Abstract
Objectives
To estimate the burden and severity of suspected reinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Methods
A retrospective cohort of members of Kaiser Permanente Southern California with PCR-positive SARS-CoV-2 infection between 1st March 2020 and 31st October 2020 was followed through electronic health records for subsequent positive SARS-CoV-2 tests (suspected reinfection) ≥90 days after initial infection, through 31st January 2021. Incidence of suspected reinfection was estimated using the Kaplan–Meier method. Cox proportional hazards models estimated the association of suspected reinfection with demographic and clinical characteristics, hospitalization, and date of initial infection.
Results
The cohort of 75 149 was predominantly Hispanic (49 648/75 149, 66.1%) and included slightly more females than males (39 736, 52.9%), with few immunocompromised patients (953, 1.3%); 315 suspected reinfections were identified, with a cumulative incidence at 270 days of 0.8% (95% confidence interval (CI) 0.7–1.0%). Hospitalization was more common at suspected reinfection (36/315, 11.4%) than initial infection (4094/75 149, 5.4%). Suspected reinfection rates were higher in females (1.0%, CI 0.8–1.2% versus 0.7%, CI 0.5–0.9%, p 0.002) and immunocompromised patients (2.1%, CI 1.0–4.2% versus 0.8%, CI 0.7–1.0%, p 0.004), and lower in children than adults (0.2%, CI 0.1–0.4% versus 0.9%, CI 0.7–1.0%, p 0.023). Patients hospitalized at initial infection were more likely to have suspected reinfection (1.2%, CI 0.6–1.7% versus 0.8%, CI 0.7–1.0%, p 0.030), as were those with initial infections later in 2020 (150-day incidence 0.4%, CI 0.2–0.5% September–October versus 0.2%, CI 0.1–0.3% March–May and 0.3%, CI 0.2–0.3% June–August, p 0.008). In an adjusted Cox proportional hazards model, being female (hazard ratio (HR) 1.44, CI 1.14–1.81), adult (age 18–39, HR 2.71, CI 1.38–5.31, age 40–59 HR 2.22, CI 1.12–4.41, age ≥60 HR 2.52, CI 1.23–5.17 versus <18 years), immunocompromised (HR 2.48, CI 1.31–4.68), hospitalized (HR 1.60, CI 1.07–2.38), and initially infected later in 2020 (HR 2.26, CI 1.38–3.71 September–October versus March–May) were significant independent predictors of suspected reinfection.
Conclusions
Reinfection with SARS-CoV-2 is uncommon, with suspected reinfections more likely in women, adults, immunocompromised subjects, and those previously hospitalized for coronavirus 2019 (COVID-19). This suggests a need for continued precautions and vaccination in patients with COVID-19 to prevent reinfection.
Keywords: COVID-19, Epidemiology, Hospitalization, Reinfection, Risk factors
Introduction
Coronavirus disease 2019 (COVID-19) causes substantial morbidity and mortality, including both immediate and long-lasting sequelae. People with initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have a reduced risk of later reinfection [1,2], but long-term follow-up after initial infection has been limited. Data are scarce on the rate of reinfection in the community and which factors increase the risk of reinfection.
Confirmation of reinfection requires sequencing virus isolated from both infections, a time-consuming and costly process. While these studies are being conducted, studies using PCR results to identify suspected reinfections ≥90 days after the initial infection [3] are also informative. Therefore, we assembled a large cohort who tested positive for SARS-CoV-2 and followed them for suspected reinfection through electronic health records (EHRs).
Methods
The study was conducted within Kaiser Permanente Southern California (KPSC), an integrated healthcare organization serving over 4.6 million members with diverse demographics similar to the southern California population [4]. Members generally receive all care within KPSC. The study cohort included all members with an initial infection (first positive SARS-CoV-2 PCR test) between 1st March 2020 and 31st October 2020, who retained membership for ≥90 days afterward. Characteristics defined at initial infection included age, sex, race/ethnicity, and immunocompromising conditions. Hospitalizations with a COVID-19 admission diagnosis were identified between 14 days after and 3 days before the positive SARS-CoV-2 test. Suspected reinfection was defined as a positive PCR test for SARS-CoV-2 ≥90 days after the first positive test in accordance with CDC investigative reinfection criteria [3], with follow-up until death, disenrollment, or 31st January 2021.
We used Kaplan–Meier analysis to estimate cumulative incidence rates and 95% confidence intervals for suspected reinfection. We used Cox proportional hazards regression to test the mutually adjusted association of age, sex, race/ethnicity, immunocompromised status, hospitalization and date of initial infection with suspected reinfection. A sensitivity analysis was conducted using a suspected reinfection definition of ≥120 days to allow for potential late viral shedding.
The study was approved by the KPSC IRB (#12454, approved 2nd May 2021), which waived the requirement for informed consent.
Results
During the cohort identification period approximately 930 000 tests were performed, of which 10.7% were positive, yielding 75 149 members who met the inclusion criteria. The cohort was predominantly Hispanic (49 648/75 149, 66.1%), with slightly more females (39 736, 52.9%) than males, 8.1% (6078) aged <18 years and 14.6% (11 009) ≥60 years. Few (953, 1.3%) had an immunocompromising condition, and relatively few (4094, 5.4%) were hospitalized at initial infection (Table 1 ).
Table 1.
Characteristics of a cohort of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rates of reinfection, and association with reinfection
| Total infected (%) | Number reinfected | Cumulative incidence of reinfection at 270 days, % (95%CI) | Unadjusted hazard ratio (95%CI) | Adjusted hazard ratio (95%CI) | 120-Day sensitivity analysis adjusted hazard ratio (95%CI)c | |
|---|---|---|---|---|---|---|
| Total | 75 149 | 315 | 0.8 (0.7–1.0) | N/A | N/A | N/A |
| Sex | ||||||
| Other/unknown | 13 (0.0%) | 0 | N/A | N/A | N/A | N/A |
| Female | 39 736 (52.9%) | 194 | 1.0 (0.8–1.2) | 1.45 (1.15– 1.82) | 1.44 (1.14–1.81) | 1.60 (1.21–2.12) |
| Male | 35 400 (47.1%) | 121 | 0.7 (0.5–0.9) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Age at infection, years | ||||||
| 0–17 | 6078 (8.1%) | 9 | 0.2 (0.1–0.4) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 18–39 | 31 697 (42.2%) | 148 | 0.9 (0.7–1.1) | 2.60 (1.32– 5.09) | 2.71 (1.38–5.31) | 4.19 (1.54–11.36) |
| 40–59 | 26 365 (35.1%) | 106 | 0.8 (0.6–1.0) | 2.15 (1.09– 4.24) | 2.22 (1.12–4.41) | 2.74 (0.99–7.54) |
| 60+ | 11 009 (14.6%) | 52 | 0.9 (0.5–1.2) | 2.49 (1.23– 5.06) | 2.52 (1.23–5.17) | 3.41 (1.20–9.74) |
| Race/ethnicity | ||||||
| Asian/Pacific Islander | 4242 (5.6%) | 11 | 0.5 (0.2–0.9) | 0.66 (0.34– 1.30) | 0.68 (0.35–1.33) | 0.69 (0.29–1.61) |
| Black | 4185 (5.6%) | 19 | 0.9 (0.3–2.6) | 1.31 (0.76– 2.27) | 1.25 (0.72–2.17) | 1.30 (0.65–2.62) |
| Hispanic | 49 648 (66.1%) | 227 | 0.9 (0.7–1.1) | 1.37 (0.97– 1.92) | 1.40 (0.99–1.97) | 1.59 (1.03–2.48) |
| Other/unknown | 4820 (6.4%) | 19 | 0.7 (0.2–1.2) | 1.22 (0.70– 2.11) | 1.30 (0.75–2.27) | 1.53 (0.78–3.00) |
| White | 12 254 (16.3%) | 39 | 0.7 (0.4–1.1) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Immunocompromisedb | ||||||
| Not immunocompromised | 74 196 (98.7%) | 305 | 0.8 (0.7–1.0) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Immunocompromised | 953 (1.3%) | 10 | 2.1 (0.1–4.2) | 2.46 (1.31– 4.61) | 2.48 (1.31– 4.68) | 2.36 (1.04–5.36) |
| Date of initial infection | ||||||
| March–May 2020 | 8545 (11.4%) | 62 | 0.2 (0.1–0.3)a | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| June–August 2020 | 49 887 (66.4%) | 218 | 0.3 (0.2–0.3)a | 1.28 (0.90– 1.82) | 1.32 (0.92–1.89) | 1.43 (0.93–2.18) |
| September–October 2020 | 16 717 (22.2%) | 35 | 0.4 (0.2–0.5)a | 2.13 (1.30– 3.48) | 2.26 (1.38–3.71) | 0.92 (0.27–3.12) |
| Hospitalized at initial infection | ||||||
| Hospitalized | 4094 (5.4%) | 29 | 1.2 (0.6–1.7) | 1.52 (1.04–2.24) | 1.60 (1.07–2.38) | 1.29 (0.76–2.19) |
| Non-hospitalized | 71 055 (94.6%) | 286 | 0.8 (0.7–1.0) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
Adjusted models include all factors in the table, mutually adjusted for each other.
Estimate at 150 days due to insufficient follow-up for 270-day estimate in later groups.
Diagnosed with HIV/AIDS, leukaemia, lymphoma, congenital immunodeficiencies, asplenia or hyposplenia prior to initial infection.
Sensitivity analysis defining reinfection as a positive test ≥120 days after initial infection instead of ≥90 days, as in the primary analysis.
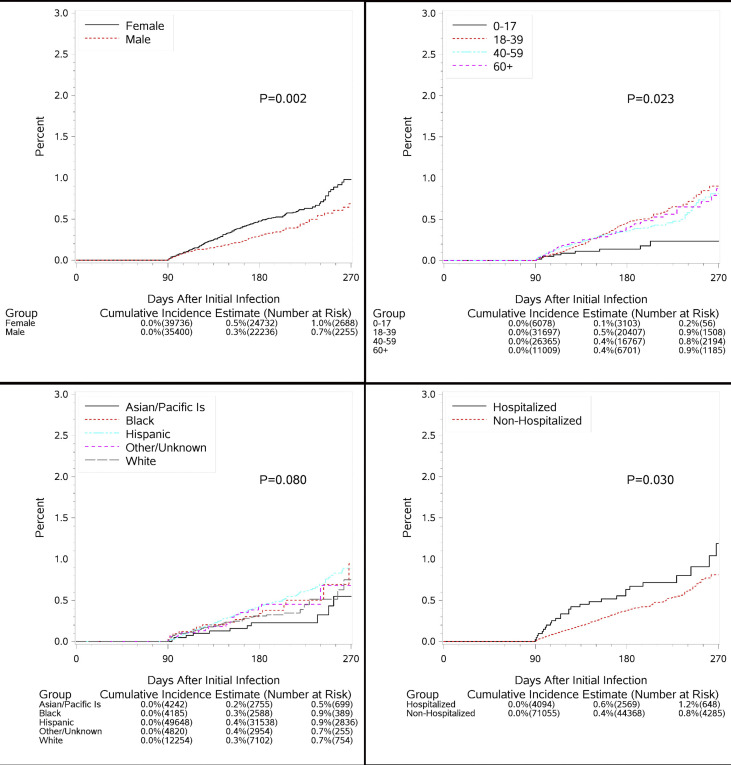
There were 315 suspected reinfections identified, with an overall cumulative incidence of 0.8% (95% confidence interval (CI) 0.7–1.0%) at 270 days following initial infection. Suspected reinfection rates were higher in females (1.0%, CI 0.8–1.2% versus 0.7%, CI 0.5–0.9%, p 0.002, Fig. 1 ), and highest among Hispanics (0.9%, CI 0.7–1.1%) and Blacks (0.9%, CI 0.3–2.6%) compared to Whites (0.7%, CI 0.4–1.1%) and Asian/Pacific Islanders (0.5%, CI 0.2–0.9%, p 0.008). Children <18 years old had a much lower rate of suspected reinfection (0.2%, CI 0.1–0.4%) than adults (0.8%, CI 0.6–1.0% 40–59 years, 0.9%, CI 0.7–1.1% 18–39 years, and 0.9%, CI 0.5–1.2% ≥ 60 years, p 0.023). Those with immunocompromising conditions were more likely to have suspected reinfection (2.1%, CI 1.0–4.2% versus 0.8%, CI 0.7–1.0%, p 0.004), as were those hospitalized for initial infection (1.2%, CI 0.6–1.7% versus 0.8%, CI 0.7–1.0%, p 0.003), and whose initial infections were later in 2020 (150-day incidence 0.4%, CI 0.2–0.5% September–October versus 0.2%, CI 0.1–0.3% March–May and 0.3%, CI 0.2–0.3% June–August, p 0.008, Table 1).
Fig. 1.
Cumulative incidence of suspected reinfection by sex, age, race/ethnicity and hospitalization at initial infection.
In adjusted analyses, females had significantly increased rates of suspected reinfection (HR 1.45, CI 1.15–1.82, p 0.002), as did immunocompromised patients (HR 2.48, CI 1.31–4.68), hospitalized patients (HR 1.60, CI 1.07–2.38, p 0.021), and those with initial infections later in 2020 (HR 2.26, CI 1.38–3.71, p 0.001 for September–October and 1.32, CI 0.92–1.89, p 0.130 for June–August versus March–May). Adults were significantly more likely to have a suspected reinfection than children (age 18–39: HR 2.71, CI 1.38–5.31, age 40–59: HR 2.22, CI 1.12–4.41, age ≥60: HR 2.52, CI 1.23–5.17 versus <18 years). A sensitivity analysis starting 120 days after initial infection yielded results which mirrored the primary findings, with some attenuation of the effect of hospitalization (adjusted HR 1.29, CI 0.76–2.19).
Hospitalization for suspected reinfection was more common than hospitalization at initial infection, with 36 of 315 (11.4%) suspected reinfections versus 4094 of 75 149 (5.4%) initial infections. Hospitalization at suspected reinfection was also more common among those hospitalized at initial infection (7/29, 24.1% versus 29/257, 10.1%, p 0.024).
Discussion
Our results suggest that SARS-CoV-2 reinfection, while uncommon, can occur. Our estimated suspected reinfection rates were slightly higher than those found in a recent study in Denmark [2], though there were minor methodological differences, and the COVID-19 surge in California during our study period was more severe than the surge occurring in the Denmark study [2,6].
Suspected reinfection rates at 150 days post-infection were higher among those with more recent initial infections. This could be due to increased testing or community transmission, or initial infection could provide less protection against variants circulating in late autumn [5,6]. We observed much lower suspected reinfection rates in children than in adults. This could be due to a differing immune response in children, and possible protection from childhood vaccines [7,8] which could strengthen immunity following initial SARS-CoV-2 infection. Reinfections in children, like initial infections, could also be more likely to be asymptomatic and undetected in the absence of systematic screening. We observed higher rates of suspected reinfection in women and Hispanic patients, due in part to demographic disparities in infection risk [9,10].
Patients with immunocompromising conditions and those hospitalized for their initial infection also had higher rates of suspected reinfection. While we cannot rule out continued viral shedding in some cases, prolonged viral shedding for ≥90 days is uncommon [11,12]; Munker et al. found no viral shedding beyond 60 days even in severe disease [11]. A slightly higher rate of testing during follow-up among hospitalized patients (0.37 versus 0.30 tests/month) could also contribute to this finding. Prolonged viral shedding has been observed among immunocompromised individuals [13] who could also be more susceptible to reinfection.
We observed a higher rate of hospitalization at suspected reinfection compared to initial infection. Although data are scarce on reinfection severity in the general population, Cavanaugh et al. reported that five skilled nursing facility residents with mild initial infections had severe reinfection [14]. Limited evidence suggests that mild infection results in a shorter-lived antibody response [15]. However, we saw increased risk of hospitalization at suspected reinfection among patients hospitalized at initial infection. This may be because patients with COVID-19 were more likely to be hospitalized if they had high-risk conditions, which may have also increased susceptibility for reinfection.
Our study has potential limitations. We did not perform genetic sequencing to verify new infection or viral culture to demonstrate live virus at suspected reinfection, and Ct values, close-contact exposure history, and symptoms were unavailable. Instead, we applied the CDC investigative criteria for reinfection [3]; studies have shown little evidence of viral shedding beyond 90 days [11,12], and sensitivity analysis using ≥120 days yielded similar results. Hospitalization defined as COVID-19 diagnosis on admission likely included some admissions for other reasons. This may have over-estimated COVID-19 hospitalization at both initial infection and suspected reinfection. Additionally, during the study period testing focused primarily on symptomatic patients, limiting detection of asymptomatic initial infections and suspected reinfections. If reinfections are more likely to be asymptomatic, we would have underestimated the reinfection rate. Tests done outside of KPSC were not included; if asymptomatic persons were more likely to be tested outside of KPSC, and reinfections were more likely to be asymptomatic, this would overestimate hospitalizations and underestimate the suspected reinfection rate.
Reinfection with SARS-CoV-2 is uncommon, with suspected reinfections more likely in women, adults, immunocompromised patients, and those with prior COVID-19 hospitalization. This may have important public health implications, suggesting a need for continued precautions and vaccination in COVID-19 patients to prevent reinfection.
Transparency declaration
The authors declare that they have no conflicts of interest. No external funding was received for this study.
Author contributions
JS contributed to the conception and design, analysis and interpretation of data, and drafting of the article. KB contributed to the conception and design, interpretation of data, and critical revision of the article. HF contributed to the conception and design, interpretation of data, and critical revision of the article. BB contributed to the conception and design, interpretation of data, and critical revision of the article. BA contributed to the conception and design, interpretation of data, and critical revision of the article. ST contributed to the conception and design, interpretation of data, and critical revision of the article.
Editor: A. Huttner
References
- 1.Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen C.H., Michlmayr D., Gubbels S.M., Mølbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yahav D., Yelin D., Eckerle I., Eberhardt C.S., Wang J., Cao B., et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect. 2021;27:315–318. doi: 10.1016/j.cmi.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koebnick C., Langer-Gould A.M., Gould M.K., Chao C.R., Iyer R.L., Smith N., et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Davis B.D., Chen S.S., Sincuir Martinez J.M., Plummer J.T., Vail E. Emergence of a novel SARS-CoV-2 variant in Southern California. JAMA. 2021;325:1324–1326. doi: 10.1001/jama.2021.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracking COVID-19 in California - coronavirus COVID-19 response. https://covid19.ca.gov/state-dashboard/
- 7.Beric-Stojsic B., Kalabalik-Hoganson J., Rizzolo D., Roy S. Childhood immunization and COVID-19: an early narrative review. Front Publ Health. 2020;8:587007. doi: 10.3389/fpubh.2020.587007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisberg S.P., Connors T.J., Zhu Y., Baldwin M.R., Lin W.H., Wontakal S., et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nau C., Bruxvoort K., Navarro R.A., Chevez S.G., Hogan T.A., Ironside K.R., et al. COVID-19 inequities across multiple racial and ethnic groups: results from an integrated health care organization. Ann Intern Med. 2021:M20–M8283. doi: 10.7326/M20-8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munker D., Osterman A., Stubbe H., Muenchhoff M., Veit T., Weinberger T., et al. Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients. Eur Respir J. 2021;58:2002724. doi: 10.1183/13993003.02724-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cevik M., Tate M., Lloyd O., Maralo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aydillo T., Gonzalez-Reiche A.S., Aslam S., de Guchte A.V., Khan Z., Obla A., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavanaugh A.M., Thoroughman D., Miranda H., Spicer K. Suspected recurrent SARS-CoV-2 infections among residents of a skilled nursing facility during a second COVID-19 outbreak -Kentucky, July-November 2020. MMWR Morb Mortal Wkly Rep. 2021;70:273–277. doi: 10.15585/mmwr.mm7008a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]