Abstract
STUDY QUESTION
Is ovarian stimulation with follitropin delta in its individualised fixed-dose regimen at least as efficacious as follitropin alfa in a conventional dosing regimen in Asian population?
SUMMARY ANSWER
Ovarian stimulation with individualised follitropin delta dosing resulted in a non-inferior ongoing pregnancy rate, a significantly higher live birth rate and a significantly lower incidence of early ovarian hyperstimulation syndrome (OHSS) and/or preventive interventions compared to conventional follitropin alfa dosing.
WHAT IS KNOWN ALREADY
Previous randomised controlled trials conducted in Japan as well as in Europe, North- and South America have demonstrated that ovarian stimulation with the individualised follitropin delta dosing regimen based on serum anti-Müllerian hormone (AMH) level and body weight modulated the ovarian response and reduced the risk of OHSS without compromising pregnancy and live birth rates.
STUDY DESIGN, SIZE, DURATION
Randomised, controlled, multi-centre, assessor-blind trial conducted in 1009 Asian patients from mainland China, South Korea, Vietnam and Taiwan, undergoing their first IVF/ICSI cycle. Randomisation was stratified by age (<35, 35–37, 38–40 years). The primary endpoint was ongoing pregnancy rate assessed 10–11 weeks after embryo transfer in the fresh cycle (non-inferiority limit −10.0%; analysis adjusted for age stratum).
PARTICIPANTS/MATERIALS, SETTING, METHODS
The follitropin delta treatment consisted of a fixed daily dose individualised according to each patient’s initial AMH level and body weight (AMH <15 pmol/l: 12 μg; AMH ≥15 pmol/l: 0.19 to 0.10 μg/kg; min-max 6–12 μg). The follitropin alfa dose was 150 IU/day for the first 5 days with subsequent potential dose adjustments according to individual response. A GnRH antagonist protocol was applied. OHSS was classified based on Golan’s system. Women with an ongoing pregnancy were followed until live birth and 4 weeks after.
MAIN RESULTS AND THE ROLE OF CHANCE
The number of oocytes retrieved was significantly (P < 0.001) lower with individualised follitropin delta versus conventional follitropin alfa (10.0 ± 6.1 versus 12.4 ± 7.3). Nevertheless, compared to the conventional dosing approach, the individualised follitropin delta dosing regimen resulted in on average 2 more oocytes (9.6 ± 5.3 versus 7.6 ± 3.5) in potential low responders as indicated by AMH <15 pmol/l, and on average 3 fewer oocytes (10.1 ± 6.3 versus 13.8 ± 7.5) in potential high responders as indicated by AMH ≥15 pmol/l. Among women with AMH ≥15 pmol/l, excessive response occurred less frequently with individualised follitropin delta than with follitropin alfa (≥15 oocytes: 20.2% versus 39.1%; ≥20 oocytes: 6.7% versus 18.5%; both P < 0.001). The incidence of early OHSS and/or preventive interventions for early OHSS was significantly (P = 0.004) reduced from 9.6% with follitropin alfa to 5.0% with individualised follitropin delta. The total gonadotropin use was significantly (P < 0.001) reduced from an average of 109.9 ± 32.9 μg (1498 ± 448 IU) follitropin alfa to 77.5 ± 24.4 μg follitropin delta. Non-inferiority of follitropin delta in its individualised dosing regimen to conventional follitropin alfa was established with respect to the primary endpoint of ongoing pregnancy rate which was 31.3% with follitropin delta compared to 25.7% with follitropin alfa (estimated mean difference 5.4% [95% CI: −0.2%; 11.0%]). The live birth rate was significantly higher at 31.3% with individualised follitropin delta compared to 24.7% with follitropin alfa (estimated mean difference 6.4% [95% CI: 0.9%; 11.9%]; P = 0.023). The live birth rate for each stratum were as follows for follitropin delta and follitropin alfa, respectively; <35 years: 31.0% versus 25.0%, 35–37 years: 35.3% versus 26.7%, 38–40 years: 20.0% versus 14.3%.
LIMITATIONS, REASONS FOR CAUTION
The trial only covered the clinical outcome of one treatment cycle with fresh cleavage-stage embryo transfers.
WIDER IMPLICATIONS OF THE FINDINGS
The present trial shows that in addition to reducing the early OHSS risk, follitropin delta in its individualised fixed-dose regimen has the potential to improve the success rate in fresh cycles across all ages and with a lower gonadotropin consumption compared to conventional follitropin alfa dosing.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by Ferring Pharmaceuticals. J.Q., Y.Z., X.L., T.H., H.-Y.H. and S.-H.K. have received institutional (not personal) clinical trial fees from Ferring Pharmaceuticals. M.G., B.M. and J.-C.A. are employees of Ferring Pharmaceuticals. J.-C.A. has pending and issued patent applications in the WO 2013/020996 and WO 2019/043143 patent families that comprise allowed and granted patent rights related to follitropin delta.
TRIAL REGISTRATION NUMBER
NCT03296527 (clinicaltrials.gov).
TRIAL REGISTRATION DATE
28 September 2017
DATE OF FIRST PATIENT’S ENROLMENT
1 December 2017
Keywords: anti-Müllerian hormone, follitropin delta, individualised dosing, live birth, ovarian stimulation
Introduction
Though it is well established that the oocyte yield in patients undergoing ovarian stimulation is positively correlated with the extent of exposure to exogenous FSH, the relation between number of oocytes retrieved and treatment outcomes, especially efficacy endpoints, is more complex. The optimal ovarian response to gonadotropins balances the chance of obtaining a live birth against patient discomfort and iatrogenic safety risks. As patient characteristics differ, selecting the most appropriate gonadotropin dose for each patient to achieve an adequate ovarian response requires consideration. The concept of individualised gonadotropin dosing has always generated interest, and clinicians have been customising their approaches based on their clinical experience. However, in order for these dose recommendations to be objective and consistent, attention should be given to the most relevant quantitative parameters such as those influencing drug exposure as well as to predictors of ovarian response to gonadotropins (Fauser et al., 2008; Nelson et al., 2009; Fleming et al., 2013; Nelson, 2013; Broer et al., 2014; La Marca and Sunkara, 2014).
Follitropin delta is a novel recombinant FSH (rFSH) preparation expressed in a human cell line (PER.C6®) and developed with an individualised dosing regimen based on each woman’s serum anti-Müllerian hormone (AMH) concentration and body weight in an effort to guide clinicians to achieve an ovarian response that can minimise the risk of ovarian hyperstimulation syndrome (OHSS) without compromising efficacy. Two randomised controlled trials (RCTs) have shown that the individualised follitropin delta dosing regimen, through modulation of the ovarian response, can reduce the OHSS risk versus other rFSH preparations, while providing at least the same live birth rates (Nyboe Andersen and Nelson et al., 2017; Ishihara and Arce, 2021).
The follitropin delta dosing algorithm establishes an individualised dose based on the woman’s body weight and serum AMH level. Populations may differ on these patient characteristics, and it therefore makes sense to validate the dosing regimen across diverse ethnicities and geographical regions. In fact, Asian women are typically characterised by lower BMI (WHO Expert Consultation, 2004) and different body composition for the same BMI (Deurenberg et al., 2002) compared to European women. Furthermore, substantially lower AMH levels in Chinese women have been reported in comparison to Caucasian women (Bleil et al., 2014) and European women at the same age (Nelson et al., 2020). In addition to diversity in population characteristics, clinical practices differ across geographical regions, such as day of transfer, number of embryos transferred and other important aspects potentially influencing success rates, which further stresses the need for customised regional clinical trials.
Although a trial has been performed in Japanese women (Ishihara and Arce, 2021), most of the data validating the individualised dosing approach are from a large trial conducted in predominantly Caucasian women (Nyboe Andersen and Nelson et al., 2017). Therefore, the aim of this large, randomised, controlled study was to investigate follitropin delta in its novel individualised algorithm-based dosing regimen in the Asian patient population, and to compare its efficacy and safety to follitropin alfa in its recommended conventional dosing approach.
Materials and methods
Trial design
This was a randomised, controlled, assessor-blind, parallel groups, multi-centre, non-inferiority trial comparing the efficacy and safety of follitropin delta in its individualised fixed-dose regimen with follitropin alfa in a conventional adjustable dosing regimen in Asian women undergoing their first ovarian stimulation cycle. The trial was conducted at 26 investigational sites (Supplementary Table SI) in the following countries/regions: mainland China, South Korea, Taiwan and Vietnam. The trial period was from 1 December 2017 to 3 January 2020, with pregnancy follow-up completed on 1 September 2020. The trial (number 000145) was approved by an Independent Ethics Committee at each centre and conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation Guidelines for Good Clinical Practice and applicable regulatory requirements. All participants provided written, informed consent.
Trial population
Eligible trial participants included Asian reproductive-aged women between the ages of 20 and 40 years, with a BMI between 17.5 and 32.0 kg/m2 and undergoing their first ovarian stimulation cycle for IVF/ICSI. Eligibility criteria included women diagnosed with tubal infertility, unexplained infertility, endometriosis stage I/II or with partners diagnosed with male factor infertility. Additionally, women had to have regular menstrual cycles of 24–35 days, presence of both ovaries and early follicular phase FSH serum levels of 1–15 IU/l. Women with endometriosis stage III/IV, history of recurrent miscarriage and with one or more follicles ≥10 mm observed prior to randomisation were excluded. There was no eligibility criterion limiting the serum AMH level at screening. A full description of inclusion/exclusion criteria is listed in Supplementary Table SII.
Treatment randomisation and blinding
Women were randomised in a 1:1 ratio to treatment with either follitropin delta (Rekovelle; Ferring Pharmaceuticals, Saint-Prex, Switzerland) or follitropin alfa (Gonal-f; Merck Serono, Geneva, Switzerland) via a central computer-generated randomisation list, prepared by an independent statistician. The randomisation was stratified by centre and age (<35, 35–37 and 38–40 years) using a block size of four. All investigators, embryologists and central laboratory personnel were blinded to the individual treatment allocation.
Trial procedures
Women randomised to follitropin delta (Rekovelle, 72 μg/2.16 ml) administered a single fixed daily subcutaneous dose in the abdomen, which was determined by their serum AMH level at screening and body weight at randomisation. For women with AMH <15 pmol/l, the daily follitropin delta dose was 12 μg, irrespective of body weight. For women with AMH ≥15 pmol/l, the daily follitropin delta dose was on a continuous scale ranging from 0.19 to 0.10 μg/kg, i.e. dependent on serum AMH and body weight. The minimum and maximum allowed daily doses were 6 μg and 12 μg, respectively. The follitropin delta dose was calculated in the electronic case record form, as per the dosing algorithm (Supplementary Table SIII). The assigned daily dose was fixed throughout the stimulation period (i.e. no dose adjustments during stimulation).
Women randomised to follitropin alfa (Gonal-f, 900 IU/1.5 ml) administered a daily subcutaneous dose of 150 IU (expressed also as 11 μg of follitropin alfa (European Medicines Agency. Gonal-f, 2021)) for the first 5 days, which is the lowest approved starting dose in mainland China, South Korea, Vietnam and Taiwan; thereafter, the dose could be adjusted up or down by 75 IU according to the individual response during stimulation, with 450 IU as the maximum daily dose allowed.
On days 2–3 of the menstrual cycle, women were randomised to ovarian stimulation with either follitropin delta or follitropin alfa. To prevent a premature LH surge, a GnRH antagonist (cetrorelix acetate, Cetrotide; Merck Serono, Geneva, Switzerland) was initiated on stimulation day 6 at a daily dose of 0.25 mg and continued throughout the stimulation period.
Triggering of final follicular maturation was done as soon as ≥3 follicles with a diameter ≥17 mm were observed. Triggering could also be done in case 1 or 2 follicles with a diameter ≥17 mm were observed and the investigator judged that ≥3 follicles with a diameter ≥17 mm could not be reached and that triggering was preferred instead of cycle cancellation. For patients with <25 follicles with a diameter ≥12 mm, triggering was performed with hCG (choriogonadotropin alfa, Ovitrelle; Merck Serono, Geneva, Switzerland) at a single dose of 250 μg. In case of excessive ovarian response (≥25 follicles with a diameter ≥12 mm), patients with 25–35 follicles with diameter ≥12 mm could either be administered a GnRH agonist (triptorelin acetate, Gonapeptyl; Ferring Pharmaceuticals, Saint-Prex, Switzerland) at a single dose of 0.2 mg, or have the cycle cancelled as per the investigator’s judgement, whereas for patients with >35 follicles with a diameter ≥12 mm the cycle was to be cancelled.
Blood samples were collected throughout the trial for analysis of AMH, FSH, LH, estradiol, inhibin B, inhibin A and progesterone at central laboratories. The serum concentration of AMH was measured at screening using the Elecsys® AMH Plus immunoassay from Roche Diagnostics (Rotkreuz, Switzerland). Serum samples for assessment of endocrine parameters (estradiol, inhibin B, inhibin A and progesterone) were collected on stimulation day 1, stimulation day 6 and end-of-stimulation, and analysed at central laboratories. Information on the sensitivity and precision of the validated methods are presented in Supplementary Table SIV.
Oocyte retrieval took place 36 h (±2 h) after triggering of final follicular maturation and the oocytes could be inseminated by IVF or ICSI, using ejaculated sperm from partner or sperm donor. In line with local clinical practice at the time of initiating the trial, single or double embryo transfer was performed on day 3 after oocyte retrieval. Women <35 years had single embryo transfer if a good-quality embryo (defined as an embryo with ≥6 blastomeres and ≤20% fragmentation, without signs of multinucleation) was available; otherwise, double embryo transfer was performed (and single transfer if two embryos were not available). Women ≥35 years had double embryo transfer (and single transfer if two embryos were not available). Remaining embryos could be cryopreserved in accordance with local guidelines and/or regulations. For women who underwent triggering of final follicular maturation with GnRH agonist, no transfer took place in the current fresh cycle and the embryos available could be cryopreserved according to local practice. All cryopreserved embryos could be used by the women after completion of the trial, in accordance with local guidelines and/or regulations.
Vaginal progesterone gel (Crinone; Merck Serono, Geneva, Switzerland) at a daily dose of 90 mg was provided for luteal phase support from the day of oocyte retrieval or the day after and at least until clinical pregnancy. Thereafter, the investigator could decide to continue luteal phase support up to the ongoing pregnancy visit, according to local practice or stop if menses, negative βhCG or pregnancy loss occurred. Patients with an ongoing pregnancy were followed up until delivery and for 4 weeks after. Adverse events were recorded from the signed informed consent until the end-of-trial visit. Local tolerability at the injection site was assessed by the woman three times daily, i.e. immediately, 30 min and 24 h after each injection of follitropin delta or follitropin alfa. The injection site reactions (redness, pain, itching, swelling and bruising) were rated as none, mild, moderate or severe.
Trial outcomes
The primary endpoint was ongoing pregnancy rate, defined as at least one intrauterine viable foetus 10–11 weeks after transfer. Prespecified secondary efficacy endpoints and assessments covered pregnancy outcomes such as positive βhCG rate (i.e. positive serum βhCG test 13–15 days after transfer), clinical pregnancy rate (i.e. at least one gestational sac 5–6 weeks after transfer), ongoing implantation rate (i.e. the number of intrauterine viable foetuses 10–11 weeks after transfer divided by the number of embryos transferred), live birth rate (i.e. the birth of at least one live neonate) and live birth rate at 4 weeks after birth (i.e. the presence of at least one live neonate 4 weeks after birth). Ovarian response parameters included number of oocytes retrieved, ovarian response within 8–14 oocytes, excessive ovarian response in at-risk population (i.e. ≥15 or ≥20 oocytes retrieved for women with AMH ≥15 pmol/l), endocrine profile at end-of-stimulation (estradiol, inhibin B, inhibin A and progesterone), fertilisation rate (i.e. the number of oocytes with two pronuclei divided by the number of oocytes retrieved), number of embryos on day 3 after oocyte retrieval, average daily dose of gonadotropin, total gonadotropin dose and duration of stimulation. Prespecified safety endpoints included adverse events, early OHSS (i.e. onset ≤9 days after triggering of final follicular maturation), preventive interventions for early OHSS (i.e. cycle cancellation due to excessive ovarian response or embryo transfer cancellation due to excessive ovarian response/OHSS risk, or triggering of final follicular maturation with GnRH agonist), late OHSS (i.e. onset >9 days after triggering of final follicular maturation) and injection site reactions. The grading (1, 2, 3, 4, or 5) and categorisation of mild, moderate or severe OHSS were based on Golan’s classification system (Golan et al., 1989).
Statistical analysis
The primary objective of this trial was to demonstrate non-inferiority of follitropin delta compared to follitropin alfa with respect to ongoing pregnancy rate. Based on a prespecified non-inferiority limit of −10.0% (absolute) and an assumed ongoing pregnancy rate of 32.2% in both treatment groups, the trial was designed to have 90% power achieving the primary objective. The non-inferiority limit was considered reasonable since further tightening of the non-inferiority limit would lead to a substantial increase in sample size with only limited impact on the precision of the estimate. The mean difference in ongoing pregnancy rates (follitropin delta-follitropin alfa) was estimated using the Mantel-Haenszel method, combining risk differences across age strata, and the 95% CI was calculated. If the lower limit of the two-sided 95% CI was greater than the non-inferiority limit (−10.0%) for both the full analysis set (FAS), i.e. all randomised and exposed women and the per-protocol analysis set, then non-inferiority was established. The FAS excluded two women randomised but not exposed to study drug, whereas the per-protocol analysis set excluded additional 39 women with major protocol deviations with a potential impact on the primary endpoint.
Positive βhCG, clinical pregnancy, ongoing implantation and live birth rates as well as live birth rate at 4 weeks after birth were compared between groups in a similar manner as the primary endpoint, i.e. using the Mantel-Haenszel method to combine risk differences across age strata along with the two-sided 95% CI and the P-value for no difference. The number of oocytes retrieved, fertilisation rate and the number of embryos on day 3 were compared between treatment groups using the van Elteren test adjusted for screening AMH concentration (<15 pmol/l or ≥15 pmol/l) and using the Wilcoxon’s test to compare treatments within each AMH category. Excessive ovarian response in at-risk population was compared between treatment groups using the Fisher’s exact test. The endocrine parameters (estradiol, inhibin B, inhibin A and progesterone) were compared between treatment groups using multiplicative analysis of covariance models with treatment and age stratum as fixed factors and the concentration on stimulation day 1 as covariate. The average daily dose, the duration of stimulation and the total gonadotropin dose were compared between treatment groups using the van Elteren test adjusted for age stratum. Elevated progesterone (>3.18 nmol/l at end-of-stimulation) and OHSS endpoints were compared between treatment groups using a logistic regression model with treatment and age stratum as fixed factors. The analyses included all randomised and exposed women, except for the analyses of fertilisation rate, excessive ovarian response and ongoing implantation rate, which are based on patients with at least one oocyte retrieved, patients with at least one oocyte retrieved or having cycle cancellation due to poor or excessive ovarian response, and patients with transfer, respectively. All statistical analyses were performed in SAS (SAS Institute Inc, version 9.4, Cary, NC, USA).
Results
Baseline characteristics
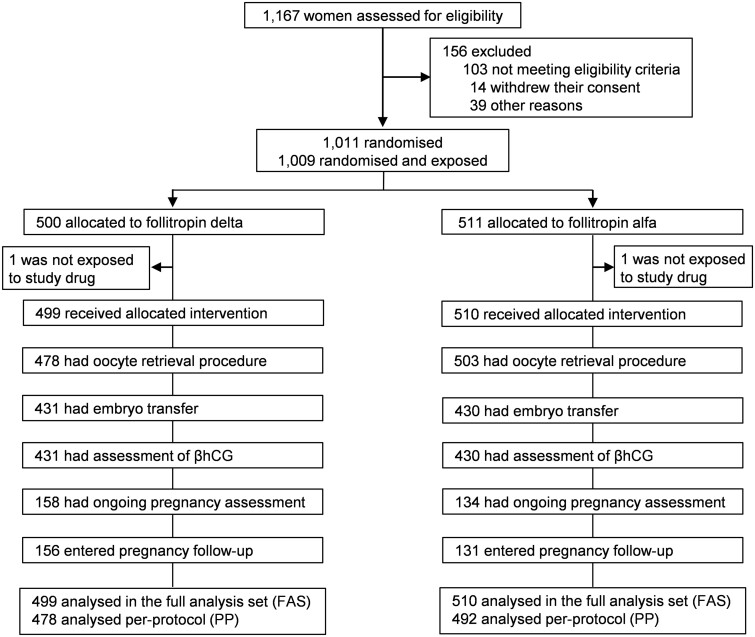
As depicted in the trial and participant flowchart, a total of 1009 women were randomised and exposed, of whom 499 were treated with follitropin delta in its individualised fixed-dose regimen and 510 with follitropin alfa in a conventional and adjustable dosing regimen (Fig. 1). The baseline patient characteristics were comparable between treatment groups including mean age and age distribution, body weight, BMI, duration of infertility, primary infertility and reasons for infertility, antral follicle count, serum AMH concentration and distribution and serum concentrations of FSH, LH, estradiol, inhibin B, inhibin A and progesterone (Table I).
Figure 1.
Trial and participant flow. βhCG, beta unit of human chorionic gonadotropin; FAS, full analysis set (defined as all women randomised and exposed); PP, per-protocol (defined as all women randomised and exposed, except those excluded as a result of major protocol deviations).
Table I.
Baseline patient characteristics.
| Characteristic | Treatment group |
|
|---|---|---|
| Individualised follitropin delta (N = 499) | Conventional follitropin alfa (N = 510) | |
| Age | ||
| All patients (years) | 31.1 ± 3.7 | 31.2 ± 3.8 |
| <35 | 394 (79.0) | 396 (77.6) |
| 35–37 | 85 (17.0) | 86 (16.9) |
| 38–40 | 20 (4.0) | 28 (5.5) |
| Body weight (kg) | 55.7 ± 8.1 | 55.8 ± 8.1 |
| BMI (kg/m2) | 21.8 ± 2.7 | 21.8 ± 2.8 |
| Infertility history | ||
| Duration of infertility (months) | 43.1 ± 27.0 | 43.7 ± 28.1 |
| Primary infertility | 324 (64.9) | 305 (59.8) |
| Reason for infertility | ||
| Unexplained infertility | 125 (25.1) | 128 (25.1) |
| Tubal infertility | 220 (44.1) | 232 (45.5) |
| Male factor | 143 (28.7) | 144 (28.2) |
| Endometriosis stage I/II | 7 (1.4) | 2 (0.4) |
| Other | 4 (0.8) | 4 (0.8) |
| Endometrial thickness (mm) | 6.2 ± 2.2 | 6.0 ± 2.0 |
| Ovarian volume (cm3) | 5.1 ± 2.7 | 5.0 ± 2.5 |
| AFCa | 14.8 ± 6.5 | 14.5 ± 6.2 |
| Endocrine profileb | ||
| AMH (pmol/l)c | 23.4 (16.1–32.9) | 22.6 (15.3–33.2) |
| AMH <15 pmol/l | 109 (21.8) | 118 (23.1) |
| AMH ≥15 pmol/l | 390 (78.2) | 392 (76.9) |
| FSH (IU/l) | 7.3 (6.3–8.5) | 7.3 (6.2–8.3) |
| LH (IU/l) | 3.8 (3.0–4.9) | 3.8 (2.8–5.1) |
| Estradiol (pmol/l) | 139.3 (111.8–165.7) | 143.4 (113.4–178.3) |
| Inhibin B (ng/l) | 79.0 (62.0–99.0) | 81.0 (63.0–102.0) |
| Inhibin A (ng/l) | 5.0 (4.0–6.5) | 5.3 (4.0–6.5) |
| Progesterone (nmol/l) | 0.8 (0.8–2.1) | 0.8 (0.8–2.1) |
Values are means ± SD, median (25th–75th percentiles) or n (%), unless otherwise stated. Data are for all patients unless otherwise stated.
AFC, antral follicle count; AMH, anti-Müllerian hormone; N, total number of patients; n, number of patients with observations.
This measurement reports the total number of antral follicles with a diameter of ≥2 mm for both ovaries combined, assessed by transvaginal ultrasound on the day of starting ovarian stimulation.
The AMH values are based on the screening samples, while the remaining endocrine parameters are based on the samples taken on stimulation day 1 before start of stimulation.
The serum concentration of AMH was assessed by a central laboratory using the Elecsys® AMH Plus immunoassay from Roche Diagnostics.
Ovarian stimulation
Table II shows the ovarian response to stimulation for both treatment groups. The daily starting dose was on average 8.5 ± 2.4 μg follitropin delta for women who received an individualised dose while all women in the conventional follitropin alfa group started on 150 IU (11 μg). Dose adjustments were recommended by the investigators at a similar incidence in the two groups, with a recommendation for dose increases on stimulation day 6 made for 45.3% (226/499) of the women in the individualised follitropin delta group (not carried out due to fixed-dose regimen) and 43.9% (224/510) of the women in the follitropin alfa group (carried out due to the adjustable dosing regimen). The proportion of women with dose increase requests per age stratum on stimulation day 6 were as follows in the individualised follitropin delta group and follitropin alfa group, respectively; <35 years: 43.7% (172/394) versus 42.7% (169/396), 35–37 years: 51.8% (44/85) versus 46.5% (40/86), 38–40 years: 50.0% (10/20) versus 53.6% (15/28). The average daily dose of gonadotropin across the entire stimulation cycle was 12.4 ± 1.7 µg (169.1 ± 23.1 IU) follitropin alfa which was significantly (P < 0.001) higher than the 8.5 ± 2.4 µg follitropin delta for the fixed-dose individualised regimen (Table II). The total gonadotropin use was significantly (P < 0.001) reduced from an average of 109.9 ± 32.9 μg (1498 ± 448 IU) follitropin alfa to 77.5 ± 24.4 μg follitropin delta.
Table II.
Ovarian stimulation.
| Outcome variable | Treatment group |
||
|---|---|---|---|
| Individualised follitropin delta (N = 499) | Conventional follitropin alfa (N = 510) | P-value | |
| Gonadotropin use | |||
| Average daily dose (μg/day) | 8.5 ± 2.4 | 12.4 ± 1.7 (169.1±23.1 IU/day)a | <0.001b |
| Duration of stimulation (days) | 9.2 ± 1.9 | 8.7 ± 1.6 | 0.001b |
| Total dose (μg) | 77.5 ± 24.4 | 109.9 ± 32.9 (1498±448 IU)a | <0.001b |
| Endocrine profilec | |||
| Estradiol (pmol/l) | 7429.3 (4786.2–10439.8) | 9055.8 (6214.2–12964.3) | <0.001d |
| Inhibin B (ng/l) | 1020.0 (689.0–1488.0) | 1101.0 (738.0–1665.0) | 0.001d |
| Inhibin A (ng/l) | 361.7 (252.8–525.6) | 447.4 (307.5–630.9) | <0.001d |
| Progesterone (nmol/l) | 2.4 (1.7–3.5) | 3.2 (2.2–4.4) | <0.001d |
| Oocytes and embryos | |||
| All patients | |||
| Oocytes retrieved | 10.0 ± 6.1 | 12.4 ± 7.3 | <0.001e |
| Fertilisation ratef | 64 ± 23 | 64 ± 21 | 0.819e |
| Embryos on day 3 | 7.0 ± 4.6 | 8.7 ± 5.5 | <0.001e |
| AMH <15 pmol/l | |||
| Oocytes retrieved | 9.6 ± 5.3 | 7.6 ± 3.5 | 0.004g |
| Fertilisation ratef | 66 ± 22 | 67 ± 22 | 0.516g |
| Embryos on day 3 | 6.8 ± 3.7 | 5.6 ± 2.9 | 0.016g |
| AMH ≥15 pmol/l | |||
| Oocytes retrieved | 10.1 ± 6.3 | 13.8 ± 7.5 | <0.001g |
| Fertilisation ratef | 63 ± 23 | 63 ± 21 | 0.539g |
| Embryos on day 3 | 7.0 ± 4.8 | 9.6 ± 5.7 | <0.001g |
| OHSS | |||
| Preventive interventions for early OHSSh | 6 (1.2) | 18 (3.5) | 0.012i |
| Early OHSSj | 20 (4.0) | 33 (6.5) | 0.075i |
| Early moderate/severe OHSS | 18 (3.6) | 24 (4.7) | 0.365i |
| Early OHSS and/or preventive interventionsk | 25 (5.0) | 49 (9.6) | 0.004i |
| Early moderate/severe OHSS and/or preventive interventions | 23 (4.6) | 40 (7.8) | 0.029i |
Values are means ± SD, median (25th–75th percentiles) or n (%), unless otherwise stated. Data are for all patients unless otherwise stated.
AMH, anti-Müllerian hormone; N, total number of patients; n, number of patients with observations; OHSS, ovarian hyperstimulation syndrome.
150 IU is equivalent to 11 μg follitropin alfa (European Medicines Agency. Gonal-f, 2021).
The P-value is based on a van Elteren test adjusted for age stratum.
At end-of-stimulation.
The P-value is for the test of equal mean effect and end-of-stimulation based on multiplicative analysis of covariance (ANCOVA) with treatment and age stratum as fixed factors and the concentration on stimulation day 1 as covariate.
The P-value is based on a van Elteren test adjusted for AMH category.
For all patients relative to oocytes retrieved.
The P-value is based on a Wilcoxon’s test.
Preventive interventions included cycle cancellation due to excessive ovarian response and triggering of final follicular maturation with GnRH agonist.
The P-value is for the test of an odd ratio equal to 1 based on a logistic regression model adjusted for age stratum.
Onset ≤9 days after triggering of final follicular maturation.
Three patients developed early OHSS despite preventive interventions: one in the individualised dosing group and two in the conventional dosing group.
At end-of-stimulation, significantly (all P ≤ 0.001) lower serum levels of estradiol, inhibin B, inhibin A and progesterone were observed with individualised follitropin delta than with follitropin alfa (Table II). The proportion of patients with elevated progesterone was 29.5% (147/499) with follitropin delta and 48.8% (249/510) with follitropin alfa (P < 0.001). Consistent with the differences in endocrine profile, the number of oocytes retrieved was significantly (P < 0.001) lower in the individualised follitropin delta group compared to the follitropin alfa group (Table II). Nevertheless, compared to the conventional dosing approach, the individualised follitropin delta dosing regimen resulted in on average 2 more oocytes (9.6 ± 5.3 versus 7.6 ± 3.5) in potential low responders as indicated by AMH <15 pmol/l and on average 3 fewer oocytes (10.1 ± 6.3 versus 13.8 ± 7.5) in potential high responders as indicated by AMH ≥15 pmol/l. Among women with AMH ≥15 pmol/l, excessive response occurred significantly (P < 0.001) less frequent with individualised follitropin delta than with follitropin alfa (≥15 oocytes: 20.2% [78/386] versus 39.1% [152/389]; ≥20 oocytes: 6.7% [26/386] versus 18.5% [72/389]). The proportion of women achieving an ovarian response ranging between 8 and 14 oocytes retrieved was 46.7% with individualised follitropin delta and 42.6% with follitropin alfa.
The average fertilisation rate was 64% in both treatment groups (Table II). The average number of embryos on day 3 after oocyte retrieval was 7.0 ± 4.6 for individualised follitropin delta and 8.7 ± 5.5 for follitropin alfa. The distinct modulation of ovarian response observed for the number of oocytes retrieved was also noted for the number of embryos on day 3, as potential low responders in the individualised follitropin delta group had on average 1.2 more embryos compared to the follitropin alfa group (6.8 ± 3.7 versus 5.6 ± 2.9), while potential high responders treated with an individualised follitropin delta dose had on average 2.6 fewer embryos than those following a conventional dosing approach with follitropin alfa (7.0 ± 4.8 versus 9.6 ± 5.7). The proportion of patients undergoing embryo transfer was 86.4% (431/499) in the individualised follitropin delta group and 84.3% (430/510) in the follitropin alfa group, and the distribution of single and multiple embryo transfers was similar in the two groups, with single embryo transfers accounting for 73.1% (315/431) of the transfers in the individualised follitropin delta group and 73.0% (314/430) in the follitropin alfa group.
Clinical outcomes: efficacy and safety
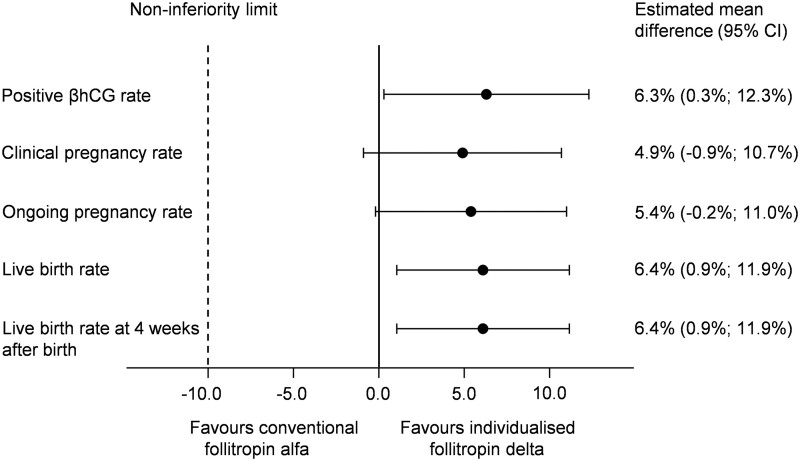
Treatment outcome data are presented in Fig. 2 and Table III. Non-inferiority of follitropin delta in its individualised fixed-dose regimen to follitropin alfa in a conventional adjustable dosing regimen was established with respect to the primary endpoint of ongoing pregnancy rate. For all patients who started a cycle, the ongoing pregnancy rate was 31.3% (156/499) with individualised follitropin delta compared to 25.7% (131/510) with follitropin alfa (estimated mean difference of 5.4% [95% CI: −0.2%; 11.0%]). Consistent results were observed in the per-protocol analysis set, where the ongoing pregnancy rate was 30.1% (144/478) with individualised follitropin delta and 25.2% (124/492) with follitropin alfa (estimated mean difference of 4.8% [95% CI: −0.8%; 10.4%]). The ongoing implantation rate was 30.7% (168/548) with individualised follitropin delta and 25.8% (141/546) with follitropin alfa (estimated mean difference of 4.4% [95% CI: −0.9%; 9.7%]).
Figure 2.
Clinical efficacy outcomes. The estimated mean differences (follitropin delta-follitropin alfa) for all clinical efficacy outcomes are presented together with the corresponding 95% CIs for equal effect using the Mantel-Haenszel method to combine the risk differences across age strata. βhCG, beta unit of human chorionic gonadotropin.
Table III.
Clinical efficacy outcomes.
| Outcome variable | Treatment group |
|||
|---|---|---|---|---|
| Individualised follitropin delta (N = 499) | Conventional follitropin alfa (N = 510) | Estimated mean difference (95% CI)a | P-valuea | |
| Positive βhCG rateb | 208 (41.7) | 180 (35.3) | 6.3% (0.3%; 12.3%) | 0.039 |
| Clinical pregnancy ratec | 180 (36.1) | 159 (31.2) | 4.9% (−0.9%; 10.7%) | 0.099 |
| Ongoing pregnancy rated,e | 156 (31.3) | 131 (25.7) | 5.4% (−0.2%; 11.0%) | 0.057 |
| Live birth ratef | 156 (31.3) | 126 (24.7) | 6.4 % (0.9%; 11.9%) | 0.023 |
| Live birth rate at 4 weeks after birthg | 156 (31.3) | 126 (24.7) | 6.4 % (0.9%; 11.9%) | 0.023 |
Values are n (%) unless otherwise stated.
βhCG, beta unit of human chorionic gonadotropin; N, total number of patients; n, number of patients with observations.
The estimated mean difference (follitropin delta-follitropin alfa) is presented together with the corresponding 95% CIs and the P-value for equal effect using the Mantel-Haenszel method to combine the risk differences across age strata.
Positive serum βhCG test 13–15 days after transfer according to the local laboratory’s reference ranges.
At least one gestational sac 5–6 weeks after transfer.
At least one intrauterine viable foetus 10–11 weeks after transfer.
The non-inferiority limit for the difference between the two treatments was pre-specified at −10.0% for the ongoing pregnancy rate.
The birth of at least one live neonate.
At least one live neonate 4 weeks after birth.
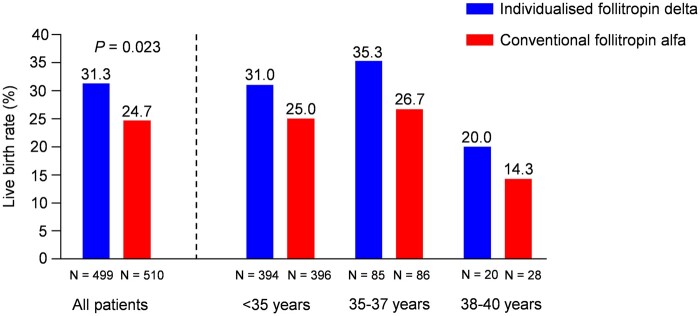
The results for ongoing pregnancy rate were on the border of superiority of follitropin delta over follitropin alfa, as indeed was shown for both earlier and later pregnancy outcomes which favoured the individualised follitropin delta dosing approach. The positive βhCG rate was 41.7% (208/499) with individualised follitropin delta, which was significantly (P = 0.039) higher than 35.3% (180/510) with follitropin alfa. The live birth rate was also significantly (P = 0.023) higher at 31.3% with individualised follitropin delta compared to 24.7% with follitropin alfa (Fig. 3, Table III). The live birth rates for each age stratum were as follows for individualised follitropin delta and follitropin alfa, respectively; <35 years: 31.0% (122/394) versus 25.0% (99/396), 35–37 years: 35.3% (30/85) versus 26.7% (23/86), 38–40 years: 20.0% (4/20) versus 14.3% (4/28) (Fig. 3). There were no neonatal deaths between birth and 4 weeks after birth in any of the treatment groups, and the live birth rates at 4 weeks after birth therefore remained the same as the live birth rates.
Figure 3.
Live birth rate for overall population and by age stratum. The blue (individualised follitropin delta) and red (conventional follitropin alfa) bars display the live birth rates for all patients who started stimulation and by each of the age randomisation strata (<35, 35–37 and 38–40 years). N, total number of patients.
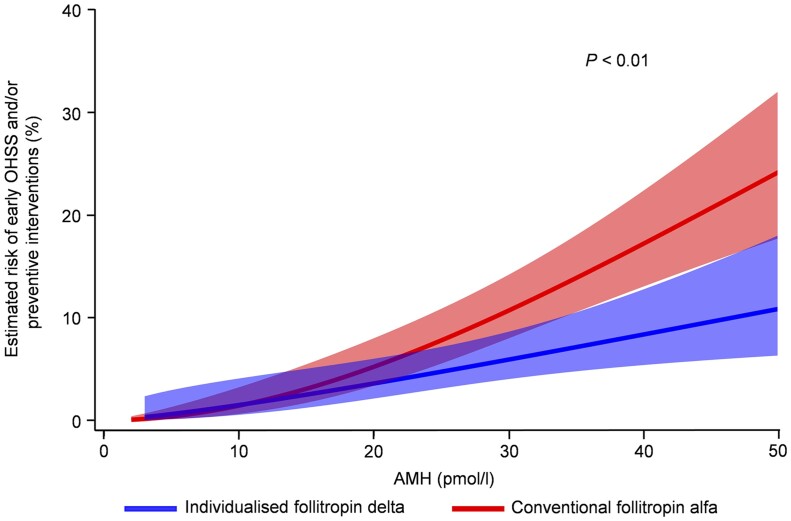
The incidence of early OHSS and/or preventive interventions for early OHSS was significantly (P = 0.004) reduced by 51% [95% CI: 19%; 70%] from an overall incidence of 9.6% (49/510) with conventional follitropin alfa to 5.0% (25/499) with individualised follitropin delta (Table II). The incidence of early moderate/severe OHSS and/or preventive interventions was significantly reduced by 44% (P = 0.029) from an overall incidence of 7.8% with the conventional dosing to 4.6% with the individualised dosing. Figure 4 shows how the risk of experiencing early OHSS and/or requiring preventive interventions for early OHSS differed between treatment groups with increasing levels of serum AMH. Overall, hospitalisation due to early OHSS occurred for three women in the individualised follitropin delta group and for five women in the follitropin alfa group. The incidence of late OHSS was not significantly different between the two treatment groups, occurring at a rate of 4.0% with individualised follitropin delta and 2.0% with follitropin alfa, with all cases occurring in pregnant women.
Figure 4.
Estimated risk of early OHSS (any grade) and/or preventive interventions for early OHSS by AMH. The solid blue (individualised follitropin delta) and red (conventional follitropin alfa) lines are based on a logistic regression model with treatment, log(AMH) and the treatment*log(AMH) interaction. The shadings represent 95% CIs for the estimated risks. The analysis of treatment difference indicates evidence of a benefit of follitropin delta over follitropin alfa (P < 0.01). AMH, anti-Müllerian hormone; OHSS, ovarian hyperstimulation syndrome.
The incidence of adverse drug reactions was 15.2% (76/499) for individualised follitropin delta and 17.1% (87/510) for follitropin alfa with the most commonly reported adverse drug reactions beside OHSS being as follows, respectively: pelvic discomfort (4.4% versus 5.3%), pelvic pain (1.2% versus 1.2%) and breast swelling (1.0% versus 0.8%). Based on all assessments, injection site reactions were recorded at a frequency of <1% in both treatment groups with the majority being mild, with moderate events only accounting for <0.1%, and no severe events.
Discussion
The current study is the first RCT investigating the use of individualised gonadotropin dosing in Asian IVF/ICSI patients and adequately powered for pregnancy outcomes. It demonstrated the efficacy and safety of follitropin delta in Asian women undergoing ovarian stimulation, and clinically validated the appropriateness of the individualised follitropin delta fixed-dose regimen in a wider Asian population beyond the confirmation already reported for Japanese patients (Ishihara and Arce, 2021). In addition to establishing non-inferiority of individualised follitropin delta to conventional follitropin alfa with respect to the primary endpoint of ongoing pregnancy rate, the present trial furthermore showed that the individualised follitropin delta dosing approach has the potential for efficacy benefits beyond the recognised advantages in safety and treatment efficiency (Nyboe Andersen and Nelson et al., 2017; Bosch et al., 2019; Ishihara and Arce, 2021). Women using the individualised follitropin delta dosing regimen achieved significantly higher live birth rates compared to the women treated with a conventional dosing regimen; a trend observed already for the earlier pregnancy parameter of positive βhCG rate. The live birth rate observed for individualised follitropin delta was significantly higher than for follitropin alfa with a relative increase of more than 25%, representing a clinically relevant improvement. This improvement was consistent across all age strata in the trial, indicating that the efficacy reported for the overall population was not driven by a specific age group, but rather a general finding. In this context, it should be mentioned that the dose selection for the control group was appropriate considering that more than 95% of the women in the present trial were younger than 39 years, for whom the optimal dose has been recommended to be 150 IU since it balances best the probability of pregnancy and the risk of complications (Sterrenburg et al., 2011). In general, the trial methodology was very robust, including prespecified definitions and standardisation of key criteria and policies as well as the blinding of all assessors. Furthermore, the mandatory follow-up to live birth and 4 weeks after birth also ensured comprehensive data collection.
This RCT compared two different gonadotropins used in different dosing regimens, with follitropin delta in an individualised and fixed-dose regimen and follitropin alfa in a conventional with a standard starting dose regimen with possibility for later adjustments, and both components may contribute to the efficacy and safety observations. The individualised follitropin delta dosing algorithm has been developed following pharmacokinetic and pharmacodynamic modelling and simulation (Arce et al., 2016) and has been prospectively validated (Nyboe Andersen and Nelson et al., 2017). The resultant personalised doses are expected to provide an ovarian response that minimises the safety risks, primarily by avoiding hyperresponse, while not compromising the probability of transfer in the fresh cycle or the availability of embryos/blastocysts for later frozen cycles. This present trial corroborated that a higher oocyte yield is not associated with a better outcome in fresh cycles, and also found that the higher gonadotropin consumption with the conventional dosing approach resulted in a higher occurrence of progesterone rises which previously has been linked to reduced chance of pregnancy (Bosch et al., 2010). Furthermore, the normalisation of the ovarian response observed with follitropin delta in the potential low responders stresses the importance of selecting the appropriate starting dose. The trial findings substantiated that an adequate number of oocytes and embryos can be achieved with an appropriate starting dose maintained throughout stimulation in a fixed-dose regimen. Indeed, embryo availability was ensured with the individualised follitropin delta dosing algorithm that independent of the patient’s AMH achieved an average of 7.0 embryos from a single stimulation cycle, which appears to be a sufficient number allowing for transfer in the fresh cycle and cryopreservation for use in later frozen cycles.
From a safety perspective, there was a decrease of approximately 50% in the incidence of early OHSS and/or preventive interventions with the individualised follitropin delta dosing regimen compared to the conventional follitropin alfa dosing regimen, with a significant reduction also when excluding the mild OHSS cases. The early OHSS risk increased with increasing serum AMH levels, and the reduction observed with individualised follitropin delta can be attributed to the ovarian response in the patients with normal and high ovarian reserve, both in terms of the average number of oocytes retrieved (10 oocytes) and the reduction in excessive response. The improved safety outcome observed in this trial is consistent with the previous RCTs showing an approximately 25–50% relative reduction in the early OHSS risk with individualised follitropin delta compared to a conventional dosing approach, with the magnitude influenced by the susceptibility of the patients enrolled in the different trials (Nyboe Andersen and Nelson et al., 2017; Ishihara and Arce, 2021). When interpreting the early OHSS risk data, it is important to keep in mind that the patients in the control group started ovarian stimulation with 150 IU/day follitropin alfa, which is the lowest approved starting dose in all participating countries. Interestingly, although it was an option to reduce the daily follitropin alfa dose during stimulation, the opposite adjustment in the form of dose increases was very frequent, including in the young women below 35 years who generally would be expected to not require high gonadotropin doses to achieve an appropriate ovarian response. Late OHSS occurred in both treatment groups in this trial, but these events happened exclusively in pregnant women and are therefore not easily preventable.
The outcome of the present large RCT fulfils all expectations for individualised gonadotropin dosing in IVF/ICSI patients, and contributes with further evidence of the clinical benefits associated with the individualised dosing approach for ovarian stimulation. In addition to the recognised improvements in terms of reduced risk of OHSS and reduced gonadotropin use, this study showed not only non-inferiority with respect to ongoing pregnancy rate but also a statistically significant and clinically relevant advantage in live birth rate for the individualised follitropin delta dosing algorithm over conventional dosing. All pregnancy outcomes, from positive βhCG rate onwards, were indicative of better efficacy with follitropin delta in its individualised fixed-dose regimen compared to the conventional follitropin alfa dosing regimen, including significantly higher live birth rate and live birth rate at 4 weeks after birth. These findings stress the power of prospective RCTs and they represent another step forward in the clinical adaptation of stratified medicine within assisted reproductive technologies, demonstrating the value of determining the gonadotropin dose for ovarian stimulation on the basis of objective biomarkers assessed for each individual patient.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
The authors thank the investigators at the participating sites for their efforts and support, and Anca Lymperopoulou and Lisbeth Helmgaard (Global Medical Writing, Ferring Pharmaceuticals, Denmark) for assistance in writing the manuscript. The authors also acknowledge Yu Zhao Bagger and Rita Lobo (Reproductive Medicine & Maternal Health, Ferring Pharmaceuticals, Denmark) and Nephele Wu (China Medical Science, Ferring Pharmaceuticals, China) for medical monitoring as well as other members of the clinical team at Ferring Pharmaceuticals.
Authors’ roles
J.Q. was the lead investigator of the trial and critically revised the manuscript for important intellectual content. J.-C.A. provided substantial contribution to the trial design and data interpretation and drafted the manuscript. M.G. performed the statistical analysis. All authors have reviewed the manuscript and approved the final version.
Funding
This study was funded by Ferring Pharmaceuticals.
Conflict of interest
J.Q., Y.Z., X.L., T.H., H.-Y.H. and S.-H.K. have received institutional (not personal) clinical trial fees from Ferring Pharmaceuticals. M.G., B.M. and J.-C.A. are employees of Ferring Pharmaceuticals. J.-C.A. has pending and issued patent applications in the WO 2013/020996 and WO 2019/043143 patent families that comprise allowed and granted patent rights related to follitropin delta.
References
- Arce J-C, Klein BM, Erichsen L.. Using AMH for determining a stratified gonadotropin dosing regimen for IVF/ICSI and optimizing outcomes. In: Seifer DB, Tal R (eds). Anti-Müllerian Hormone: Biology, Role in Ovarian Function and Clinical Significance. Hauppauge, NY: Nova Science Publishers, Inc, 2016, 83–102. [Google Scholar]
- Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI.. Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril 2014;101:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch E, Havelock J, Martin FS, Rasmussen BB, Klein BM, Mannaerts B, Arce J-C; ESTHER-2 Study Group. Follitropin delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind Phase 3 safety trial. Reprod Biomed Online 2019;38:195–205. [DOI] [PubMed] [Google Scholar]
- Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, Pellicer A.. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod 2010;25:2092–2100. [DOI] [PubMed] [Google Scholar]
- Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM.. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014;20:688–701. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Deurenberg-Yap M, Guricci S.. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002;3:141–146. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Gonal-f. Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/gonal-f-epar-product-information_en.pdf (15 March 2021, date last accessed).
- Fauser BCJM, Diedrich K, Devroey P; Evian Annual Reproduction Workshop Group 2007. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update 2008;14:1–14. [DOI] [PubMed] [Google Scholar]
- Fleming R, Broekmans F, Calhaz-Jorge C, Dracea L, Alexander H, Nyboe Andersen A, Blockeel C, Jenkins J, Lunenfeld B, Platteau P. et al. Can anti-Müllerian hormone concentrations be used to determine gonadotrophin dose and treatment protocol for ovarian stimulation? Reprod Biomed Online 2013;26:431–439. [DOI] [PubMed] [Google Scholar]
- Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E.. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv 1989;44:430–440. [DOI] [PubMed] [Google Scholar]
- Ishihara O, Arce J-C; Follitropin Delta Phase 3 Trial (STORK) Group. Individualized follitropin delta dosing reduces OHSS risk in Japanese IVF/ICSI patients: a randomized controlled trial. Reprod Biomed Online 2021;42:909–914. [DOI] [PubMed] [Google Scholar]
- La Marca A, Sunkara SK.. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update 2014;20:124–140. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Aijun S, Ling Q, Tengda X, Wei X, Yan D, Yanfang W, Zenghui T, Xinqi C, Fraser A. et al. Ethnic discordance in serum anti-Müllerian hormone in healthy women: a population study from China and Europe. Reprod Biomed Online 2020;40:461–467. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, Mitchell P, Ambrose P, Fleming R.. Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod 2009;24:867–875. [DOI] [PubMed] [Google Scholar]
- Nelson SM.Biomarkers of ovarian response: current and future applications. Fertil Steril 2013;99:963–969. [DOI] [PubMed] [Google Scholar]
- Nyboe Andersen A, Nelson SM, Fauser BCJM, García-Velasco JA, Klein BM, Arce J-C; ESTHER-1 Study Group. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril 2017;107:387–396. [DOI] [PubMed] [Google Scholar]
- Sterrenburg MD, Veltman-Verhulst SM, Eijkemans MJC, Hughes EG, Macklon NS, Broekmans FJ, Fauser BCJM.. Clinical outcomes in relation to the daily dose of recombinant follicle-stimulating hormone for ovarian stimulation in in vitro fertilization in presumed normal responders younger than 39 years: a meta-analysis. Hum Reprod Update 2011;17:184–196. [DOI] [PubMed] [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.