Abstract
Docetaxel-based therapy has been applied to kill cancers including lung and breast cancers but frequently causes peripheral neuropathy such as mechanical allodynia. Lack of effective drugs for chemotherapy-induced peripheral neuropathy (CIPN) treatment leads us to find novel drugs. Here, we investigated whether and how novel anticancer herbal prescription SH003 alleviates mechanical allodynia in mouse model of docetaxel-induced neuropathic pain. Docetaxel-induced mechanical allodynia was evaluated using von Frey filaments. Nerve damage and degeneration in paw skin of mice were investigated by immunofluorescence staining. Neuroinflammation markers in bloodstream, lumbar (L4-L6) spinal cord, and sciatic nerves were examined by ELISA or western blot analysis. Docetaxel (15.277 mg/kg) was intravenously injected into the tail vein of C57BL/6 mice, and mechanical allodynia was followed up. SH003 (557.569 mg/kg) was orally administered at least 60 min before the mechanical allodynia test, and von Frey test was performed twice. Docetaxel injection induced mechanical allodynia, and SH003 administration restored withdrawal threshold. Meanwhile, degeneration of intraepidermal nerve fibers (IENF) was observed in docetaxel-treated mice, but SH003 treatment suppressed it. Moreover, docetaxel injection increased levels of TNF-α and IL-6 in plasma and expressions of phospho-NF-κB and phospho-STAT3 in both of lumbar spinal cord and sciatic nerves, while SH003 treatment inhibited those changes. Taken together, it is worth noting that TNF-α and IL-6 in plasma and phospho-NF-κB and phospho-STAT3 in spinal cord and sciatic nerves are putative biomarkers of docetaxel-induced peripheral neuropathy (DIPN) in mouse models. In addition, we suggest that SH003 would be beneficial for alleviation of docetaxel-induced neuropathic pain.
1. Introduction
Cancer patients receive chemotherapy to kill malignant tumors and improve survival rate whereas it unfortunately causes severe side effects [1, 2]. CIPN is one of the painful side effects occurring in approximately 30–70% of patients and characterized by damaged peripheral neurons [3–6]. The main symptoms include pain, muscle weakness, and muscle spasms, resulting in a decrease in the quality of life [7]. Taxanes (paclitaxel and docetaxel) are useful anticancer drugs but cause CIPN [8]. Docetaxel is one of the cytotoxic anticancer drugs and exhibits an anticancer effect by binding to tubulin, resulting in impairment of microtubule homeostasis and mitotic arrest [9]. US Food and Drug Administration (FDA) approved docetaxel as an anticancer drug against multiple types of cancers including non-small-cell lung and breast cancers [10, 11]. Of note, docetaxel is associated with acute pain syndrome in several cancer patients [12–17]. Development of painful symptoms by DIPN may lead to discontinuation of cancer treatment regardless of therapeutic benefit of chemotherapy [18–20]. Until now, there have been no effective therapeutic options for DIPN in cancer patients [21]. Thus, this drives us to find novel medicines for treatment of DIPN.
Cancer chemotherapy typically damages the distal sensory neurons of hands or feet with severe pain [22]. Nonclinical studies have demonstrated that peripheral nervous system tissues, which are mainly damaged in CIPN model, include sciatic nerves, lumbar spinal cord, and dorsal root ganglion (DRG) [23, 24]. While the mechanism underlying CIPN is still unclear, several CIPN studies have demonstrated that NF-κB has been suggested to be one of the readouts for neuropathic pain [8, 25]. It was reported that activation of NF-κB is associated with spinal cord and sciatic nerve injury in rodent model [26]. Moreover, inhibition of NF-κB pathway can ameliorate chronic pain in neuropathic pain rodent model. Besides NF-κB, STAT3 pathway has also been suggested to be involved in neuropathic pain. It has been reported that paclitaxel treatment induces neuropathic pain with increased expression of phosphorylated JAK2 and STAT3 [27]. Furthermore, another study demonstrated that chemotherapeutic agent bortezomib-induced mechanical allodynia is associated with STAT3 activation in DRG [28]. Moreover, TNF-α-mediated activation of STAT3 plays a critical role in CIPN [29]. Therefore, activation of NF-κB and STAT3 can be regarded as a biomarker for CIPN. However, there is no evidence to support the fact that those biomarkers are associated with DIPN.
Novel herbal prescription SH003 is the traditional Chinese medicine (TCM) theory-based anticancer drugs composed of Astragalus membranaceus, Angelica gigas, and Trichosanthes kirilowii Maximowicz. The anticancer effect of SH003 against breast, lung, and prostate cancer has been demonstrated through several in vitro and in vivo studies [30–38]. The safety of SH003 was proved by phase I clinical study for solid tumors, and phase II clinical study for wild-type EGFR lung cancer patients is in progress [39–41]. Meanwhile, phase I/II clinical study of SH003 combined with docetaxel for breast and lung cancer patients is also in progress (NCT01755923, https://clinicaltrials.gov). While it is a crucial point that TCM-based herbal medicines and supplements are the representative alternative treatment for improving health problems including cancer-related side effects [42–46], we needed to investigate whether novel herbal medicine SH003 alleviates docetaxel-mediated adverse effects in both nonclinical and clinical studies.
The purpose of the present study was to evaluate the mitigative effect of SH003 on DIPN in C57BL/6 mice. Intravenous injection of docetaxel decreased the withdrawal threshold of von Frey filament compared to Control group whereas oral administration of SH003 relieved this symptom. ELISA analysis showed that levels of TNF-α and IL-6 in plasma were upregulated by docetaxel injection while SH003 treatment revered it. Docetaxel-induced upregulation of phospho-NF-κB and phospho-STAT3 expression in sciatic nerve and spinal cord was inhibited by SH003 treatment. Histological analysis of mouse foot pad skin indicated that administration of SH003 relieved docetaxel-induced damage on peripheral nerve fibers. Therefore, the present study suggests that SH003 is applicable to alleviate DIPN.
2. Materials and Methods
2.1. SH003 and Docetaxel
SH003 powder was prepared as described previously. In brief, Astragalus membranaceus (333 g), Angelica gigas (333 g), and Trichosanthes kirilowii Maximowicz (333 g) were mixed and then extracted with 10 times volume of 30% ethanol at 100°C for 3 h. This process was performed 2 times. The extract was dried at reduced pressure (40 Torr) at 60°C for 18 h. Dried SH003 was stored at −20°C. Docetaxel was purchased from Sigma-Aldrich (#01885–25MG-F, St. Louis, MO, USA). Docetaxel was dissolved in DMSO and stored at −20°C.
2.2. Determination of Drug Dose
No-observed-adverse-effect level (NOAEL) of SH003 determined by phase I clinical trials was 4,800 mg/day [41]. Maximum tolerable dose (MTD) of docetaxel in cancer patients was 75 mg/m2 [40]. Animal equivalent dose was calculated by the following equation [47]:
| (1) |
Human and mice weights are 65 kg and 0.02 kg, respectively. Since there is an individual difference in metabolic rate, exponent for body surface area is 0.75. Thus, animal equivalent dose of SH003 and docetaxel was determined as 557.569 mg/kg and 15.277 mg/kg, respectively.
2.3. Docetaxel-Induced Neuropathy Mouse Model and Experiment Schedule
All procedures in animal experiments were approved by Kyung Hee University Institutional Animal Care and Use Committee (KHU-IACUC; KHSASP-19-322). C57BL/6N mice (5 weeks old) were purchased from Nara Biotech (Seoul, South Korea). Mice were housed under constant temperature (24 ± 2°C) with light-dark cycle, and food and water were freely available. Two experiments were performed. The timelines of experiment “1” and experiment “2” are shown in Figure 1.
Figure 1.
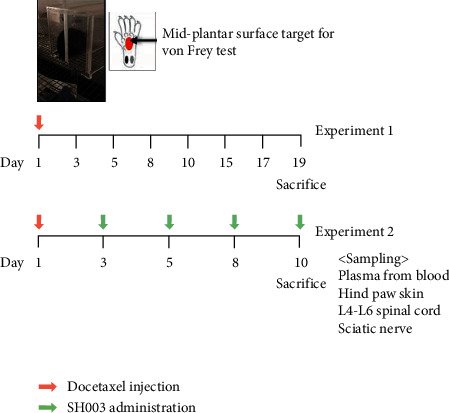
Timelines of the in vivo experimental design.
In experiment “1,” mice were randomly divided into different treatment groups as follows: Control (n = 6) and Docetaxel (n = 6). Before injection of docetaxel on the 1st day, baseline withdrawal threshold in each mouse was determined. Mice were treated with a single intravenous injection of docetaxel to model DIPN, and neuropathic symptom was followed up.
In experiment “2,” mice were randomly divided into different groups as follows: “Control” (n = 5), “Docetaxel” (n = 5), “Docetaxel + SH003” (n = 5). After determination of baseline withdrawal threshold by von Frey test on the 1st day, mice in “Docetaxel” and “Docetaxel + SH003” were intravenously injected with 50 μL docetaxel while mice in “Control” were injected with the same volume of DMSO, which did not exceed 5 mL/kg according to guidelines [48]. SH003 was orally administered at least 60 min before the performance of mechanical allodynia test. SH003 administration and von Frey filament test were performed 2-3 times a week. At the end of the experiment, mice were euthanized by 100% carbon dioxide in chamber by 30–70%/min filling rate, followed by cervical dislocation. The hind paw skin of mice was collected for staining of IENF. For evaluation of the inflammatory damage on peripheral neurons, lumbar (L4-L6) spinal cord and both left and right sciatic nerves were collected.
2.4. Measurement of Mechanical Allodynia
Mechanical allodynia was evaluated using von Frey filaments (JD-SI-11F, Jeungdo B and P, Seoul, South Korea) according to standard operating procedure from the Jackson Laboratory mouse neurobehavioral phenotyping facility. In brief, the room atmosphere was maintained with constant temperature (24 ± 2°C), humidity (50 ± 20%), and lighting (400–500 lux) without noise. Prior to the start of von Frey test, mice were individually placed on stainless steel mesh floor with acrylic cover (L∗W∗H= 10∗8∗7 cm) and left without disturbance for a minimum of 30 min to acclimate. The midplantar surface of left hind paw of mice was poked with filament (0.4 g) for 3 seconds until it bends, and each application was presented at least 3 times. If there was no response with the starting filament (0.4 g), it was changed to the next highest filament (0.6 g) in the series and continuously moved through the series in order until a withdrawal response was observed or the 2 g filament was presented. If there was response with the starting filament, it was moved to the next lowest filament in the series until a withdrawal response was not observed or the 0.02 g filament was presented. Licking and shaking of the affected paw were determined as the withdrawal responses. The whole procedure from acclimation to determination of withdrawal responses was repeated once again. Mechanical allodynia threshold values from two independent experiments were recorded and averaged in each group.
2.5. Immunofluorescence Staining of IENF
The hind paw skin was isolated from all mice and immediately fixed with 4% paraformaldehyde for 6 h. Fixed skin samples were dehydrated using 30% sucrose solution for 16 h. For the cryosection, dehydrated tissues were embedded in OCT compound and frozen at liquid nitrogen. Frozen sections of 20 μm were stained with anti-PGP9.5 antibody (1 : 800, ab8189, Abcam, Cambridge, UK) and IgG secondary antibody conjugated with Alexa Fluor 488 (1 : 2,000, A28175, Invitrogen Co., Carlsbad, CA, USA). The images were acquired using Zeiss LSM 5 PASCAL confocal laser scanning microscope system (Carl Zeiss AG, Oberkochen, Germany) at a magnification of 10x.
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of inflammatory cytokines TNF-ɑ and IL-6 were assessed using a DuoSet ELISA kit (555268 and 555240, respectively, BD Biosciences, San Diego, USA) according to the manufacturer's instructions. In brief, the whole blood was collected from left ventricle and transferred to EDTA tube (367835, BD Biosciences, San Diego, USA). After centrifugation at 2,000 rpm for 10 min, plasma from blood was prepared and stored in a deep freezer at −80°C until use. 96-well plates were coated with capture antibody in ELISA coating buffer and incubated overnight at 4°C. The plates were then washed with wash buffer and subsequently blocked with assay diluent at RT for 1 h. Diluted standard and plasma samples were added to plates and incubated at RT for 2 h. Detection antibody and streptavidin-conjugated horseradish peroxidase were added to the plates, followed by incubation at RT for 1 h. Tetramethylbenzidine substrate was added and incubated at RT for 0.5 h. The reaction was ended by adding stop buffer. The optical density was measured at 450 nm on an automated ELISA reader (Versa Max, Molecular Devices, CA, USA).
2.7. Western Blot Analysis
Total protein from lumbar (L4-L6) spinal cord and sciatic nerves were extracted using RIPA buffer (R2002, Biosesang, Gyeonggi-do, South Korea) with protease and phosphatase inhibitors. The same amount of proteins was quantified by Bradford protein assay (Bio-Rad, Hercules, CA, USA). The proteins were separated by 8 or 15% SDS-PAGE, followed by transfer to nitrocellulose membranes. After blocking with blocking buffer including 1% (w/v) skim milk and 1% (w/v) BSA at RT for 1 hour, the membrane was incubated with anti-NF-κB (#8242), anti-phospho-NF-κB (#3033), anti-STAT3 (#4904), anti-phospho-STAT3 (#9145), and anti-GAPDH (#5174) at 4°C for 16–24 h. All antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Horseradish peroxidase- (HRP-) conjugated secondary IgG antibodies (Calbiochem, San Diego, CA, USA) were further incubated with the membrane at RT for 1 h. An enhanced chemiluminescence kit (DG-WP100, DoGen, Seoul, South Korea) was used for detection of HRP signal.
2.8. Statistical Analysis
Statistical analysis was performed using Prism (GraphPad, San Diego, CA, USA). The differences of means between the groups were analyzed by one-way or two-way ANOVA using Tukey's or Sidak's multiple comparisons test, respectively. P value < 0.05 means statistically significant difference. Results were represented as mean ± standard deviation (SD) or standard error of the mean (SEM).
3. Results and Discussion
3.1. Intravenous Injection of DIPN in C57BL/6 Mice
Docetaxel is a well-known anticancer drug with severe side effects including CIPN with pain in the hands and feet [49,50]. The present study was performed to model DIPN. Docetaxel was intravenously injected into mice tail vein, and mechanical allodynia threshold by von Frey test was followed up. On the 5th day, mechanical withdrawal threshold of Docetaxel group began to be lower than that of Control group (Figure 2(a)). On the 10th day, the biggest difference of mechanical allodynia threshold between Control group and Docetaxel group occurred, while there were no differences after the 15th day (Figure 2(a)). Drug treatment did not affect body weights of mice until 19 days (Figure 2(b)). Therefore, we determined to perform further efficacy study until the 10th day. It is common for taxane-based adjuvant chemotherapy to induce acute pain syndrome [51]. In fact, the incidence of taxane acute pain syndrome is more common with docetaxel than paclitaxel whereas docetaxel triggers less peripheral neuropathy than paclitaxel [16, 52]. Docetaxel-mediated pain flare occurs within 4–5 days after docetaxel chemotherapy and lasts about 4 days [52]. Consistent with clinical findings, in vivo model for docetaxel-related acute pain syndrome was successfully developed by a single intravenous injection of docetaxel at MTD.
Figure 2.
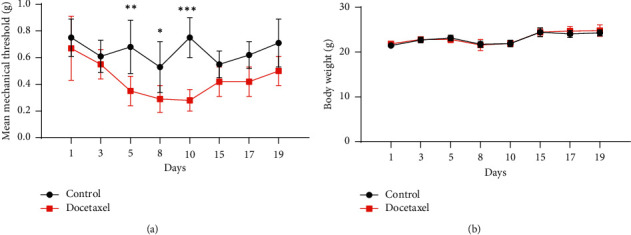
Docetaxel injection induced mechanical allodynia in C57BL/6 mice. The mice were divided into “Control” (n = 6) and “Docetaxel” (n = 6). Docetaxel was intravenously injected via tail vein of C57BL/6 mice on the 1st day, and mechanical allodynia threshold by von Frey filament was followed up. Mean mechanical threshold (a) and body weight (b). The differences of means between the groups were analyzed by two-way ANOVA using Sidak's multiple comparisons test (∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001; Docetaxel vs. Control).
3.2. Oral Administration of SH003 Mitigated Docetaxel-Induced Mechanical Allodynia at Hind Paws of C57BL/6 Mice
Next, we further examined whether SH003 mitigates docetaxel-induced mechanical allodynia. Although mechanical threshold of “Docetaxel” was higher than that of “Docetaxel + SH003” on the 3rd day, the threshold value of two groups was not statistically different with “Control.” While docetaxel treatment induced mechanical allodynia, SH003 treatment relieved it on the 8th and 10th day (Figure 3(a)). Drug treatment did not affect body weights of mice until 10 days (Figure 3(b)). The data showed that SH003 administration mitigated DIPN. SH003 is a TCM-based novel herbal mixture for treating several malignant cancers including breast, prostate, and lung cancers. Anticancer effect and mode of action have been clearly demonstrated by numerous nonclinical studies [30–38]. In fact, conventional chemodrugs have adverse side effects because of their cytotoxicity to healthy cells as well as cancer cells regardless of therapeutic benefit. Our previous study reported that SH003 is safe in rats according to toxicity studies with Good Laboratory Practice (GLP) regulations [36]. Oral administration of SH003 at various doses (500; 1,000; 2,000 mg/kg) did not show any problem in mortality, food intake, haematological value, organ weight, histopathology, etc. In sum, we suggest that SH003 is safe and effective herbal medicine for treatment of DIPN as well as cancer.
Figure 3.
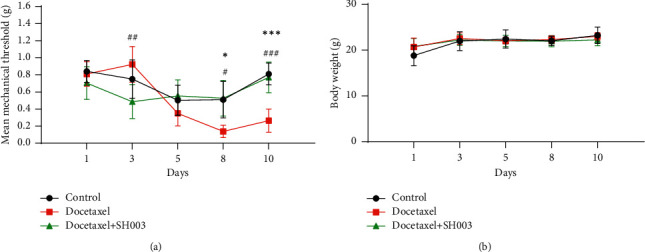
Effect of SH003 administration on docetaxel-induced mechanical allodynia in C57BL/6 mice. The mice were divided into Control (n = 5), Docetaxel (n = 5), and Docetaxel + SH003 (n = 5) groups. Docetaxel was intravenously injected via tail vein of C57BL/6 mice on the 1st day, and mechanical allodynia threshold by von Frey filament was followed up. From 3rd day, SH003 was orally administered at least 60 min before the performance of mechanical allodynia test. Mean mechanical threshold (a) and body weight (b). Data are presented as mean ± SEM. The differences of means between the groups were analyzed by two-way ANOVA using Sidak's multiple comparisons test (∗P < 0.05, Docetaxel vs. Control; ∗∗∗P < 0.001, Docetaxel vs. Control; #P < 0.05, Docetaxel + SH003 vs. Docetaxel; ###P < 0.001, Docetaxel + SH003 vs. Docetaxel).
3.3. SH003 Administration Inhibited Docetaxel-Induced Reduction of IENF at Hind Paws of C57BL/6 Mice
IENF are bare nerve endings within junction between dermis and epidermis and play a role in transmission of peripheral pain [53, 54]. Clinically, chemotherapy damages distal IENF, resulting in loss of IENF in hands and feet in patients with CIPN. [55–59]. In the rodent CIPN model, degeneration of IENF in paw skin is detected although the contribution of IENF loss to the pathobiology of CIPN is not fully elucidated [60–62]. Of note, chemotherapeutic drugs including paclitaxel and oxaliplatin induce loss of IENF with severe pain in rodent model while prevention of IENF loss inhibits neuropathic pain [53, 55, 63]. Thus, the reduction of IENF loss could be regarded as one of therapeutic markers in CIPN model. We further investigated the therapeutic effect of SH003 on IENF degeneration in mice paw skin. Compared to Control, IENF seem to be fragmented and degenerated (white arrow) in docetaxel-treated mice, whose morphological changes indicate axonal degeneration (Figures 4(a) and 4(b)). SH003 treatment decreased docetaxel-induced histopathological changes whereas a layer (yellow arrow) was thickened (Figure 4(c)). This layer located in the mid-epidermis is stratum lucidum containing dead keratinocytes and is present in the palms and soles [64]. The thickness of this layer is regulated by the rate of mitosis of the epidermal cells [65]. Stratum lucidum layer contains a large amount of eleidin which is known to protect skins by blocking infiltration of water [66]. However, the role of stratum lucidum in neuropathic pain is unknown yet. Although further studies are still needed to investigate the possible role of components in stratum lucidum in DIPN model, our data suggest that histological change in stratum lucidum is one of the therapeutic markers for SH003 on DIPN.
Figure 4.
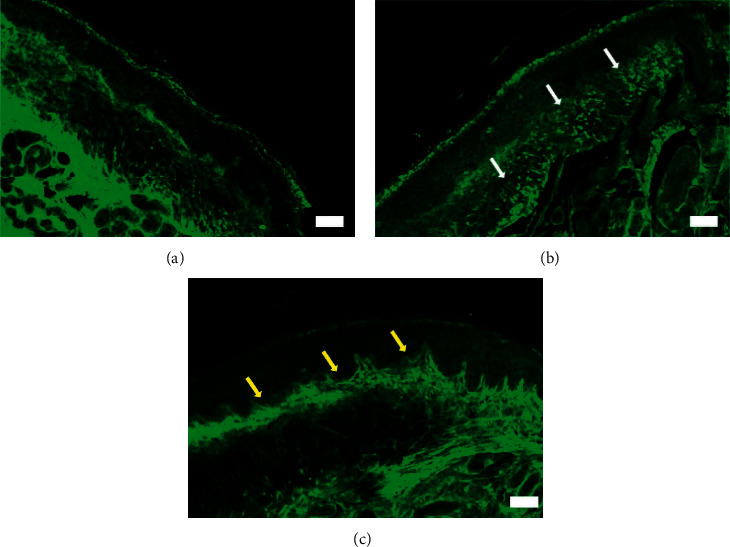
Effect of SH003 administration on degeneration of IENF induced by docetaxel. The hind paw skins were isolated and prepared for immunofluorescence staining of IENF in Control (a), Docetaxel (b), and Docetaxel + SH003 (c) groups. The representative images of PGP 9.5-labeled IENF in paw skins were acquired using Zeiss LSM5 PASCAL confocal laser scanning microscope system. White scale bar indicates 50 μm (magnification: 10x). White and yellow arrows indicate the degenerated IENF and stratum lucidum layer, respectively.
3.4. Oral Administration of SH003 Inhibited Docetaxel-Induced Production of Proinflammatory Cytokines in Bloodstream of C57BL/6 Mice
In CIPN model, pain mediators including inflammatory cytokines are increased in peripheral nerve spinal cord and sciatic nerves [67]. In vivo studies demonstrated that levels of TNF-α and IL-6 are elevated in CIPN animal model [68, 69]. It was also demonstrated that neutralizing antibodies against TNF-α or IL-6 reduce chemotherapy-induced allodynia [69, 70]. The present study investigated whether SH003 reduces TNF-α and IL-6 in DIPN model. Docetaxel injection increased levels of TNF-α and IL-6 in plasma sample (Figures 5(a) and 5(b)). The present data showed that DIPN is associated with inflammatory neuropathy [8]. In SH003 group, the inflammatory level increased by docetaxel was reduced as much as Control group (Figures 5(a) and 5(b)). We also demonstrated that SH003 inhibition of DIPN is mediated by relieving inflammation.
Figure 5.
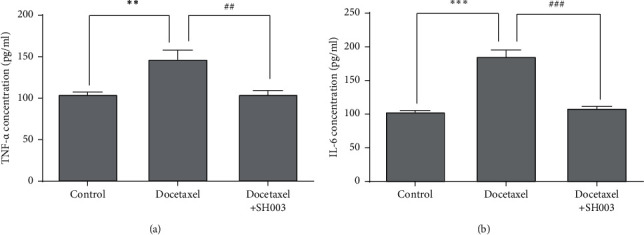
Effect of SH003 administration on the expression of TNF-α and IL-6 in plasma of DIPN mice. Plasma sample was prepared, and levels of inflammatory cytokines TNF-α (a) and IL-6 (b) were assessed using a DuoSet ELISA kit according to the manufacturer's instructions. Data are presented as mean ± SD of the mean (n = 5 for each group). The differences of means between the groups were analyzed by one-way ANOVA using Tukey's post hoc test (∗∗P < 0.001, Docetaxel vs. Control; ∗∗∗P < 0.001, Docetaxel vs. Control; ##P < 0.01, Docetaxel + SH003 vs. Docetaxel; ###P < 0.001, Docetaxel + SH003 vs. Docetaxel).
3.5. Oral Administration of SH003 Inhibited Docetaxel-Induced Phosphorylation of NF-κB and STAT3 in Both L4-L6 Spinal Cord and Sciatic Nerve of C57BL/6 Mice
NF-κB and STAT3 would be one of the readouts for CIPN [8, 25–29]. Moreover, TNF-α and IL-6 are involved in the activation of NF-κB and STAT3 in neuroinflammation [71–73]. Thus, we further examined whether SH003 treatment inhibits NF-κB and STAT3 in both of lumbar spinal cord and sciatic nerves. Docetaxel treatment increased the expression of phospho-NF-κB and phospho-STAT3 in both L4-L6 spinal cords and sciatic nerves whereas SH003 treatment reversed it (Figures 6(a) and 6(b)). The present study suggested that activation of NF-κB and STAT3 is a putative biomarker of DIPN while further studies are necessary to decipher how they are involved in the development and progression of DIPN. Moreover, SH003 inhibition of NF-κB and STAT3 shown in our data would be a hint for SH003 application to other peripheral neuropathic diseases. Deregulation of NF-κB and STAT3 appears to be involved in the progression of neuropathic pain in diabetes [74–77]. Alcoholic neuropathy is tightly linked to NF-κB deregulation [78, 79]. NF-κB deregulation is likely to be related to dysimmune neuropathies such as acute Guillain–Barré syndrome [80]. Therefore, SH003 may be applicable for treating other neuropathic diseases related to the deregulation of NF-κB and STAT3.
Figure 6.
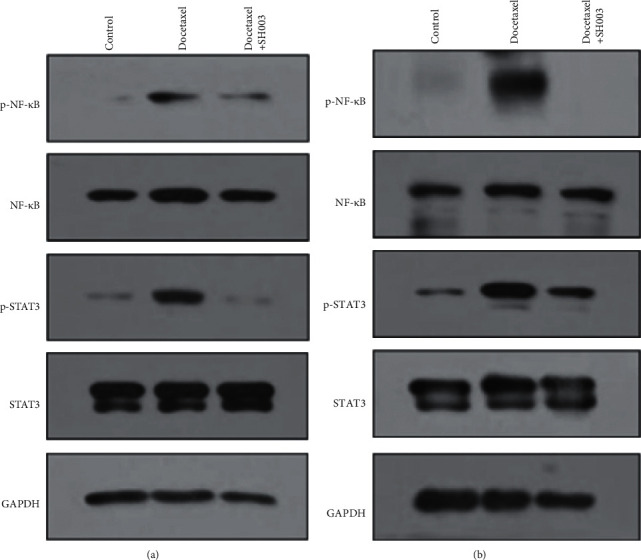
Effect of SH003 administration on the expression of NF-κB and STAT3 in lumbar (L4-L6) spinal cord and sciatic nerves of DIPN mice. At the end of the experiment, lumbar (L4-L6) spinal cord and sciatic nerves were isolated. Total proteins were extracted and separated by SDS-PAGE. Expression of NF-κB and STAT3 from spinal cord (a) and sciatic nerves (b) was analyzed by western blotting. GAPDH was used as internal marker.
4. Conclusions
Single injection of docetaxel at MTD can develop acute pain syndrome model in C57BL/6 mice. Moreover, activation of NF-κB and STAT3 in sciatic nerves and L4-L6 spinal cords and upregulation of TNF-α or IL-6 in bloodstream may be biomarkers of DIPN in mouse model. However, further studies are needed to investigate the role of those putative biomarkers in the development and progression of DIPN. Furthermore, we suggest that SH003 administration is able to relieve docetaxel-induced neuropathic pain and this efficacy needs to be monitored in current clinical studies.
Acknowledgments
This research was supported by a grant from the Korean Medicine R&D Project of the Ministry of Health and Welfare (HI18C2382) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (no. 2020R1A5A201941311).
Data Availability
All datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this study.
References
- 1.Maihofner C., Diel I., Tesch H., Quandel T., Baron R. Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Supportive Care in Cancer. 2021;29 doi: 10.1007/s00520-021-06042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu B., Wang N., Tan H.-Y., Li S., Cheung F., Feng Y. Multi-component herbal products in the prevention and treatment of chemotherapy-associated toxicity and side effects: a review on experimental and clinical evidences. Frontiers in Pharmacology. 2018;9:p. 1394. doi: 10.3389/fphar.2018.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seretny M., Currie G. L., Sena E. S., et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Colvin L. A. Chemotherapy-induced peripheral neuropathy: where are we now? Pain. 2019;160(1):S1–S10. doi: 10.1097/j.pain.0000000000001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao T., Goloubeva O., Pelser C., et al. A pilot study of acupuncture in treating bortezomib-induced peripheral neuropathy in patients with multiple myeloma. Integrative Cancer Therapies. 2014;13(5):396–404. doi: 10.1177/1534735414534729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham J. E., Kelechi T., Sterba K., Barthelemy N., Falkowski P., Chin S. H. Case report of a patient with chemotherapy-induced peripheral neuropathy treated with manual therapy (massage) Supportive Care in Cancer. 2011;19(9):1473–1476. doi: 10.1007/s00520-011-1231-8. [DOI] [PubMed] [Google Scholar]
- 7.Brami C., Bao T., Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Critical Reviews in Oncology. 2016;98:325–334. doi: 10.1016/j.critrevonc.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zajaczkowska R., Kocot-Kepska M., Leppert W., Wrzosek A., Mika J., Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. International Journal of Molecular Sciences. 2019;20(6) doi: 10.3390/ijms20061451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhtar E., Adhami V. M., Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Molecular Cancer Therapeutics. 2014;13(2):275–284. doi: 10.1158/1535-7163.mct-13-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engels F. K., Sparreboom A., Mathot R. A. A., Verweij J. Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. British Journal of Cancer. 2005;93(2):173–177. doi: 10.1038/sj.bjc.6602698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montero A., Fossella F., Hortobagyi G., Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. The Lancet Oncology. 2005;6(4):229–239. doi: 10.1016/s1470-2045(05)70094-2. [DOI] [PubMed] [Google Scholar]
- 12.Fazio R., Quattrini A., Bolognesi A., et al. Docetaxel neuropathy: a distal axonopathy. Acta Neuropathologica. 1999;98(6):651–653. doi: 10.1007/s004010051132. [DOI] [PubMed] [Google Scholar]
- 13.Chan Y.-N., Jheng Y.-W., Wang P.-J., Chen C.-Y., Lin M.-W., Wang Y.-J. Taxane-induced peripheral neuropathy: objective and subjective comparison between paclitaxel and docetaxel in patients with breast cancer. Clinical Journal of Oncology Nursing. 2019;23(5):494–501. doi: 10.1188/19.cjon.494-501. [DOI] [PubMed] [Google Scholar]
- 14.Tofthagen C., McAllister R. D., Visovsky C. Peripheral neuropathy caused by Paclitaxel and docetaxel: an evaluation and comparison of symptoms. Journal of the advanced practitioner in oncology. 2013;4(4):204–215. doi: 10.6004/jadpro.2013.4.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckhoff L., Knoop A. S., Jensen M.-B., Ejlertsen B., Ewertz M. Risk of docetaxel-induced peripheral neuropathy among 1,725 Danish patients with early stage breast cancer. Breast Cancer Research and Treatment. 2013;142(1):109–118. doi: 10.1007/s10549-013-2728-2. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes R., Mazzarello S., Hutton B., et al. Taxane acute pain syndrome (TAPS) in patients receiving taxane-based chemotherapy for breast cancer-a systematic review. Supportive Care in Cancer. 2016;24(8):3633–3650. doi: 10.1007/s00520-016-3256-5. [DOI] [PubMed] [Google Scholar]
- 17.Chiu N., Zhang L., Dent R., et al. A prospective study of docetaxel-associated pain syndrome. Supportive Care in Cancer. 2018;26(1):203–211. doi: 10.1007/s00520-017-3836-z. [DOI] [PubMed] [Google Scholar]
- 18.Verweij J., Clavel M., Chevalier B. Paclitaxel (TaxolTM) and docetaxel (TaxotereTM): not simply two of a kind. Annals of Oncology. 1994;5(6):495–505. doi: 10.1093/oxfordjournals.annonc.a058903. [DOI] [PubMed] [Google Scholar]
- 19.Gilron I., Bailey J. M., Tu D., Holden R. R., Weaver D. F., Houlden R. L. Morphine, gabapentin, or their combination for neuropathic pain. New England Journal of Medicine. 2005;352(13):1324–1334. doi: 10.1056/nejmoa042580. [DOI] [PubMed] [Google Scholar]
- 20.Ewertz M., Qvortrup C., Eckhoff L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncologica. 2015;54(5):587–591. doi: 10.3109/0284186x.2014.995775. [DOI] [PubMed] [Google Scholar]
- 21.Henke C. C., Cabri J., Fricke L., et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Supportive Care in Cancer. 2014;22(1):95–101. doi: 10.1007/s00520-013-1925-1. [DOI] [PubMed] [Google Scholar]
- 22.Sisignano M., Baron R., Scholich K., Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nature Reviews Neurology. 2014;10(12):694–707. doi: 10.1038/nrneurol.2014.211. [DOI] [PubMed] [Google Scholar]
- 23.Han Y., Smith M. T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN) Frontiers in Pharmacology. 2013;4:p. 156. doi: 10.3389/fphar.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoke A., Ray M. Rodent models of chemotherapy-induced peripheral neuropathy. ILAR Journal. 2014;54(3):273–281. doi: 10.1093/ilar/ilt053. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A. S., Berg S., Alkass K., et al. NF-kappaB-Associated pain-related neuropeptide expression in patients with degenerative disc disease. International Journal of Molecular Sciences. 2019;20(3) doi: 10.3390/ijms20030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma W., Bisby M. A. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Research. 1998;797(2):243–254. doi: 10.1016/s0006-8993(98)00380-1. [DOI] [PubMed] [Google Scholar]
- 27.Brandolini L., Benedetti E., Ruffini P. A., et al. CXCR1/2 pathways in paclitaxel-induced neuropathic pain. Oncotarget. 2017;8(14):23188–23201. doi: 10.18632/oncotarget.15533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C.-C., Huang Z.-X., Li X., et al. Upregulation of NLRP3 via STAT3-dependent histone acetylation contributes to painful neuropathy induced by bortezomib. Experimental Neurology. 2018;302:104–111. doi: 10.1016/j.expneurol.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Li Y. Y., Li H., Liu Z. L., et al. Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Molecular Pain. 2017;13 doi: 10.1177/1744806917747425.1744806917747425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi Y. K., Cho S. G., Woo S. M., et al. Herbal extract SH003 suppresses tumor growth and metastasis of MDA-MB-231 breast cancer cells by inhibiting STAT3-IL-6 signaling. Mediators of Inflammation. 2014;2014 doi: 10.1155/2014/492173.492173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi E. K., Kim S.-M., Hong S.-W., et al. SH003 selectively induces p73-dependent apoptosis in triple-negative breast cancer cells. Molecular Medicine Reports. 2016;14(4):3955–3960. doi: 10.3892/mmr.2016.5722. [DOI] [PubMed] [Google Scholar]
- 32.Choi H. S., Kim M. K., Lee K., et al. SH003 represses tumor angiogenesis by blocking VEGF binding to VEGFR2. Oncotarget. 2016;7(22):32969–32979. doi: 10.18632/oncotarget.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y.-J., Choi Y. K., Lee K. M., Cho S.-G., Kang S.-Y., Ko S.-G. SH003 induces apoptosis of DU145 prostate cancer cells by inhibiting ERK-involved pathway. BMC Complementary and Alternative Medicine. 2016;16(1):p. 507. doi: 10.1186/s12906-016-1490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo S.-M., Kim A. J., Choi Y. K., Shin Y. C., Cho S.-G., Ko S.-G. Synergistic effect of SH003 and doxorubicin in triple-negative breast cancer. Phytotherapy Research. 2016;30(11):1817–1823. doi: 10.1002/ptr.5687. [DOI] [PubMed] [Google Scholar]
- 35.Choi H. S., Cho S. G., Kim M. K., et al. SH003 enhances paclitaxel chemosensitivity in MCF-7/PAX breast cancer cells through inhibition of MDR1 activity. Molecular and Cellular Biochemistry. 2017;426(1-2):1–8. doi: 10.1007/s11010-016-2875-y. [DOI] [PubMed] [Google Scholar]
- 36.Choi Y. K., Cho S.-G., Choi Y.-J., et al. SH003 suppresses breast cancer growth by accumulating p62 in autolysosomes. Oncotarget. 2017;8(51):88386–88400. doi: 10.18632/oncotarget.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo H. S., Ku J. M., Lee H. J., et al. SH003 reverses drug resistance by blocking signal transducer and activator of transcription 3 (STAT3) signaling in breast cancer cells. Bioscience Reports. 2017;37(6) doi: 10.1042/BSR20170125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim T. W., Cheon C., Ko S.-G. SH003 activates autophagic cell death by activating ATF4 and inhibiting G9a under hypoxia in gastric cancer cells. Cell Death & Disease. 2020;11(8):p. 717. doi: 10.1038/s41419-020-02924-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheon C., Kang S., Ko Y., et al. Single-arm, open-label, dose-escalation phase I study to evaluate the safety of a herbal medicine SH003 in patients with solid cancer: a study protocol. BMJ Open. 2018;8(8) doi: 10.1136/bmjopen-2017-019502.e019502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheon C., Ko S. G. Phase I study to evaluate the maximum tolerated dose of the combination of SH003 and docetaxel in patients with solid cancer: a study protocol. Medicine. 2020;99(38) doi: 10.1097/MD.0000000000022228.e22228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheon C., Ko S. G. A phase I study to evaluate the safety of the herbal medicine SH003 in patients with solid cancer. Integrative Cancer Therapies. 2020;19 doi: 10.1177/1534735420911442.1534735420911442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdel-Aziz S. M., Aeron A., Garg N. Microbes in Food and Health. Berlin, Germany: Springer; 2016. p. p. 1. online resource (X, 362 pages 331 illustrations, 321 illustrations in color.) [Google Scholar]
- 43.Hsiao W., Liu L. The role of traditional Chinese herbal medicines in cancer therapy - from TCM theory to mechanistic insights. Planta Medica. 2010;76(11):1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 44.Liou K. T., Chen C., Emard N., Lynch K. A., Hou Y. N., Mao J. J. Herbal topical analgesic for pain management: perspectives from cancer patients. Pain Medicine. 2021;22 doi: 10.1093/pm/pnab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J.-W., Lee W. B., Kim W., Min B.-I., Lee H., Cho S.-H. Traditional herbal medicine for cancer pain: a systematic review and meta-analysis. Complementary Therapies in Medicine. 2015;23(2):265–274. doi: 10.1016/j.ctim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Yu S., Peng H. D., Ju D. W., et al. Mechanisms of treatment of cancer pain with a topical Chinese herbal formula in rats. Chinese Medical Journal. 2009;122(17):2027–2031. [PubMed] [Google Scholar]
- 47.Nair A., Jacob S. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Workman P., Aboagye E. O., Aboagye E. O., et al. Guidelines for the welfare and use of animals in cancer research. British Journal of Cancer. 2010;102(11):1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilkens P. H. E., Verweij J., Vecht C. J., Stoter G., van den Bent M. J. Clinical characteristics of severe peripheral neuropathy induced by docetaxel (Taxotere) Annals of Oncology. 1997;8(2):187–190. doi: 10.1023/a:1008245400251. [DOI] [PubMed] [Google Scholar]
- 50.Roglio I., Bianchi R., Camozzi F., et al. Docetaxel-induced peripheral neuropathy: protective effects of dihydroprogesterone and progesterone in an experimental model. Journal of the Peripheral Nervous System. 2009;14(1):36–44. doi: 10.1111/j.1529-8027.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 51.Loprinzi C. L., Reeves B. N., Dakhil S. R., et al. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. Journal of Clinical Oncology. 2011;29(11):1472–1478. doi: 10.1200/jco.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asthana R., Zhang L., Wan B. A., et al. Pain descriptors of taxane acute pain syndrome (TAPS) in breast cancer patients-a prospective clinical study. Supportive Care in Cancer. 2020;28(2):589–598. doi: 10.1007/s00520-019-04845-7. [DOI] [PubMed] [Google Scholar]
- 53.Boyette-Davis J., Dougherty P. M. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Experimental Neurology. 2011;229(2):353–357. doi: 10.1016/j.expneurol.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siau C., Xiao W., Bennett G. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Experimental Neurology. 2006;201(2):507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyette-Davis J., Xin W., Zhang H., Dougherty P. M. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152(2):308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannoccaro M. P., Donadio V., Gomis Pèrez C., Borsini W., Di Stasi V., Liguori R. Somatic and autonomic small fiber neuropathy induced by bortezomib therapy: an immunofluorescence study. Neurological Sciences. 2011;32(2):361–363. doi: 10.1007/s10072-010-0475-2. [DOI] [PubMed] [Google Scholar]
- 57.Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. European Journal of Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 58.Periquet M. I., Novak V., Collins M. P., et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology. 1999;53(8):p. 1641. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 59.Krøigård T., Schrøder H. D., Qvortrup C., et al. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. European Journal of Neurology. 2014;21(4):623–629. doi: 10.1111/ene.12353. [DOI] [PubMed] [Google Scholar]
- 60.Yang W., Sung K., Zhou F., et al. Targeted mutation (R100W) of the gene encoding NGF leads to deficits in the peripheral sensory nervous system. Frontiers in Aging Neuroscience. 2018;10:p. 373. doi: 10.3389/fnagi.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geisler S., Doan R. A., Strickland A., Huang X., Milbrandt J., DiAntonio A. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain: A Journal of Neurology. 2016;139(Pt 12):3092–3108. doi: 10.1093/brain/aww251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marmiroli P., Nicolini G., Miloso M., Scuteri A., Cavaletti G. The fundamental role of morphology in experimental neurotoxicology: the example of chemotherapy-induced peripheral neurotoxicity. Italian journal of anatomy and embryology. 2012;117(2):75–97. [PubMed] [Google Scholar]
- 63.Kyte S. L., Toma W., Bagdas D., et al. Nicotine prevents and reverses paclitaxel-induced mechanical allodynia in a mouse model of CIPN. Journal of Pharmacology and Experimental Therapeutics. 2018;364(1):110–119. doi: 10.1124/jpet.117.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yousef H., Alhajj M., Sharma S. StatPearls. Treasure Island, FL, USA: StatPearls Publishing; 2021. Anatomy, skin (integument), epidermis. [PubMed] [Google Scholar]
- 65.Mehmood N., Hariz A., Templeton S., Voelcker N. An improved flexible telemetry system to autonomously monitor sub-bandage pressure and wound moisture. Sensors. 2014;14(11):21770–21790. doi: 10.3390/s141121770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McBain A. J., O’Neill C. A., Oates A. Reference Module in Biomedical Sciences. Amsterdam, Netherlands: Elsevier; 2016. Skin microbiology; pp. 734–747. [Google Scholar]
- 67.Cata J. P., Weng H. R., Lee B. N., Reuben J. M., Dougherty P. M. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiologica. 2006;72(3):151–169. [PubMed] [Google Scholar]
- 68.Muthuraman A., Singh N., Jaggi A. S. Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food and Chemical Toxicology. 2011;49(10):2557–2563. doi: 10.1016/j.fct.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 69.Kiguchi N., Maeda T., Kobayashi Y., Kondo T., Ozaki M., Kishioka S. The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. European Journal of Pharmacology. 2008;592(1-3):87–92. doi: 10.1016/j.ejphar.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Kiguchi N., Maeda T., Kobayashi Y., Kishioka S. Up-regulation of tumor necrosis factor-alpha in spinal cord contributes to vincristine-induced mechanical allodynia in mice. Neuroscience Letters. 2008;445(2):140–143. doi: 10.1016/j.neulet.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Y.-Q., Liu Z., Liu Z.-H., et al. Interleukin-6: an emerging regulator of pathological pain. Journal of Neuroinflammation. 2016;13(1):p. 141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leung L., Cahill C. M. TNF-α and neuropathic pain - a review. Journal of Neuroinflammation. 2010;7(1):p. 27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fumagalli G., Monza L., Cavaletti G., Rigolio R., Meregalli C. Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.626687.626687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saleh A., Roy Chowdhury S. K., Smith D. R., et al. Diabetes impairs an interleukin-1β-dependent pathway that enhances neurite outgrowth through JAK/STAT3 modulation of mitochondrial bioenergetics in adult sensory neurons. Molecular Brain. 2013;6(1):p. 45. doi: 10.1186/1756-6606-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hur J., O’Brien P. D., Nair V., et al. Transcriptional networks of murine diabetic peripheral neuropathy and nephropathy: common and distinct gene expression patterns. Diabetologia. 2016;59(6):1297–1306. doi: 10.1007/s00125-016-3913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganesh Yerra V., Negi G., Sharma S. S., Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biology. 2013;1(1):394–397. doi: 10.1016/j.redox.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suryavanshi S. V., Kulkarni Y. A. NF-κβ: a potential target in the management of vascular complications of diabetes. Frontiers in Pharmacology. 2017;8:p. 798. doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chopra K., Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. British Journal of Clinical Pharmacology. 2012;73(3):348–362. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crews F. T., Lawrimore C. J., Walter T. J., Coleman L. G. The role of neuroimmune signaling in alcoholism. Neuropharmacology. 2017;122:56–73. doi: 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leger J. M., Larue S., Dashi F. Dysimmune neuropathies: current diagnosis and therapy. Revue du Praticien. 2008;58(17):1887–1889. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


