Objectives:
Heightened systemic inflammation is common in obese individuals and persons with HIV (PWH) and is independently associated with an increased risk of cardiovascular diseases (CVDs). We investigated the combined effect of central obesity, a surrogate measure of visceral fat and HIV on circulating levels of inflammatory cytokines among Kenyan adults.
Design:
A cross-sectional study.
Methods:
We analysed and compared data from 287 virally suppressed PWH and 277 noninfected Kenyan adults, including biomarkers of gut epithelial dysfunction (intestinal fatty acid binding protein), monocyte activation (soluble CD163 and CD14) and inflammation [interleukin (IL)-1β, IL-6, TNF-α and hsCRP] by HIV/central obesity status (HIV-positive/obese, HIV-negative/obese, HIV-positive/nonobese and HIV-negative/nonobese). Central obesity was defined as waist circumference more than 80 cm for women and more than 94 cm for men. We assessed the association of HIV/obesity status with elevated biomarkers (>75th percentile) using logistic regression.
Results:
Median age for participants was 44 years and 37% were centrally obese. Levels of all biomarkers were higher among the HIV-positive/obese compared with the HIV-negative/nonobese (P < 0.05 for all comparisons). The HIV-positive/obese group had the greatest odds of having elevated inflammatory biomarkers compared with other groups even after adjustment of age, BMI and other conventional CVD risk factors (P < 0.05 for all). Additional adjustment for sCD163 in the multivariate model substantially attenuated the association for HIV-positive/obesity with IL-1β, IL-6 and TNF-α but not hsCRP. The contribution of HIV-positive/obesity to inflammation was independent of the degree of immunosuppression.
Conclusion:
Central obesity is prevalent among virally suppressed African PWH and is associated with greater inflammation and monocyte activation independent of other comorbidities and HIV-specific factors.
Keywords: Africa, HIV, inflammation, microbial translocation, monocyte activation, obesity, sCD163
Introduction
Antiretroviral therapy (ART) has greatly reduced mortality associated with HIV/AIDS in low-resource settings including Kenya [1]. However, after ART, most persons with HIV (PWH) gain weight, and while weight gain after ART initiation is associated with a survival advantage among normal weight individuals, it may exacerbate risk for cardiovascular disease (CVD) and other non-AIDS comorbidities [2–8].
Elevated levels of pro-inflammatory cytokines [interleukin (IL)-1 β, IL -6, tumour necrosis factor (TNF)-α and high-sensitivity C-reactive protein (hsCRP)] and monocyte activation biomarkers [soluble CD163 (sCD163) and sCD14] are associated with an increased risk of developing CVD [9–17]. We have recently shown that African PWH and the obese adults exhibit high levels of these biomarkers even after ART compared with the noninfected adults [10,16]. The pathophysiology of HIV-associated immune activation and inflammation is still unclear and may be multifactorial; however, hsCRP has been shown to correlate with visceral fat mass in PWH implying that visceral adiposity may be a risk factor [18]. The heightened inflammatory state observed in obese individuals regardless of the HIV status is thought to be due to increased inflammatory cytokine production by the immune cells that have infiltrated the adipose tissue, adipocyte hypertrophy and the direct effect of leptin on hepatocytes to induce hsCRP production [12,13].
To date, there is limited information on the combined effect of HIV and central obesity on biomarkers of inflammation after viral suppression especially from the low and middle-income countries. Previous studies have reported high levels of circulating markers of inflammation among generally obese PWH, but other studies have found modest effects [19–21]. It is important to note that most of these studies did not include women or HIV-negative adults for comparison and used BMI as a measure of adiposity. Waist circumference is a superior surrogate marker of visceral adipose tissues compared with BMI [22] and although both subcutaneous and visceral adipose tissues have been associated with cardiometabolic diseases, high visceral adipose tissue is more strongly correlated with cardiometabolic diseases than its counterpart [23,24]. In fact, waist circumference is a superior predictor of health risks than BMI [25–29].
In this present study, we sought to investigate the combined effects of central obesity and HIV on biomarkers of inflammation, monocyte activation and gut enterocyte damage among Kenyan adults. We hypothesized that central obesity and HIV would both be associated with inflammation independent of each other, but their joint presence would be associated with greater levels of these biomarkers.
Materials and methods
Study setting and participants
We analysed data from 287 PWH and 277 HIV-negative participants enrolled between 2017 and 2018 in an observational cross-sectional study in western Kenya. The cohort was established to assess risk factors for CVD in PWH on long-term ART. Recruitment and study procedures have been described elsewhere [10,30,31]. Briefly, the study is composed of a convenience sample of PWH and HIV-negative men and women at least 30 years old enrolled from the Kisumu District Hospital HIV clinic and voluntary HIV testing centres. Data were collected through a standardized questionnaire and medical chart abstraction, while anthropometric measurements and serum collection were performed as part of the study visit. Mid-waist circumference was measured in cm by a validated WHO STEPwise survey protocol [32]. Central obesity was defined based on International Diabetes Federation (IDF) definition as waist circumference more than 0.80 m for women and greater than 0.94 m for men, considered as indicative of an increased risk of cardiometabolic diseases [33]. Overweight referred to BMI greater than or equal to 25 to 29.9 kg/m2 and general obesity was BMI greater than or equal to 30 kg/m2.
Laboratory testing consisted of CD4+ cell counts, HIV viral load, inflammatory biomarkers, fasting glucose and lipids [total cholesterol, triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol]. Individuals with a recorded history of CVD, neoplasia, active infection or those who at the time of recruitment were on medications that could influence their immune status were excluded from this analysis.
Immunological biomarker analysis
Serum levels of IL-1β, IL-6 and TNF-α were measured using multiplex ELISA (Meso-Scale Discovery, Rockville, Maryland, USA). Samples with a coefficient of variation greater than 30% were rerun and the duplicate and the lower coefficient of variation was averaged for the analyses. hsCRP testing was performed using an automated Beckman Coulter AU5812. Serum levels of intestinal fatty acid binding protein (I-FABP), sCD163 and sCD14 were measured using commercially available ELISA assays (Quantikine ELISA kit; R&D Systems, Minneapolis, Minnesota, USA). The inter-assay coefficients of variation were less than 11%. All samples were tested centrally at University of Washington (Seattle, Washington, USA). Assays were performed in duplicate and in accordance with manufacturers’ protocols. These markers were selected because they have been shown to be associated with cardiometabolic diseases or death and were likely to be produced by adipose tissue-resident immune cells [34–37].
Statistical analysis
We first compared demographic and clinical characteristics within and across groups (HIV-positive/obese, HIV-negative/obese, HIV-positive/nonobese and HIV-negative/nonobese) using t-tests, Wilcoxon rank sum tests or Kruskal--Wallis test for continuous variables and X2 test or Fisher test for categorical variables. We used univariable and multivariable logistic regression to estimate the association between obesity/HIV-infection comorbidity and prevalence of elevated biomarkers [highest quartile (>75th percentile) vs. lower three quartiles] before and after adjustment of clinical characteristics. We defined elevated biomarkers as levels more than 75% because with the exception of hsCRP, it is still unclear what thresholds are associated with clinical events for these biomarkers. This is also consistent with other studies that have looked at the association between biomarkers with health risks using biomarker quartiles [35,38,39]. hsCRP was categorized according to clinical risk, and the cut off of 2 mg/l was used as previously described [40]. Models were adjusted for demographic characteristics (age and sex), and traditional CVD risk factors (hypertension, hypercholesterolemia and alcohol use status) and further adjusted for BMI. These covariates were selected based on prior work suggesting an association between covariates and the biomarkers [10,16,41]. Multivariable models were sequentially adjusted for each of the biomarkers; I-FABP, sCD14 and sCD163, respectively. In separate analyses among PWH, we further adjusted for potential HIV-specific confounding factors, such as current and nadir CD4+ T-cell count, duration of ART intake, ART regimen (efavirenz vs. nevirapine vs. protease inhibitor-based regimen). We also conducted multivariable linear regression analysis to evaluate the association between (log-transformed) biomarkers and HIV status in separate models for each biomarker, and adjusted for age, sex, hypertension, cholesterols, waist circumference and alcohol use status. We report the exponentiated β-coefficients and their calculated 95% confidence intervals (95% CIs) representing the fold increase/decrease in biomarker level. An interaction term between sex and HIV status was introduced in the model to test the difference of these associations by sex. Finally, in a sensitivity analysis, we repeated models after excluding individuals with hsCRP greater than 10 mg/l (n = 50), as this could indicate the presence of an acute infection [42,43], and the underweight individuals with BMI less than 18.5 kg/m2 (n = 51). Significance was set at a P value less than 0.05. Analyses were performed using STATA version 13 (San Antonio, Texas, USA).
Ethics statement
The Ethics and Research Committee of Kenyatta National Hospital and the Institutional Review Board at University of Washington approved the study. All participants provided written informed consent.
Results
Study participants characteristics
Sociodemographic and clinical characteristics are summarized in Table 1, stratified by HIV and central obesity status. The median (IQR) age for all participants was 44 (35–54) years, half were female (82% of those with obesity were female). The prevalence of abdominal obesity was lower in PWH relative to the HIV-negative participants (30 vs. 40%; P = 0.001; sup Table 1). PWH with central obesity were less likely to have an obese BMI (BMI > 30 kg/m2) compared with their HIV-negative counterparts (22 vs. 44%; P < 0.0001). Centrally obese participants were more likely to have hypertension and dyslipidaemia, and less likely to report current alcohol and tobacco use compared with the nonobese (Table 1). The mean CD4+ T-cell count for the PWH was 520 cells/μl and was not statistically different between the obese and nonobese groups (P = 0.07). The majority (97%) of PWH were virally suppressed (HIV RNA < 1000 copies/ml). All PWH were on co-trimoxazole prophylaxis and ART consisting of a nonnucleoside reverse transcriptase inhibitor (NNRTI) backbone of efavirenz (45%) or nevirapine (42%) with lamivudine as well as tenofovir or zidovudine; only 13% were on protease inhibitors, and none were on integrase inhibitors. The mean duration of ART across groups was 8 years.
Table 1.
Characteristics of participants stratified by HIV and obesity status.
| HIV-negative | HIV-positive | |||||
| Centrally obese (n = 111) | Nonobese (n = 166) | P | Centrally obese (n = 85) | Nonobese (n = 202) | P | |
| Demographics | ||||||
| Age (years) | 44 (34, 55) | 36 (31, 52) | 0.01 | 45 (39,55) | 45 (40,53) | 0.44 |
| Female | 90 (81) | 49 (30) | <0.001 | 72 (84) | 71 (35) | <0.001 |
| Cardiovascular risk factors | ||||||
| Current smoker | 2 (2) | 6 (4) | 0.04 | 0 (0) | 8 (4) | 0.02 |
| Current alcohol use | 17 (13) | 14 (10) | 0.008 | 9 (11) | 42 (21) | 0.06 |
| Diabetes | 5 (5) | 4 (2) | 0.33 | 2 (2) | 1 (1) | 0.16 |
| Hypertension | 37 (33) | 23 (14) | <0.001 | 16 (19) | 19 (9) | 0.03 |
| Other characteristics | ||||||
| BMI (kg/m2) | 29 (26, 34) | 21 (20, 24) | <0.001 | 27 (25, 29) | 21 (19, 23) | <0.001 |
| BMI category (kg/m2) | <0.001 | <0.001 | ||||
| Underweight <18.5 | 0 | 19 (11) | 0 | 32 (16) | ||
| Normal weight (18.5–24.9) | 21 (19) | 121 (74) | 21 (25) | 156 (77) | ||
| Overweight (25.0–29.9) | 41 (37) | 24 (14) | 45 (53) | 9 (5) | ||
| General obesity (>30) | 49 (44) | 2 (1) | 19 (22) | 5 (2) | ||
| Waist circumference, cm | 93 ± 11 | 77 ± 7 | <0.001 | 96 ± 11 | 77 ± 8 | <0.001 |
| Laboratory values | ||||||
| HDL-cholesterol (mg/dl) | 50 ± 10 | 51 ± 15 | 0.74 | 53 ± 14 | 55 ± 17 | 0.50 |
| LDL- cholesterol (mg/dl) | 106 ± 31 | 90 ± 30 | <0.001 | 104 ± 35 | 91 ± 29 | 0.001 |
| Triglycerides (mg/dl) | 74 (60, 101) | 72 (55, 93) | 0.003 | 86 (68, 121) | 79 (60, 106) | 0.09 |
| Total cholesterol (mg/dl) | 172 ± 35 | 157 ± 37 | <0.001 | 178 ± 44 | 165 ± 35 | 0.009 |
| HIV related characteristics | ||||||
| Nadir CD4+ T-cell count (cells/μl) | 458 ± 276 | 370 ± 225∗ | 0.006 | |||
| CD4+ T-cell count (cells/μl) | 556 ± 197 | 505 ± 235 | 0.08 | |||
| Viral suppression RNA < 1000 copies/ml | 84 (97) | 197 (97) | 0.22 | |||
| Total ART duration (years) | 9 (9,10) | 8 (4,11) | 0.43 | |||
Data reported as mean ± standard deviation, percentage or median [interquartile range (IQR)].
BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PI, protease inhibitors. P value for comparisons between obese and nonobese participants (data were analysed in HIV positive and HIV negative separately).
Difference in biomarkers levels according to HIV status and sex
Median levels of all biomarkers, including sCD14, sCD163, I-FABP, IL-1β, hsCRP, TNF-α and IL-6, were significantly higher in PWH than in the HIV-negative participants (Sup Table 2). When comparing biomarkers by sex and HIV status, men with HIV demonstrated significantly higher levels of IL-1β, TNF-α and IL-6 than the women with HIV and men and women without HIV (Sup Figure 1). In contrast, levels of sCD163 were significantly higher among women with HIV than the men with HIV and persons without HIV. sCD14 and I-FABP levels did not differ significantly between HIV-positive men and women. After adjustment for age, sex and traditional CVD risk factors, HIV infection remained associated with higher levels of all biomarkers in men with HIV compared with men without HIV (P < 0.05; Sup Table 3). However, women with HIV had higher adjusted levels of sCD14, sCD163, I-FABP, TNF-α and IL-6 but not IL-1β or hsCRP than the uninfected women (P < 0.05). There was evidence of an interaction between HIV and sex for sCD163 (P = 0.03) but not for other biomarkers (Sup Table 3).
Intestinal barrier dysfunction and monocyte/macrophage activation biomarkers
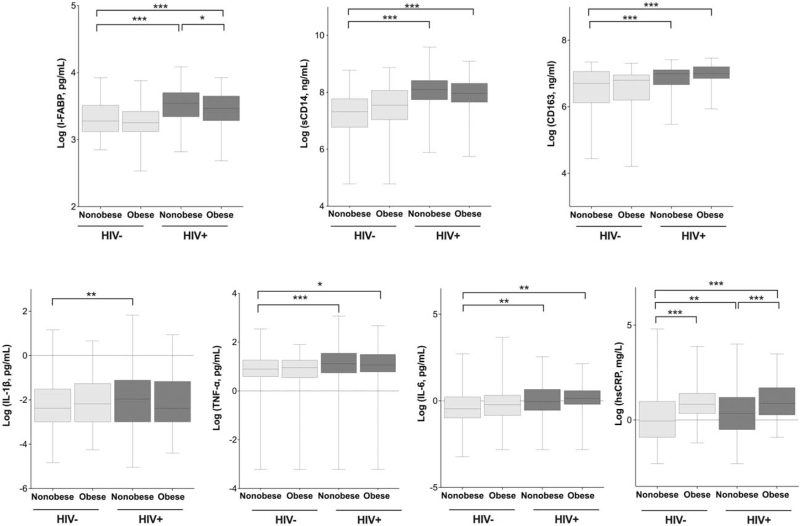
Median levels of sCD14, sCD163 and I-FABP biomarkers were significantly higher among the HIV-positive/obese than among the HIV-negative/nonobese (P < 0.001; Fig. 1). After controlling for clinical factors including BMI, HIV-positive/obese status remained associated with higher odds of elevated sCD14 [odds ratio (OR), 6.58; P < 0.001], sCD163 (OR, 2.45; P = 0.04), and there was a trend for I-FABP (OR 1.94, 95% CI: 0.99–4.21) compared with HIV-negative/nonobese (Table 2).
Fig. 1.
Serum levels of biomarkers by HIV serostatus and obesity status.
All biomarkers were log transformed. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Table 2.
Association of HIV and obesity group with elevated biomarkers.
| I-FABPb OR (95% CI) | sCD14b OR (95% CI) | sCD163b OR (95% CI) | TNF-αc OR (95% CI) | IL-6c OR (95% CI) | IL-1βc OR (95% CI) | hsCRPc OR (95% CI) | |
| Stratified by HIV and obesity status | |||||||
| Unadjusted | |||||||
| HIV-negative, nonobese | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| HIV-positive, nonobese | 2.90 (1.74–4.82) | 7.73 (4.15–13.92) | 1.73 (1.11–3.31) | 1.96 (1.61–4.50) | 1.87 (1.17–2.99) | 1.62 (0.96–2.71) | 1.44 (0.93–2.23) |
| HIV-negative, obese | 0.40 (0.18–0.89) | 0.76 (0.29–1.79) | 0.77 (0.30–1.77) | 0.74 (0.36–1.53) | 0.94 (0.53–1.70) | 1.50 (0.82–2.74) | 3.37 (2.04–5.58) |
| HIV-positive, obese | 2.10 (1.14–3.88) | 5.79 (2.86–11.06) | 2.27 (1.08–4.76) | 1.90 (0.97- 3.51) | 1.77 (0.99–3.18) | 1.61 (0.85–3.06) | 3.58 (2.07–6.19) |
| Fully adjusteda | |||||||
| HIV-negative, nonobese | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| HIV-positive, nonobese | 2.58 (1.52–4.36) | 7.57 (4.05–14.12) | 1.93 (1.01–3.82) | 2.45 (1.45–4.14) | 1.95 (1.19–3.13) | 1.98 (1.15–3.43) | 1.26 (0.80–2.01) |
| HIV-negative, obese | 0.37 (0.15–0.84) | 0.76 (0.30–1.92) | 0.65 (0.26–1.63) | 1.35 (0.64–2.82) | 1.25 (0.63–2.46) | 3.47 (1.71–7.08) | 2.13 (1.58–5.07) |
| HIV-positive, obese | 1.80 (0.92–3.49) | 5.73 (2.62–11.65) | 2.18 (1.07–4.90) | 2.86 (1.35–6.06) | 2.70 (1.35–5.39) | 4.06 (2.02–8.77) | 2.99 (1.59–5.62) |
| Fully adjusted and BMI | |||||||
| HIV-negative, nonobese | |||||||
| HIV-positive, nonobese | 2.51 (1.39–4.09) | 7.43 (3.86–13.89) | 1.81 (1.02–6.10) | 2.66 (1.53–5.13) | 2.24 (1.25–4.04) | 1.89 (1.01–3.56) | 1.61 (0.94–2.77) |
| HIV-negative, obese | 0.47 (0.19–1.16) | 1.06 (0.38–2.97) | 0.78 (0.27–2.22) | 1.60 (0.65–3.95) | 1.62 (0.67–3.44) | 4.35 (1.83–9.84) | 2.21 (1.11–4.40) |
| HIV-positive, obese | 1.94 (0.99–4.21) | 6.58 (3.17–13.72) | 2.45 (1.38–5.10) | 3.91 (1.62–9.46) | 3.01 (1.37–6.54) | 4.37 (1.78–9.17) | 2.31 (1.11–4.79) |
Obesity is defined as waist circumference of ≥80 cm for women and ≥94 cm for men.
hsCRP, high-sensitivity C-reactive protein; I-FABP, intestinal fatty acid-binding protein; IL-1β, interleukin 1beta; IL-6, interleukin 6; sCD14, cluster of differentiation 14; sCD163, cluster of differentiation 163; TNF-α, tumour necrosis factor alpha.
Fully adjusted for age, sex, HDL, triglycerides, LDL, hypertension and alcohol use status.
n = 541.
n = 564.
Inflammatory biomarkers
In unadjusted analysis, there was significant higher median levels of hsCRP, TNF-α and IL-6 among HIV-positive/nonobese and the HIV-positive /obese groups relative to the HIV-negative/nonobese (Fig. 1). Median levels of hsCRP were also significantly higher among the HIV-positive /obese than among HIV-positive /nonobese participants. Levels of IL-1β were comparable between HIV-positive /obese and the HIV-negative/nonobese group (Fig. 1). When we adjusted for traditional CVD risk factors, such as age, sex, BMI, alcohol use, hypertension, triglycerides and HDL, HIV-positive /obese continued to have a significantly greater odds for elevated IL-6 (OR, 3.01; P = 0.004), hsCRP (OR, 2.31; P = 0.001), TNF-α (OR, 3.91; P = 0.01) and IL-1β (OR, 4.37; P < 0.001) compared with the HIV-negative/nonobese (Table 2). There were no significant changes in the OR for the biomarkers among HIV-positive /obese individuals in analyses that stratified PWH by current CD4+ T-cell count (≤ or >200) or ART duration (≤ or >4 years; data not shown). Our analysis by [highest quartile (>75th percentile) vs. lower three quartiles] mimicked the linear relationships of HIV/obese status with inflammatory cytokines (data not presented). We also found no meaningful differences in these relationships in a sensitivity analysis that excluded those with hsCRP of greater than 10 mg/l or underweight individuals with BMI 18.5 kg/m2 or less (data not shown).
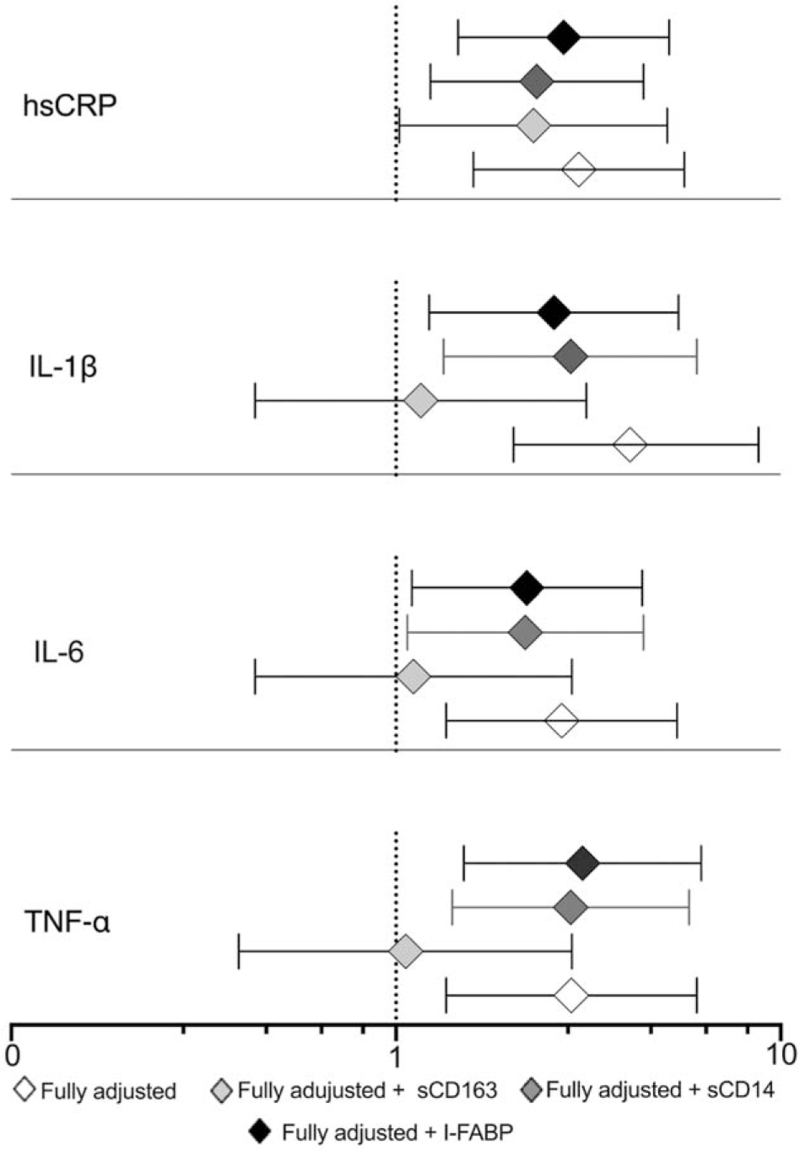
Given the findings above, we evaluated whether monocyte/macrophage activation (sCD163 and sCD14) or gut epithelial damage (I-FABP) were potential mediators of the association between HIV-positive /obesity and inflammation. Additional adjustment for sCD163, a monocyte activation biomarker, in the multivariable model used for Table 2 did change the association of HIV-positive /obese with IL-6 (OR, 1.19; P = 0.72), TNF-α (OR, 1.11; P = 0.84) and IL-1β (OR, 1.07; P = 0.89), but not hsCRP (OR, 2.43; P = 0.03, Fig. 2). By contrast, adjustment for sCD14 and I-FABP among those with HIV and obesity had minimal effect on the association with the inflammation biomarkers.
Fig. 2.
Association of central obesity with biomarkers among PWH adjusted for demographic, and CVD risk factors plus markers of intestinal barrier dysfunction and monocyte activation.
Fully adjusted for age, sex, HDL, triglycerides, hypertension, BMI and alcohol use status.
Discussion
We found a high prevalence of central obesity in our cohort of normal/overweight virally suppressed Kenyan PWH. Importantly, PWH with central obesity exhibited immune dysregulation as reflected by elevated biomarkers of inflammation and monocyte activation. The associations were robust to adjustment for age, sex, BMI and traditional CVD risk factors. sCD163 strongly attenuated the association of HIV-positive /obese with IL-6, TNF-α and IL-1β but not that of hsCRP. Our data imply that abdominal adiposity promotes HIV-associated immune activation, and this may in part be due macrophage activation. To our knowledge, this is the first study to assess the contribution of HIV and obesity to inflammation and immune activation in a virally suppressed African cohort of PWH.
We have previously shown that HIV was associated with greater inflammation and immune activation in our study cohort [10,16]. The present study expands on our data and those from prior studies in the USA and Europe that found greater inflammation and monocyte/macrophage activation among obese PWH [12,19–21]. In the Fat Redistribution and Metabolic Change in HIV-Infection cohort, each two-fold increase in visceral adipose tissue was associated with a 17% higher serum CRP level [44], after adjustment for the Framingham Risk Score factors. Another study among veteran men reported that HIV/obese status had positive associations with IL-6 elevation particularly among the PWH with detectable viremia [45]. However, some differences between our data and these prior studies may be explained by the way central obesity was categorized (quartiles vs. means), the referent group (HIV-negative/nonobese vs. HIV-positive /nonobese) and the study population. Despite these differences, most of these previous studies reported increase in biomarkers of inflammation among obese PWH, which is consistent to the findings of the current study.
IL-1β is one of the major inflammatory cytokines produced by macrophages and a key contributor to the pathogenesis of atherosclerosis [46]. Recently, Hoel et al.[47] demonstrated that activation of IL-1β was associated with an approximately 1.5-fold increased risk of myocardial infarction among PWH. Indeed, the Canakinumab Anti-inflammatory Thrombosis Outcome Study demonstrated a decrease in the risk of cardiovascular events in individuals with established CVD after IL-1β inhibition with canakinumab [48]. Thus, our findings of higher prevalence of elevated IL-1β among obese adults in our cohort is of concern for this particularly healthy population of PWH. Our findings are novel as there is a lack of studies comparing IL-1β by HIV/obesity status. Longitudinal studies are needed to further confirm these findings.
Intestinal fatty acid binding protein (I-FABP) is a cytosolic protein found in enterocytes of the gastrointestinal tract and is involved in the translocation of fatty acids from the apical membrane of enterocytes to the endoplasmic reticulum. It is highly expressed in disease conditions wherein the intestinal wall is injured by pathogens such as HIV and is currently used as a marker of intestinal damage in PWH [49]. Higher circulating levels of I-FABP are associated with increased mortality in PWH [50,51]. On the contrary, CD14 is a glycoprotein and a co-receptor that mediates the interaction of lipopolysaccharide (LPS), a major constituent of bacterial endotoxin, with monocytes/macrophages. Our findings of lower levels of sCD14 and I-FABP among obese PWH compared with the nonobese PWH in our study may reflect improvement of malnutrition-associated enteropathy commonly observed in PWH in resource limited settings [52]. Nonetheless, we still observed significantly higher rates of elevated I-FABP and sCD14 in the HIV-positive/obese and HIV-positive/nonobese compared with HIV-negative participants, suggesting that these markers do not fully normalize after ART. Our results are consistent with previous published data that reported similar associations between body mass index and sCD14 and I-FABP [19–21,53]. Our study and those previously published are unusual in that we observe a decrease in sCD14 and I-FABP, while hsCRP increased among obese patients, whereas the SMART study reported a positive correlation between hsCRP and sCD14 [36]. The negative association between abdominal obesity with I-FABP and sCD14 levels in PWH needs to be further explored because unlike the previous studies, we included multiple markers of monocyte/macrophage and individuals from sub-Saharan Africa who are also at high risk of coinfections that could potentially induce inflammation. Notably, we did not establish a correlation between sCD14 with sCD163 in PWH suggesting that the differences we observe between sCD14 and sCD163 may correspond to different pathways leading to their activation in African PWH or perhaps the availability of sCD14 in the blood.
Although the exact mechanism underlying the association between adiposity and inflammation is not fully understood, the fact that sCD163, a marker of macrophage activation, attenuated the association between obesity and IL-1β, IL-6 and TNF-α in PWH suggests that macrophages play an import role [12,13,54,55]. Indeed, in human or animal models, obesity results in an increase in macrophage infiltration and activation in the adipose tissues [12,56,57], consistent with the increase in inflammatory cytokines produced by these cells. Our data also suggest that there may be more than one pathway that leads to inflammation in addition to macrophage activation and microbial translocation, possibly through greater expression of pro-inflammatory cytokines by stromal vascular cells and hypertrophied adipocytes and/or by leptin-induced CRP expression in hepatocytes. Additional adjustment with sCD14, a known receptor for LPS and an indirect marker of microbial translocation or I-FABP, marker of gut epithelial damage, did not attenuate the association between obese status with inflammatory cytokines. Furthermore, these associations between abdominal obesity and inflammatory cytokines were independent of HIV specific factors (ART regimen, duration of HIV treatment or the level of immunosuppression) in our PWH, implying that factors other than HIV virus may also play an important role. Other potential sources of inflammation in obese PWH that require further study include nonmeasured coinfections highly endemic in this population such as tuberculosis and cytomegalovirus.
Our study has several strengths. It is the first study to assess the contribution of HIV and central obesity on markers of inflammation and monocyte activation in Sub-Saharan Africa. Our study included a large diverse panel of inflammatory biomarkers not previously reported, a relatively large sample size and robust clinical data on comorbid diseases that likely modulate inflammation. We enrolled an equal number of men and women, making our study results more generalizable. The relatively sex-matched HIV-negative persons allowed for direct comparison between groups. We acknowledge certain limitations to our study. This was a cross-sectional study and therefore we cannot exclude the possibility of nonmeasured confounding factors. We also lack cell surface immune activation marker data for comparison with the plasma cytokine data. At the time of recruitment, none of the PWH were on integrase strand transfer inhibitor (INSTI) treatment, making our results less generalizable for PWH currently on INSTI.
Our data suggest that central obesity is common in African PWH specially among the normal and overweight individuals. Centrally obese PWH with well controlled HIV are at the highest risk for elevated inflammation and immune activation and could be targeted to reduce the risk of CVD. As the prevalence of obesity increase in Africa [58], more prospective studies are needed to define health risks associated with elevated biomarkers among Africans with and those without HIV infection.
Acknowledgements
We thank Geoffrey Omondi, Juliet Aldo and Lindsay Kendall for their contribution. This project was supported by National Institutes of Health (NIH) grant R21TW010459, AI027757 and R21TW010459-02S1; The GlaxoSmithKline R&D grant number 3001388945; and EDCTP2 programme grant TMA-2016-1598-Kenya CVHIV. T.T. is supported by NIH grant 1K01HL147723 from NHLBI. The funders did not participate in data collection or any activity that is directly related to the execution of the research. T.T., C.F., S.P., S.P. contributed to the conception and design of the study, the supervision, data acquisition, analysis and interpretation, and the critical revision of the manuscript. J.Z., C.W., J.W., O.C., P.M., B.C., J.M., J.O., S.M. contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
Conflicts of interest
All authors declare no financial conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Slaymaker E, Todd J, Marston M, Calvert C, Michael D, Nakiyingi-Miiro J, et al. How have ART treatment programmes changed the patterns of excess mortality in people living with HIV? Estimates from four countries in East and Southern Africa. Glob Health Action 2014; 7:22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013; 14:195–207. [DOI] [PubMed] [Google Scholar]
- 3.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow FC, Price RW, Hsue PY, Kim AS. Greater risk of stroke of undetermined etiology in a contemporary HIV-infected cohort compared with uninfected individuals. J Stroke Cerebrovasc Dis 2017; 26:1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol 2013; 2013:697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrin M, Tate JP, Akgün KM, Butt AA, Crothers K, Freiberg MS, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temu TM, Zifodya JS, Polyak SJ, Wagoner J, Wanjalla CN, Masyuko S, et al. Antiretroviral therapy reduces but does not normalize immune and vascular inflammatory markers in adults with chronic HIV infection in Kenya. AIDS 2020; 35:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mave V, Erlandson KM, Gupte N, Balagopal A, Asmuth DM, Campbell TB, et al. Inflammation and change in body weight with antiretroviral therapy initiation in a multinational cohort of HIV-infected adults. J Infect Dis 2016; 214:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koethe JR, Heimburger DC, PrayGod G, Filteau S. From wasting to obesity: the contribution of nutritional status to immune activation in HIV infection. J Infect Dis 2016; 214: Suppl 2: S75–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest 2017; 127:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013; 14:232–244. [DOI] [PubMed] [Google Scholar]
- 15.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 2007; 116:1234–1241. [DOI] [PubMed] [Google Scholar]
- 16.Masyuko SJ, Page ST, Polyak SJ, Kinuthia J, Osoti AO, Otieno FC, et al. Human immunodeficiency virus is associated with higher levels of systemic inflammation among Kenyan adults despite viral suppression. Clin Infect Dis 2020; ciaa1650.doi:10.1093/cid/ciaa1650 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fjeldborg K, Christiansen T, Bennetzen M, J. Møller H, Pedersen SB, Richelsen B. The macrophage-specific serum marker, soluble CD163, is increased in obesity and reduced after dietary-induced weight loss. Obesity (Silver Spring) 2013; 21:2437–2443. [DOI] [PubMed] [Google Scholar]
- 18.Koethe JR, Bian A, Shintani AK, Boger MS, Mitchell VJ, Erdem H, et al. Serum leptin level mediates the association of body composition and serum C-reactive protein in HIV-infected persons on antiretroviral therapy. AIDS Res Hum Retroviruses 2012; 28:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koethe JR, Dee K, Bian A, Shintani A, Turner M, Bebawy S, et al. Circulating interleukin-6, soluble CD14, and other inflammation biomarker levels differ between obese and nonobese HIV-infected adults on antiretroviral therapy. AIDS Res Hum Retroviruses 2013; 29:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conley LJ, Bush TJ, Rupert AW, Sereti I, Patel P, Brooks JT, et al. Obesity is associated with greater inflammation and monocyte activation among HIV-infected adults receiving antiretroviral therapy. AIDS 2015; 29:2201–2207. [DOI] [PubMed] [Google Scholar]
- 21.Taylor BS, So-Armah K, Tate JP, Marconi VC, Koethe JR, Bedimo RJ, et al. HIV and obesity comorbidity increase interleukin 6 but not soluble CD14 or D-dimer. J Acquir Immune Defic Syndr 2017; 75:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020; 16:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010; 18:2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Levy D, Long MT. Visceral and intrahepatic fat are associated with cardiometabolic risk factors above other ectopic fat depots: the Framingham Heart Study. Am J Med 2018; 131:684–692. e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baik I, Ascherio A, Rimm EB, Giovannucci E, Spiegelman D, Stampfer MJ, et al. Adiposity and mortality in men. Am J Epidemiol 2000; 152:264–271. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005; 366:1640–1649. [DOI] [PubMed] [Google Scholar]
- 27.Lofgren I, Herron K, Zern T, West K, Patalay M, Shachter NS, et al. Waist circumference is a better predictor than body mass index of coronary heart disease risk in overweight premenopausal women. J Nutr 2004; 134:1071–1076. [DOI] [PubMed] [Google Scholar]
- 28.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116:39–48. [DOI] [PubMed] [Google Scholar]
- 29.Canoy D, Cairns BJ, Balkwill A, Wright FL, Green J, Reeves G, et al. Coronary heart disease incidence in women by waist circumference within categories of body mass index. Eur J Prev Cardiol 2013; 20:759–762. [DOI] [PubMed] [Google Scholar]
- 30.Masyuko SJ, Page ST, Kinuthia J, Osoti AO, Polyak SJ, Otieno FC, et al. Metabolic syndrome and 10-year cardiovascular risk among HIV-positive and HIV-negative adults: a cross-sectional study. Medicine (Baltimore) 2020; 99:e20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temu TM, Polyak SJ, Zifodya JS, Wanjalla CN, Koethe JR, Masyuko S, et al. Endothelial dysfunction is related to monocyte activation in antiretroviral-treated people with HIV and HIV-negative adults in Kenya. Open Forum Infect Dis 2020; 7:ofaa425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Center. APHR. Kenya STEPwise survey for non communicable diseases risk factors 2015 report. Ministry of Health, Kenya; 2015. Available at https://www.who.int/ncds/surveillance/steps/Kenya_2015_STEPS_Report.pdf [Accessed 23 May 2021] [Google Scholar]
- 33. Federation TID. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. 2006. Available at https://www.idf.org/ [Accessed 23 May 2021] [Google Scholar]
- 34.Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016; 30:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borges Á, O’Connor JL, Phillips AN, Neaton JD, Grund B, Neuhaus J, et al. Interleukin 6 is a stronger predictor of clinical events than high-sensitivity C-reactive protein or D-dimer during HIV infection. J Infect Dis 2016; 214:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. A systematic review. PLoS One 2016; 11:e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vos AG, Hulzebosch A, Grobbee DE, Barth RE, Klipstein-Grobusch K. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One 2017; 12:e0169986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 2007; 115:1528–1536. [DOI] [PubMed] [Google Scholar]
- 41.Borges AH, O’Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ, et al. Factors associated with plasma IL-6 levels during HIV infection. J Infect Dis 2015; 212:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fonseca FA, Izar MC. High-sensitivity C-reactive protein and cardiovascular disease across countries and ethnicities. Clinics (Sao Paulo) 2016; 71:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link?. J Am Coll Cardiol 2013; 62:397–408. [DOI] [PubMed] [Google Scholar]
- 44.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol 2006; 163:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012; 55:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 2018; 281:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoel H, Ueland T, Knudsen A, Kjær A, Michelsen AE, Sagen EL, et al. Soluble markers of interleukin 1 activation as predictors of first-time myocardial infarction in HIV-infected individuals. J Infect Dis 2020; 221:506–509. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 49.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 50.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.León A, Leal L, Torres B, Lucero C, Inciarte A, Arnedo M, et al. Association of microbial translocation biomarkers with clinical outcome in controllers HIV-infected patients. AIDS 2015; 29:675–681. [DOI] [PubMed] [Google Scholar]
- 52.Welsh FK, Farmery SM, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, et al. Gut barrier function in malnourished patients. Gut 1998; 42:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheru LT, Park EA, Saylor CF, Burdo TH, Fitch KV, Looby S, et al. I-FABP is higher in people with chronic HIV than elite controllers, related to sugar and fatty acid intake and inversely related to body fat in people with HIV. Open Forum Infect Dis 2018; 5:ofy288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanjalla CN, McDonnell WJ, Koethe JR. Adipose tissue T cells in HIV/SIV infection. Front Immunol 2018; 9:2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 2017; 127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Godfrey C, Bremer A, Alba D, Apovian C, Koethe JR, Koliwad S, et al. Obesity and fat metabolism in human immunodeficiency virus-infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis 2019; 220:420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel P, Rose CE, Collins PY, Nuche-Berenguer B, Sahasrabuddhe VV, Peprah E, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. AIDS 2018; 32: Suppl 1: S5–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.