 0.32–3.36); P = 0.96; Supplementary Table 2]. There were no significant associations between the three stratification factors and the primary outcome. ITT best-case scenario and per-protocol analyses yielded similar results (Supplementary Tables 3 and 4). We refrained from the Cox proportional hazards regression analysis included in the statistical analysis plan because of not meeting test assumptions and the limited number of censored cases.
Causative human papillomavirus genotype in recurrent high-grade anal intraepithelial neoplasia lesions
For 83.3% (35/42) of participants with recurrent HGAIN in the qHPV group and 66.7% (24/36) in the placebo group, we could identify the causative HPV type of the lesion (P = 0.18). Of qHPV recipients, 40% (14/35) had recurrent lesion(s) caused by vaccine HPV types, versus 60% (21/35) caused by nonvaccine types, compared with 37.5% (9/24) versus 62.5% (15/24) for the placebo recipients (P = 0.85). More inconclusive results (i.e. nondiagnostic sample: cut through the lesion or HPV-positive but untypable) were found in the placebo group: 16.7% (7/42) for qHPV versus 33.3% (12/36) for placebo (P = 0.09).
Human papillomavirus type-specific antibody response
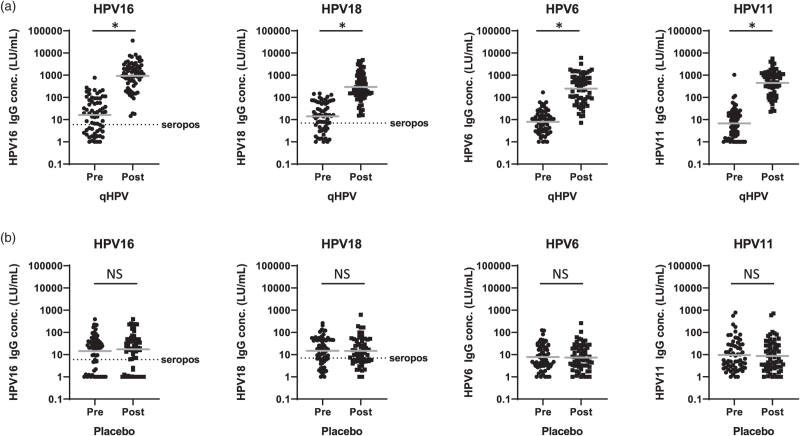
All participants were included in the analysis to measure antibody response. In the qHPV group a significant increase in geometric mean concentrations was observed for type-specific antibodies against vaccine types HPV16, 18, 6, and 11 (for all P < 0.001; Fig. 3; Supplementary Table 5). There was no increase in antibody levels in the placebo group (for all P > 0.10). About two-thirds of men were already seropositive before vaccination: in the qHPV group 66% (42/64) for HPV16 and 70% (45/64) for HPV18, compared to 68% (42/62) and 69% (43/62) in the placebo group, respectively (Supplementary Table 6). After three vaccinations with qHPV, antibody levels for HPV16 and 18 were above seropositivity thresholds in all patients. For cross-reactivity of nonvaccine types see Supplementary Results, Supplementary Figure 1, Supplementary Table 5, and Supplementary Table 6. We refrained from analyses relating the primary outcome with magnitude of HPV-specific antibody responses or HPV serostatus at baseline, as numbers per separate vaccine type were small.
Fig. 3.
Human papillomavirus type-specific IgG antibody concentrations before first (pre) and 3 months after last vaccination (post).
(a) qHPV group; (b) placebo group. Units: LU/ml. Gray horizontal solid bars represent geometric mean concentrations. Dotted horizontal line/seropos: seropositivity thresholds: 6 LU/ml (HPV16), 7 LU/ml (HPV18). HPV, human papillomavirus; IgG, Immunoglobulin; LU/ml, Luminex units per ml; qHPV, quadrivalent HPV vaccine. ∗P less than 0.001; NS, nonsignificant (P > 0.10; paired t test).
Safety
One or more adverse events were reported by 90.6% (58/64) of qHPV and 88.7% (55/62) of placebo recipients (P = 0.72; Supplementary Table 7). Vaccine-related adverse events were reported in 67.2% (43/64) and 61.3% (38/62) of qHPV and placebo recipients (P = 0.49). qHPV recipients reported more injection-site-related adverse events (P = 0.007). All injection-site-related adverse events in both groups were mild (grade 1). More details on (S)AEs are presented in Supplementary Table 8. No deaths occurred and none of nine SAEs were vaccine-related or study-related.
Discussion
In HIV+ MSM successfully treated for intra-anal HGAIN, vaccination with the prophylactic qHPV vaccine does not prevent short-term recurrence of HGAIN. This finding appeared consistent across all subgroups, accounting for the stratification factors. We could confirm that the qHPV vaccine is immunogenic and well tolerated in HIV+ MSM [16]. Vaccination with qHPV induced a significant increase in concentrations of type-specific antibodies against all vaccine types and the nonvaccine types 31 and 45, but two-thirds of participants were already seropositive for vaccine types HPV16 and 18 at baseline. A similar proportion of recurrent HGAIN lesions in both groups were caused by vaccine types, supporting nonefficacy. Additionally, we did not observe significant effects of qHPV vaccination on the secondary outcome prevention of LGAIN and anogenital condylomata.
Vaccination with qHPV for primary prevention of anal HPV infections and HGAIN has been proven ineffective in PWH (aged >26 years) [24] and HIV+ MSM [25]. This is in spite of the induction of adequate antibody responses by the qHPV vaccine in PWH. Efficacy as secondary prevention, that is, preventing new infections or lesions in patients with ongoing or previous HPV infection and/or resulting premalignant lesions, has been questioned [26–28]. A recent meta-analysis indicates efficacy (RR = 0.41) of qHPV vaccination as a treatment adjuvant to prevent recurrence of HGCIN [14]. However, considerable heterogeneity between studies was noted [different ages of patients (mostly younger), timing of vaccination before or after treatment and duration of follow-up], and only one included study was a randomized trial specifically designed to assess prevention of recurrent HGCIN after treatment. However, this study was neither placebo-controlled nor blinded [29]. A recent RCT could not confirm prevention of recurrent HGCIN by HPV vaccination in HIV+ women [15]. The only study in MSM evaluating qHPV as treatment adjuvant to prevent HGAIN recurrence claimed a significant reduction (hazard ratio = 0.50) of HGAIN recurrence 2 years after qHPV vaccination, but this was a nonrandomized, nonconcurrent cohort study of HIV-negative MSM, who were on average slightly younger than our study cohort [13]. We now provided evidence that qHPV vaccination as secondary prevention for HGAIN recurrence in HIV+ MSM is ineffective.
How the secondary prevention efficacy of qHPV as treatment adjuvant observed in aforementioned studies should be explained remains to be elucidated [13,14,29]. One hypothesis that has been posed in the literature suggests a ‘therapeutic’ effect of the vaccine [30]. This hypothesis seems somewhat counterintuitive, as a combination of innate and adaptive (including T-cell-mediated) immune responses is required to actively clear (residual) AIN lesions, whereas the mode of action of prophylactic (L1 VLP) qHPV vaccination relies predominantly on potent neutralizing antibodies. Although the interplay with the immune system is not fully unravelled, vaccination does also induce a L1-specific CD8+ T-cell response; however, basal keratinocytes at the site of infection do not express L1 and it has been suggested that they may, therefore, escape the immune system [31].
Another hypothesis in literature proposes a specific ‘prophylactic’ effect by preventing new lesions caused by HPV types to which patients were not previously exposed [30]. In general, antibody responses adequately correlate with the efficacy of this vaccine. Our and previous observations of nonefficacy of qHPV in PWH, in spite of adequate antibody responses, show this is not the case for PWH [24,25]. We hypothesize that the HIV infection, the MSM risk group, or the combination is likely to be the cause of this nonefficacy. If this hypothesis would hold true, younger patients could still benefit, as their probability of being previously exposed to these HPV types is generally lower, especially in MSM who often harbour many hrHPV types and anal HPV prevalence does not decrease at older age [32]. We found no significant effect in our analysis for the ‘younger’ age group (<44 years), although our population still consisted mainly of middle-aged (median age 49 years) HIV+ MSM. At baseline, two-thirds of participants were already seropositive for vaccine HPV types 16 and 18, showing previous exposure to these types. We cannot rule out that young (HIV+) MSM, possibly naive for at least some HPV types, would benefit from posttreatment vaccination, given results in women in which cohorts were generally younger. However, screening for anal cancer in HIV+ MSM, and thus treatment of HGAIN, is generally not started before the age of 30–35 years.
Deshmukh et al. modelled cost-effectiveness and concluded that posttreatment adjuvant qHPV vaccination for HIV-negative MSM aged 27 years and older is cost-effective [33], and likewise in HIV+ MSM [9]. On the basis of our findings, posttreatment vaccination is unlikely to be universally cost-effective in HIV+ MSM.
This trial is, to our knowledge, the first RCT designed to investigate in HIV+ MSM the efficacy of qHPV vaccination as posttreatment adjuvant to prevent HGAIN recurrence, confirmed by determining causal HPV types in recurrent HGAIN lesions and assessing immunogenicity with HPV type-specific antibody response. We decided to investigate the clinically relevant outcome of overall HGAIN recurrence, irrespective of possible anal HPV infections at baseline, rather than HPV type-specific efficacy.
Our study has several limitations. First, although participants were thoroughly screened by two experienced high-resolution anoscopists at enrolment and all suspected lesions were biopsied, we cannot rule out that microscopical lesions remained undetected or were misdiagnosed, although this would be equally distributed by randomization. Anal cytology in case of a negative HRA at enrolment could have slightly lowered this risk, although the diagnostic yield of additional cytology at FU18 turned out to be low. Second, as we did an ITT analysis, starting at first vaccination, a number of participants already had their recurrence at the time of third vaccination (6 months). However, also during the ensuing year, we observed no difference in recurrence rates between the two study arms. We acknowledge that the follow-up of 12 months after last vaccination is short. However, in a previous study in HIV-negative MSM, the strongest significant effect was already observed within the first year after last vaccination [13]. Moreover, during our follow-up period, we already observed recurrence rates that approximate current literature [6–8]. Previous follow-up studies after HGAIN treatment have shown recurrence rates levelling off 3 years after treatment [7]. Hypothetically, vaccination with qHPV could have long-term effects by reaching this plateau phase earlier, lowering the total number of recurrences, and/or result in less treatment-resistant recurrent lesions. For this reason, extended follow-up is planned for the study participants. Third, we were unable to determine causative HPV types in all recurrent HGAIN lesions, precluding a definite conclusion on prevention of vaccine-type recurrences. Fourth, our study does not give a decisive answer whether the nonavalent HPV vaccine, which has a wider coverage of HPV types but was not licensed yet during the preparation of this trial, would be efficacious to prevent recurrent HGAIN in HIV+ MSM, although this is not supported by the cross-reactivity we observed for HPV31 and 45. Finally, follow-up was discontinued when the primary endpoint was reached, while ideally follow-up should have been continued to assess secondary endpoints LGAIN and anogenital condylomata. Although we included a worst-case and best-case scenario, the results for these endpoints should, therefore, be interpreted with caution, as is expressed by the wide CIs.
In conclusion, the anal cancer precursor HGAIN is highly prevalent in HIV+ MSM and screening for premalignant lesions is advocated for this group. However, treatment is frustrated by high recurrence rates. In search of a strategy to reduce recurrence of HGAIN, vaccination with qHPV has been suggested. We have now provided evidence from an RCT that there is insufficient scientific rationale to support qHPV vaccination as treatment adjuvant to prevent short-term HGAIN recurrences in HIV+ MSM.
Acknowledgements
We thank all participants in the trial; H.A.M. van den Munckhof (DDL, Rijswijk) for excellent technical advice and assistance; H.E. Nobel (AMC, Amsterdam), W. Brokking (DC Klinieken Lairesse, Amsterdam), A.J.M. Toonen, L.E.H. Nené, F.M. Vermeulen (OLVG, Amsterdam) for performing HRAs; M.F. Schim van der Loeff (Public Health Service (GGD) Amsterdam) for excellent advice on data management and analysis; and F.R.M. van der Klis [National Institute for Public Health and the Environment (RIVM)] for facilitating the serological assays.
This work was supported by a research grant of ZonMw (the Netherlands Organisation for Health Research and Development) (grant no. 80-83600-98-10032) to J.M.P. and H.J.C.d.V.; the vaccines for this investigator-initiated study were donated by Merck Sharp & Dohme Corp. (MSD) in the form of a research grant from the Investigator Initiated Studies Program of MSD; and funding for a proctologic chair, needed for high-resolution anoscopy, was provided by the Maurits and Anna de Kock Foundation (nonprofit organization). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author's contributions: J.M.P. and H.J.C.d.V. are principal investigators of the study. K.C.M.G., H.J.C.d.V., and J.M.P. designed the study. K.C.M.G., R.P.v.d.Z., M.L.S.v.H., I.C., A.v.E., H.J.C.d.V., and J.M.P. were involved in clinical data and material collection. C.J.M.v.N., H.P., and W.G.V.Q. were involved in laboratory testing. K.C.M.G. and R.P.v.d.Z. managed the database. R.P.v.d.Z., V.W.J., and M.G.W.D. performed the data and statistical analyses. R.P.v.d.Z. and V.W.J. created the figures and tables. R.P.v.d.Z. and J.M.P. drafted the first version of the manuscript. All authors critically reviewed the manuscript and approved the final version. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis and believe that the manuscript represents honest work. J.M.P. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; that any discrepancies from the study as planned have been explained; and had full responsibility for the decision to submit for publication. The opinions expressed in this article are those of the authors and do not necessarily represent those of MSD or its affiliates. MSD was provided the opportunity to review a preliminary version of this manuscript in order to ensure the protection of its proprietary information and intellectual property, but the authors are solely responsible for final content and interpretation.
Data sharing statement: The study protocol and statistical analysis plan are readily publically available (see Methods). Individual participant data that underlie the results reported in this article (text, tables, figures, and appendices) and a data dictionary will be available for 5 years after publication after de-identification and at request to the corresponding author. Data will be securely transferred to researchers, who provide a methodologically sound research proposal and only to achieve aims in the approved proposal, after: approval of the ethics review board; additional approval of study participants (if applicable); and signing a data access agreement.
Conflicts of interest
M.G.W.D. has a family member working at MSD. H.J.C.d.V. received money or goods for research from Medigene, Gilead and MSD, money for presentations from Abbott and Janssen and money for advice from Medigene and Novartis. All other authors have no conflicts of interest to declare.
Preliminary results were previously presented at: International Anal Neoplasia Society Scientific Meeting in Amsterdam, The Netherlands, 1--3 November 2019 (oral presentation).
Dutch HIV Treating Physicians Scientific Meeting Amsterdam, The Netherlands, 17 January 2020 (oral presentation).
International Papillomavirus Society Conference (digital) 20–24 July 2020 (poster).
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Karien C.M. Gosens and Ramon P. van der Zee contributed equally.
Supplemental digital content is available for this article.
References
- 1.Clifford GM, Georges D, Shiels MS, Engels EA, Albuquerque A, Poynten IM, et al. A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer 2020; 148:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383. [DOI] [PubMed] [Google Scholar]
- 3.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 2010; 10:550–560. [DOI] [PubMed] [Google Scholar]
- 4.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. [DOI] [PubMed] [Google Scholar]
- 5.Palefsky JM. Anal cancer prevention in HIV-positive men and women. Curr Opin Oncol 2009; 21:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstone SE, Lensing SY, Stier EA, Darragh T, Lee JY, van Zante A, et al. A randomized clinical trial of infrared coagulation ablation versus active monitoring of intra-anal high-grade dysplasia in adults with human immunodeficiency virus infection: an AIDS Malignancy Consortium Trial. Clin Infect Dis 2019; 68:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: recurrence and incidence of cancer. Dis Colon Rectum 2014; 57:316–323. [DOI] [PubMed] [Google Scholar]
- 8.Richel O, de Vries HJ, van Noesel CJ, Dijkgraaf MG, Prins JM. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncol 2013; 14:346–353. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh AA, Cantor SB, Fenwick E, Chiao EY, Nyitray AG, Stier EA, et al. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: the time is now. Vaccine 2017; 35:5102–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbyn M, Xu L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev Vaccines 2018; 17:1085–1091. [DOI] [PubMed] [Google Scholar]
- 11.Harder T, Wichmann O, Klug SJ, van der Sande MAB, Wiese-Posselt M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med 2018; 16:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585. [DOI] [PubMed] [Google Scholar]
- 13.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis 2012; 54:891–898. [DOI] [PubMed] [Google Scholar]
- 14.Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Prophylactic HPV vaccination after conization: a systematic review and meta-analysis. Vaccine 2020; 38:6402–6409. [DOI] [PubMed] [Google Scholar]
- 15.Firnhaber C, Swarts A, Jezile V, Mulongo M, Goeieman B, Williams S, et al. HPV Vaccination prior to loop electroexcision procedure does not prevent recurrent cervical high grade squamous intraepithelial lesions in women living with HIV: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2020; ciaa1456.doi: 10.1093/cid/ciaa1456. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan Y, Liu X, Feng Y, Wu S, Jiang Y. Safety and efficacy of human papillomavirus vaccination for people living with HIV: a systematic review and meta-analysis. Int J STD AIDS 2019; 30:1105–1115. [DOI] [PubMed] [Google Scholar]
- 17.Hillman RJ, Cuming T, Darragh T, Nathan M, Berry-Lawthorn M, Goldstone S, et al. 2016 IANS International Guidelines for practice standards in the detection of anal cancer precursors. J Low Genit Tract Dis 2016; 20:283–291. [DOI] [PubMed] [Google Scholar]
- 18.Hillman RJ, Berry-Lawhorn JM, Ong JJ, Cuming T, Nathan M, Goldstone S, et al. International Anal Neoplasia Society. International Anal Neoplasia Society Guidelines for the practice of digital anal rectal examination. J Low Genit Tract Dis 2019; 23:138–146. [DOI] [PubMed] [Google Scholar]
- 19.Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. Members of the LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis 2012; 16:205–242. [DOI] [PubMed] [Google Scholar]
- 20.Richel O, Quint KD, Lindeman J, van Noesel CJ, De Koning MN, van den Munckhof HA, et al. One lesion, one virus: individual components of high-grade anal intraepithelial neoplasia in HIV-positive men contain a single HPV type. J Infect Dis 2014; 210:111–120. [DOI] [PubMed] [Google Scholar]
- 21.Scherpenisse M, Mollers M, Schepp RM, Boot HJ, de Melker HE, Meijer CJ, et al. Seroprevalence of seven high-risk HPV types in the Netherlands. Vaccine 2012; 30:6686–6693. [DOI] [PubMed] [Google Scholar]
- 22.Pasmans H, Hoes J, Tymchenko L, de Melker HE, van der Klis FRM. Changes in HPV seroprevalence from an unvaccinated toward a girls-only vaccinated population in the Netherlands. Cancer Epidemiol Biomarkers Prev 2020; 29:2243–2254. [DOI] [PubMed] [Google Scholar]
- 23.SERVICES USDOHAH. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. 2010. [Google Scholar]
- 24.Wilkin TJ, Chen H, Cespedes MS, Leon-Cruz JT, Godfrey C, Chiao EY, et al. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS Clinical Trials Group Protocol A5298. Clin Infect Dis 2018; 67:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidalgo-Tenorio C, Pasquau J, Omar-Mohamed M, Sampedro A, López-Ruz MA, López Hidalgo J, et al. Effectiveness of the quadrivalent HPV vaccine in preventing anal ≥ HSILs in a Spanish population of HIV+ MSM aged > 26 years. Viruses 2021; 13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Costa Rican HPV Vaccine Trial Group. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA 2007; 298:743–753. [DOI] [PubMed] [Google Scholar]
- 27.Haupt RM, Wheeler CM, Brown DR, Garland SM, Ferris DG, Paavonen JA, et al. FUTURE I and II Investigators. Impact of an HPV6/11/16/18 L1 virus-like particle vaccine on progression to cervical intraepithelial neoplasia in seropositive women with HPV16/18 infection. Int J Cancer 2011; 129:2632–2642. [DOI] [PubMed] [Google Scholar]
- 28.Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15-25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer 2012; 131:106–116. [DOI] [PubMed] [Google Scholar]
- 29.Pieralli A, Bianchi C, Auzzi N, Fallani MG, Bussani C, Fambrini M, et al. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch Gynecol Obstet 2018; 298:1205–1210. [DOI] [PubMed] [Google Scholar]
- 30.Ghelardi A, Parazzini F, Martella F, Pieralli A, Bay P, Tonetti A, et al. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol Oncol 2018; 151:229–234. [DOI] [PubMed] [Google Scholar]
- 31.Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer 2018; 18:240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poynten IM, Machalek D, Templeton D, Jin F, Hillman R, Zablotzska I, et al. Comparison of age-specific patterns of sexual behaviour and anal HPV prevalence in homosexual men with patterns in women. Sex Transm Infect 2016; 92:228–231. [DOI] [PubMed] [Google Scholar]
- 33.Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine 2014; 32:6941–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.