INTRODUCTION:
Risk-adapted screening combining the Asia-Pacific Colorectal Screening score, fecal immunochemical test (FIT), and colonoscopy improved the yield of colorectal cancer screening than FIT. However, the optimal positivity thresholds of risk scoring and FIT of such a strategy warrant further investigation.
METHODS:
We included 3,407 participants aged 50–74 years undergoing colonoscopy from a colorectal cancer screening trial. For the risk-adapted screening strategy, subjects were referred for subsequent colonoscopy or FIT according to their risk scores. Diagnostic performance was evaluated for FIT and the risk-adapted screening method with various positivity thresholds. Furthermore, a modeled screening cohort was established to compare the yield and cost using colonoscopy, FIT, and the risk-adapted screening method in a single round of screening.
RESULTS:
Risk-adapted screening method had higher sensitivity for advanced neoplasm (AN) (27.6%–76.3% vs 13.8%–17.3%) but lower specificity (46.6%–90.8% vs 97.4%–98.8%) than FIT did. In a modeled screening cohort, FIT-based screening would be slightly affected because the threshold varied with a reduction of 76.0%–80.9% in AN detection and 82.0%–84.4% in cost when compared with colonoscopy. By contrast, adjusting the threshold of Asia-Pacific Colorectal Screening score from 3 to 5 points for risk-adapted screening varied from an increase of 12.6%–14.1% to a decrease of 55.6%–60.1% in AN detection, with the reduction of cost from 4.2%–5.3% rising to 66.4%–68.5%.
DISCUSSION:
With an appropriate positivity threshold tailored to clinical practice, the risk-adapted screening could save colonoscopy resources and cost compared with the colonoscopy-only and FIT-only strategies.
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide (1). Screening could decrease both the incidence and mortality of CRC (2–4). However, the effectiveness of the current CRC screening strategies may be suboptimal owing to limited healthcare resources, low adherence, potential complications for colonoscopy, or the omission of advanced neoplasm (AN) for the fecal immunochemical test (FIT) (5–8). Therefore, a risk-adapted screening strategy might be a useful means to improve the efficacy and efficiency of CRC screening in the era of precision medicine (9). Accordingly, various risk-adapted screening modalities have been developed in recent years (10,11).
Risk prediction models have been proposed for risk stratification in population-based CRC screening. Despite their modest discriminative power in detecting AN, such models have the potential to be integrated into the current CRC screening modalities (12,13). For instance, the Asia-Pacific Colorectal Screening (APCS) score is one of the risk-stratification models that has shown much improved sensitivity for AN (14,15). Our previous study demonstrated that the risk-adapted screening strategy based on the APCS score had a 2-fold higher detection rate for AN than FIT-only screening with comparable colonoscopy load (16). However, the positivity threshold of risk prediction score or the quantitative FIT to define the high-risk population varies widely. Thus, a tailored threshold combination for risk prediction score and FIT needs further investigation.
This study aimed to explore the yield and health-economical expenditure of the risk-adapted screening strategy at various positivity thresholds and to compare them with FIT-only and risk assessment-only strategies.
METHODS
Study design and population
The retrospective analysis was conducted using data from the Target-C study (11,16). Briefly, the Target-C study is an ongoing multicenter randomized controlled trial comparing the effectiveness of colonoscopy, FIT, and risk-adapted screening strategy in 19,582 participants aged 50–74 years recruited from 6 study sites in China. A standardized epidemiological questionnaire survey was conducted to collect information, such as sociodemographic characteristics, living habits, and disease history. For each participant undergoing colonoscopy, a stool sample was requested before the appointment. For this study, we included all 3,407 subjects who had finished colonoscopy examination and had provided stool samples in the baseline screening phase of the Target-C study.
This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College (number: 18-013/1615). All participants provided informed consent.
Fecal sample handling and FIT
Eligible participants were asked to collect one stool specimen into a sterile container (SARSTEDT, Germany) from a single bowel movement, without any diet or medicine restrictions, within 24 hours before colonoscopy examination. The collected stool samples have been stored at −80 °C for further analysis. For this study, the frozen fecal samples were tested using the quantitative FIT (OC-Sensor; Eiken Chemical, Japan) following the manufacturer's instructions in parallel by trained staff, and the test results were recorded. The laboratory staff was blinded to the colonoscopy results.
Risk assessment and risk-adapted screening strategy
We used the modified APCS score in this study. Briefly, it incorporates 5 CRC-related risk factors and assigns respective values for each category by factor as follows: age (50–54 years = 0; 55–64 years = 1; 65–74 years = 2), sex (female = 0; male = 1), family history of CRC among first-degree relatives (no = 0; yes = 1), smoking (never = 0; current or past = 1), and body mass index (<23 = 0; ≥23 = 1) (17). According to the collected information, each participant in our study was assigned a summary score ranging from 0 to 6. Based on the risk score, a risk-adapted screening strategy was developed: subjects with scores equal to or higher than the positivity threshold were referred for colonoscopy, whereas those with scores lower than the threshold were referred for FIT and further underwent colonoscopy if the FIT results were positive.
Colonoscopy and pathology
All colonoscopy examinations were performed by experienced endoscopists following standard procedures in the designated hospitals. All subjects received adequate bowel preparation before colonoscopy. During examination, abnormal findings were checked carefully. If necessary, biopsies were conducted for pathological diagnosis. Endoscopists were blinded to the risk assessment and FIT test results. Colonoscopy and histology reports were collected and documented in the online data management system. Advanced adenoma (AA) was defined as adenomas ≥10 mm in size with villous architecture, high-grade dysplasia, or intramucosal carcinoma. AN consisted of CRC and its precancerous lesion, AA.
Screening scenario
FIT and risk-adapted screening strategies with various different thresholds were analyzed in our study, respectively. Based on the positivity threshold was commonly preset at 20 μg Hb/g for FIT and at 4 points for APCS, we establish several adjusted screening scenarios by adjusting the positivity thresholds to 10 and 30 μg Hb/g for FIT, and 3 and 5 points for APCS, respectively. Finally, we established 3 FIT-based screening scenarios with positivity thresholds of 10, 20, and 30 μg Hb/g and 9 risk-adapted screening scenarios consisting of APCS and FIT with the combinations of the respective positivity threshold.
Model setup and model parameters
We performed model calculations to estimate the expected number of detected AN, colonoscopy load, total cost, and cost per detected lesion in a 100,000 projected cohort. The model takes into account the prevalence of CRC and its most common precursor, AA, the sensitivity of APCS and FIT for detecting CRC and AA, respectively, the specificity for the absence of any AN, the participation rate of FIT, the colonoscopy adherence rate in general population, or following a positive FIT result, or assessed at high CRC risk by APCS, and the cost. The relative parameters were derived from the Target-C study, as shown in Supplementary Tables 1 and 2 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A666). The prevalence of CRC and AA refers to the endoscopy results of the respective lesion in this study (PCRC = 0.8%; PAA = 7.5%). In our calculation, the sensitivity and specificity of FIT were derived from laboratory-based testing, respectively. For colonoscopy, both the sensitivity and specificity were assumed as 100%. In addition, participation into FIT or APCS and adherence to colonoscopy were supposed not to be affected by the change in positivity thresholds within the same strategy. For economic analysis, the direct costs per AN were calculated based on the corresponding costs in China with Chinese yuan. Detailed information about unit costs is given in Supplemental Table 3 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A666).
Derivation of expected yield of screening and costs
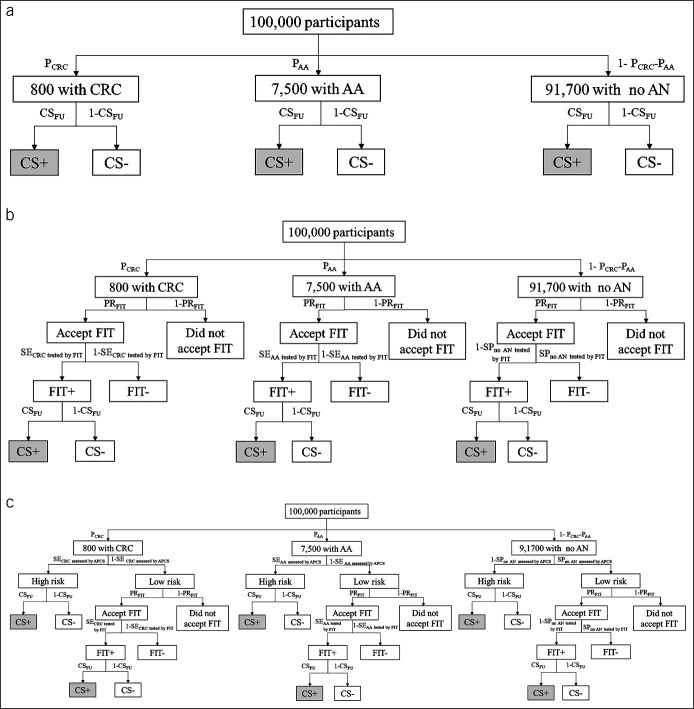
Figure 1 illustrates the derivation with a numerical example of a cohort of 100,000 participants using primary colonoscopy, FIT, and risk-adapted screening method, respectively. In the following, the expected number of CRC, AA, and no AN detected was calculated using different methods and was edited as gray-shaded cell, respectively.
Figure 1.
The screening process for one-time colonoscopy-based screening (a), one-time FIT screening (b), and risk-adapted screening method (c) in a hypothetical cohort of 100,000 participants. The gray-shaded cells indicate the CRC or AA cases detected by the screening method. AA, advanced adenoma; CRC, colorectal cancer; CSFU, colonoscopic follow-up rate; CS+/CS−, uptake of colonoscopy yes/no; FIT, fecal immunochemical test; FIT+/FIT−, positive/negative result of FIT; PCRC/PAA, prevalence of CRC/AA; PRFIT, participation rate of FIT; SECRC tested by FIT/SEAA tested by FIT, sensitivity of CRC/AA tested by FIT; SECRC assessed by APCS/SEAA assessed by APCS, sensitivity of detecting CRC/AA assessed by APCS-based risk assessment; SPno AN tested by APCS, specificity for the absence of AN assessed by APCS-based risk assessment; SPno AN tested by FIT, specificity for the absence of AN tested by FIT.
Colonoscopy-based screening.
CRC (or AA) cases or those with no AN were directly verified through screening colonoscopy. These components are given as follows:
where PCRC/PAA represents for the prevalence of CRC/AA and CSFU for the follow-up rate of colonoscopy.
FIT-based screening.
CRC (AA) cases or those with no AN were tested by FIT first, and those with positive FIT results were verified through subsequent colonoscopy. These components are given by
where PCRC/PAA represents the prevalence of CRC/AA, PRFIT the participation rate of FIT, SECRC assessed by FIT/SEAA assessed by FIT the sensitivity of CRC/AA tested by FIT, CSFU the follow-up rate of colonoscopy, and SPno AN tested by FIT the specificity for the absence of AN tested by FIT.
Risk-adapted screening.
CRC (AA) cases or those with no AN were initially assessed by the APCS system. According to the APCS score, participants assessed at high-risk were verified by subsequent colonoscopy, whereas those at low-risk were tested by subsequent FIT and were verified through subsequent colonoscopy if the FIT results were positive. These components are given by
where PCRC/PAA represents the prevalence of CRC/AA, CSFU the follow-up rate of colonoscopy, SECRC assessed by APCS/SEAA assessed by APCS the sensitivity of detecting CRC/AA assessed by APCS-based risk assessment, PRFIT the participation rate of FIT, SECRC assessed by FIT/SEAA assessed by FIT the sensitivity of CRC/AA tested by FIT, SPno AN tested by APCS the specificity for the absence of AN assessed by APCS-based risk assessment, and SPno AN tested by FIT the specificity for the absence of AN tested by FIT.
Statistical analysis
Diagnostic performance was compared among screening scenarios using FIT and risk-adapted screening methods for detecting CRC, AA, and AN according to sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with their corresponding 95% confidence intervals (CIs). The 95% CIs were calculated using the Clopper-Pearson method. Receiver operating characteristic (ROC) analysis was conducted, and the areas under the curves were determined for FIT and risk-adapted screening. To evaluate the yield and cost burden of screening, the expected number of detected AN, colonoscopy load, total cost, and cost per detected lesion were estimated and compared using screening colonoscopy as a reference in a projected cohort. Furthermore, to assess the influence of alternative participation rates on the results, we conducted sensitivity analyses in 2 other types of participation settings: higher participation setting at a perfect adherence rate (100%) and lower participation setting at rates of 20% lower than the actual rates (see Supplemental Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A666). All statistical analyses were performed using R version 5.3 and Microsoft Excel (18). P values <0.05 were considered statistically significant.
RESULTS
Participant characteristics
Among the 3,407 included participants, 1,753 (51.5%) were male and the mean age (SD) was 60.5 (6.3) years. Only a minority (10.9%) of participants had first-degree relatives with CRC. In total, 30.6% were current or former smokers and 66.2% had a body mass index ≥23. A total of 970 (28.5%) participants had a fecal hemoglobin concentration ≥ 10 μg Hb/g on FIT. As for the APCS score, 3,108 (68.1%), 792 (22.0%), and 295 (8.7%) participants had ≤ 2 or 3 points, 4 points, and ≥5 points, respectively. There were 283 (8.3%) cases of ANs, including 28 (0.8%) cases of CRC, 255 (7.5%) cases of AA, and 3,124 (91.7%) subjects with no AN in our study. The participants' characteristics are given in Table 1.
Table 1.
Baseline characteristics of 3,407 participants included in this study
| Characteristic | N (%) |
| Sex | |
| Male | 1,753 (51.5) |
| Age, yr | |
| 50–54 | 773 (22.7) |
| 55–64 | 1,558 (45.7) |
| 65–74 | 1,076 (31.6) |
| Family history of colorectal cancer among first-degree relatives | |
| Yes | 372 (10.9) |
| Smoking status | |
| Current or former smoker | 1,041 (30.6) |
| BMI, kg/m2 | |
| ≥23 | 2,257 (66.2) |
| Fecal hemoglobin concentration detected by FIT, μg Hb/g | |
| <10 | 2,437 (71.5) |
| 10–19 | 671 (19.7) |
| 20–29 | 119 (3.5) |
| ≥30 | 180 (5.3) |
| APCSa | |
| ≤2 | 1,572 (46.1) |
| 3 | 748 (22.0) |
| 4 | 792 (23.2) |
| ≥5 | 295 (8.7) |
| Findings at colonoscopy | |
| Advanced neoplasm | 283 (8.3) |
| Colorectal cancer | 28 (0.8) |
| Advanced adenoma | 255 (7.5) |
| No advanced neoplasm | 3,124 (91.7) |
| Nonadvanced adenoma | 677 (19.9) |
| Hyperplastic polyp | 261 (7.7) |
| None of the above | 2,186 (64.2) |
APCS, Asia-Pacific Colorectal Screening; BMI, body mass index; CRC, colorectal cancer; FIT, fecal immunochemical test.
The APCS score synthesizes 5 risk factors of CRC (age, sex, family history of CRC among first-degree relatives, smoking, and BMI). Each factor is allocated a score, and the cumulative score is calculated. Subjects with scores at or greater than the positivity threshold were defined as high-risk and were referred for a colonoscopy; those with scores less than the positivity threshold were defined as low-risk and were referred for FIT screening.
Comparison of test accuracy among different CRC screening strategies
Table 2 displays the variations in sensitivities, specificities, PPV, and NPV for detecting CRC, AA, and AN using FIT and risk-adapted screening scenarios under different positivity thresholds, respectively. Overall, the sensitivities for AN increased at a higher threshold while the specificities decreased at lower thresholds across scenarios.
Table 2.
Diagnostic performance of FIT and APCS combined with FIT for detecting colorectal neoplasms among 3,407 participants
| Screening strategies (positivity threshold: APCS; FIT, μg Hb/g) | Positivity rate, % | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | ||||
| CRC | AA | AN | CRC | AA | AN | ||||
| FIT | |||||||||
| None; 10 | 3.8 | 57.1 (40.0–73.1) | 12.9 (9.6–16.9) | 17.3 (13.7–21.4) | 97.4 (96.9–97.8) | 12.2 (7.8–18.0) | 25.2 (19.0–32.2) | 37.4 (30.3–44.9) | 92.9 (92.1–93.6) |
| None; 20 | 2.8 | 57.1 (40.0–73.1) | 11.0 (7.9–14.7) | 15.5 (12.1–19.5) | 98.3 (97.9–98.7) | 16.7 (10.7–24.2) | 29.2 (21.6–37.7) | 45.8 (37.1–54.7) | 92.8 (92.0–93.5) |
| None; 30 | 2.2 | 57.1 (40.0–73.1) | 9.0 (6.2–12.5) | 13.8 (10.5–17.6) | 98.8 (98.4–99.1) | 21.1 (13.7–30.2) | 30.3 (21.6–40.1) | 51.3 (41.3–61.2) | 92.7 (91.9–93.4) |
| Risk-adapted screening methoda | |||||||||
| 3; 10 | 55.3 | 85.7 (70.2–95.0) | 75.3 (70.4–79.7) | 76.3 (71.8–80.4) | 46.6 (45.1–48.1) | 1.3 (0.9–1.8) | 10.2 (9.1–11.4) | 11.5 (10.3–12.7) | 95.6 (94.6–96.4) |
| 3; 20 | 54.8 | 85.7 (70.2–95.0) | 74.5 (69.6–79.0) | 75.6 (71.1–79.8) | 47.1 (45.6–48.5) | 1.3 (0.9–1.8) | 10.2 (9.0–11.4) | 11.5 (10.3–12.7) | 95.5 (94.5–96.4) |
| 3; 30 | 54.6 | 85.7 (70.2–95.0) | 74.1 (69.2–78.6) | 75.3 (70.7–79.4) | 47.3 (45.8–48.8) | 1.3 (0.9–1.8) | 10.2 (9.0–11.4) | 11.5 (10.3–12.7) | 95.5 (94.5–96.3) |
| 4; 10 | 34.4 | 71.4 (54.3–84.9) | 57.6 (52.3–62.8) | 59.0 (54.0–63.9) | 67.9 (66.5–69.2) | 1.7 (1.1–2.5) | 12.6 (11.0–14.3) | 14.3 (12.6–16.1) | 94.8 (94.0–95.6) |
| 4; 20 | 33.8 | 71.4 (54.3–84.9) | 56.5 (51.1–61.7) | 58.0 (52.9–62.9) | 68.4 (67.0–69.8) | 1.7 (1.2–2.5) | 12.5 (10.9–14.2) | 14.3 (12.6–16.1) | 94.7 (93.9–95.5) |
| 4; 30 | 33.4 | 71.4 (54.3–84.9) | 55.7 (50.3–60.9) | 57.2 (52.2–62.2) | 68.8 (67.4–70.1) | 1.8 (1.2–2.5) | 12.5 (10.9–14.2) | 14.2 (12.6–16.1) | 94.7 (93.8–95.4) |
| 5; 10 | 12.2 | 64.3 (47.0–79.2) | 27.1 (22.5–32.0) | 30.7 (26.2–35.6) | 89.5 (88.6–90.4) | 4.3 (2.8–6.4) | 16.6 (13.7–19.9) | 21.0 (17.7–24.5) | 93.4 (92.7–94.2) |
| 5; 20 | 11.2 | 64.3 (47.0–79.2) | 25.1 (20.7–30.0) | 29.0 (24.5–33.7) | 90.4 (89.5–91.2) | 4.7 (3.1–6.9) | 16.7 (13.6–20.2) | 21.4 (18.0–25.1) | 93.4 (92.6–94.1) |
| 5; 30 | 10.7 | 64.3 (47.0–79.2) | 23.5 (19.2–28.3) | 27.6 (23.2–32.3) | 90.8 (89.9–91.6) | 4.9 (3.2–7.2) | 16.4 (13.3–19.9) | 21.3 (17.8–25.1) | 93.3 (92.5–94.0) |
AA, advanced adenoma; AN, advanced neoplasm; APCS, Asia-Pacific Colorectal Screening; CI, confidence interval; CRC, colorectal cancer; FIT, fecal immunochemical test; NPV, negative predictive value; PPV, positive predictive value.
Risk-adapted screening method was conducted based on the APCS score. Subjects with scores at or greater than the positivity threshold were defined as high-risk and were referred for a colonoscopy; those with scores less than the positivity threshold were defined as low-risk and were referred for FIT screening.
For the FIT strategy, the sensitivity, specificity, and NPV varied slightly while PPV changed greatly across different screening strategies. The sensitivity remained 57.1% (95% CI, 40.0%–73.1%), with the positivity threshold increasing from 10 μg Hb/g to 30 μg Hb/g. A slight decrease in the sensitivity was observed with a value of 12.9% (95% CI, 9.6%–16.9%) at 10 μg Hb/g drop to 9.0% (95% CI, 6.2%–12.5%) at 30 μg Hb/g for AA and 17.3% (95% CI, 13.7%–21.4%) at 10 μg Hb/g drop to 13.8% (95% CI, 10.5%–17.6%) at 30 μg Hb/g for AN, respectively. For PPV, the highest PPV for AN was observed at 51.3% (95% CI, 41.3%–61.2%) with 30 μg Hb/g while the lowest one was 37.4% (95% CI, 30.3%–44.9%) at 10 μg Hb/g.
When compared with the FIT strategy, each risk-adapted screening scenario has higher sensitivity for AN detection but lower specificity. Furthermore, great changes in sensitivity and specificity were found with APCS threshold varying; however, there existed small variation with FIT threshold changing within the same APCS score. At a threshold of 3 points for the APCS and 10 μg Hb/g for FIT, the risk-adapted screening method achieved the highest sensitivity of AN at 76.3% (95% CI, 71.8%–80.4%) but the lowest specificity at 46.6% (95% CI, 45.1%–48.1%). Meanwhile, a threshold at 5 points for the APCS and 30 μg Hb/g for FIT yielded the lowest sensitivity of 27.6% (95% CI, 23.2%–32.3%) but the highest specificity of 90.8% (95% CI, 89.9%–91.6%). By contrast, PPV changed slightly because the positivity thresholds varied.
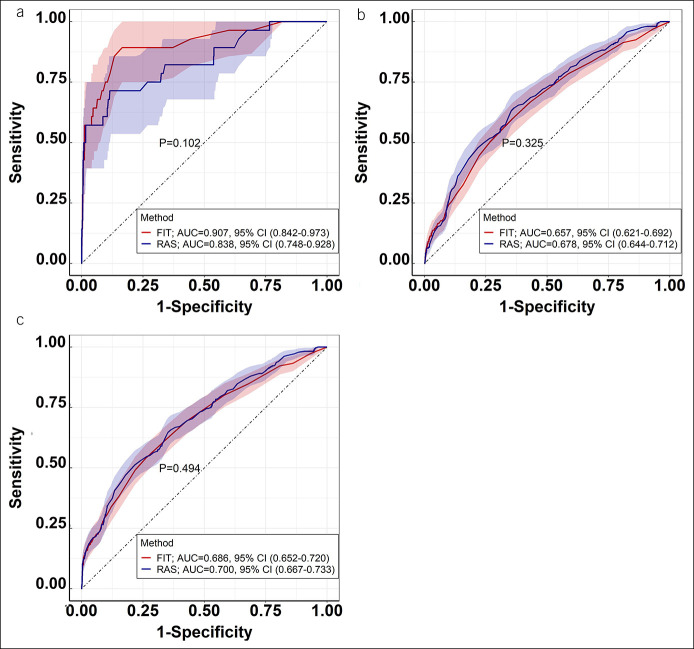
Figure 2 shows the results by ROC analyses where there was no significantly difference in area under the curve between FIT and risk-adapted screening for detecting CRC (0.907, 95% CI, 0.842–0.973 vs 0.838, 95% CI, 0.748–0.928; P = 0.102), AA (0.657, 95% CI, 0.621–0.692 vs 0.678, 95% CI, 0.644–0.712; P = 0.325), and AN (0.686, 95% CI, 0.652–0.720 vs 0.700, 95% CI, 0.667–0.733; P = 0.494), respectively.
Figure 2.
Receiver operating characteristic curves of the FIT and RAS method for detecting colorectal cancer (a), advanced adenoma (b), and advanced neoplasm (c). AUC, area under the curve; CI, confidence interval; FIT, fecal immunochemical test; RAS, risk-adapted screening.
Comparison of yield of screening and cost in the modeled cohort
In a projected screening population of 100,000 invited persons, colonoscopy, FIT, and risk-adapted screening method were evaluated concerning the diagnostic yield and cost per lesion detected across scenarios with different positivity thresholds (Table 3).
Table 3.
Diagnostic yield and cost per advanced neoplasm for screening strategies with single round of screening in a hypothetical cohort of 100,000 participants
| Positivity threshold (APCS; FIT, μg Hb/g) | No. of AN detected | Missed AN cases (%) | Total colonoscopy | Averted colonoscopy (%) | Total cost needed | Saved cost (%) | Cost per lesion detected | Reduced cost per AN (%) |
| Colonoscopy | 3,320 | Reference | 40,000 | Reference | 17,822,400 | Reference | 5,368 | Reference |
| FIT | ||||||||
| None; 10 | 798 | 76.0 | 2,133 | 94.7 | 3,211,464 | 82.0 | 4,024 | 25.0 |
| None; 20 | 718 | 78.4 | 1,591 | 96.0 | 2,956,531 | 83.4 | 4,118 | 23.3 |
| None; 30 | 634 | 80.9 | 1,250 | 96.9 | 2,777,950 | 84.4 | 4,382 | 18.4 |
| Risk-adapted screening methoda | ||||||||
| 3; 10 | 3,788 | −14.1 | 33,118 | 17.2 | 17,069,730 | 4.2 | 4,506 | 16.1 |
| 3; 20 | 3,755 | −13.1 | 32,839 | 17.9 | 16,947,009 | 4.9 | 4,513 | 15.9 |
| 3; 30 | 3,738 | −12.6 | 32,699 | 18.3 | 16,877,100 | 5.3 | 4,515 | 15.9 |
| 4; 10 | 2,905 | 12.5 | 20,506 | 48.7 | 11,845,065 | 33.5 | 4,077 | 24.0 |
| 4; 20 | 2,857 | 13.9 | 20,172 | 49.6 | 11,685,953 | 34.4 | 4,090 | 23.8 |
| 4; 30 | 2,822 | 15.0 | 19,959 | 50.1 | 11,578,930 | 35.0 | 4,103 | 23.6 |
| 5; 10 | 1,475 | 55.6 | 7,159 | 82.1 | 5,980,076 | 66.4 | 4,054 | 24.5 |
| 5; 20 | 1,394 | 58.0 | 6,653 | 83.4 | 5,740,395 | 67.8 | 4,118 | 23.3 |
| 5; 30 | 1,326 | 60.1 | 6,397 | 84.0 | 5,611,525 | 68.5 | 4,232 | 21.2 |
AA, advanced adenoma; AN, advanced neoplasm; APCS, Asia-Pacific Colorectal Screening; CRC, colorectal cancer; FIT, fecal immunochemical test.
Risk-adapted screening method was conducted based on the APCS score. Subjects with scores at or greater than the positivity threshold were defined as high-risk and were referred for a colonoscopy; those with scores less than the positivity threshold were defined as low-risk and were referred for FIT screening.
For the FIT screening strategy, the diagnostic yield and cost burden were also scarcely affected by the varying thresholds. When compared with colonoscopy-based screening as reference, it would miss 76.0%–80.9% AN detection, with a reduction of 94.7%–96.9% in colonoscopy load, 82.0%–84.4% in total cost, and 18.4%–25.0% in cost per AN.
By contrast, the related results of the risk-adapted screening method would be greatly influenced by the variation in threshold for the APCS score but for FIT. When compared with colonoscopy-based screening, risk-adapted screening methods at an APCS threshold of 3 points led to 12.6%–14.1% increased detection of AN, with 17.2%–18.3% reduction of colonoscopy load, 4.2%–5.3% total cost, and 15.9%–16.1% cost per AN. However, the results changed greatly that risk-adapted screening methods at a 4-point threshold for APCS would miss 48.7%–50.1% AN, with averting 48.5%–50.0% colonoscopy, saving 33.5%–35.0% total cost, and 23.6%–24.0% cost per AN while the respective value changing to 55.6%–60.1%, 82.1%–84.0%, 66.4%–68.5%, and 21.2%–24.5% at a 5-point threshold for APCS. However, such variation was observed with only a change in the positivity threshold of FIT. For example, with the APCS threshold fixed at 3 points, risk-adapted screening methods yield a 14.1% increase in AN detection, with 17.2% reduction of colonoscopy load, 4.2% total cost, and 16.1% cost per AN when the FIT threshold was adjusted to 10 μg Hb/g while the respective value changed slightly to 13.1%, 17.9%, 4.9%, and 15.9%, respectively, when the FIT threshold was adjusted to 30 μg Hb/g.
Sensitivity analysis
In sensitivity analyses, risk-adapted screening scenarios differed in higher and lower participation settings. In the lower participation settings (see Supplemental Table 4, Supplementary Digital Content 1, http://links.lww.com/CTG/A666), similar patterns were observed for the proportion for AN missed, averted colonoscopy, total cost, and cost per AN, respectively, when compared with in the actual participation setting. By contrast, in the higher participation settings (see Supplemental Table 5, Supplementary Digital Content 1, http://links.lww.com/CTG/A666), although the respective values increased to a greater extent, the trend of overall results was consistent with that in the actual participation setting.
DISCUSSION
This study demonstrated that the overall diagnostic performances for AN detection were similar between FIT-based and risk-adapted screening strategies according to the ROC analyses. Based on a modeled screening cohort, when compared with colonoscopy-based screening, FIT-based screening would miss more than 76% AN cases, although it saved substantial colonoscopy load and cost. By contrast, the positivity threshold, especially for the APCS, strongly affected the performance of the risk-adapted screening strategy. Adjusting the APCS threshold from 3 to 5 points for the risk-adapted screening method varied from an increase of 12.6%–14.1% to a decrease of 55.6%–60.1% in AN detection, with the reduction of cost from 4.2%–5.3% rising to 66.4%–68.5%. These findings demonstrate that implementing a novel risk-adapted screening strategy may be cost-benefit in a single CRC screening because it could lead to a remarkable reduction of colonoscopy load and cost, at a cost of an acceptable missed rate of AN, when compared with colonoscopy-based screening.
AN is the most important outcome of interest in CRC screening. In our study, FIT had limited sensitivities between 15.5% and 17.3% for AN, which was within the range of reported findings (9%–60%) (19–21). These results demonstrate that even at the decreased positivity threshold, most FIT hardly could achieve high sensitivity for detecting AN to meet the demand for the efficacy of CRC screening. However, as demonstrated by the current studies, the risk-adapted screening strategy had higher sensitivity ranging from 27.6% to 68.8%, which could be a potential tool to facilitate and improve the identifying asymptomatic subjects with AN for early colonoscopy (14,22).
Colonoscopy is still the gold standard for colorectal neoplasm diagnosis and provides an opportunity to resect suspicious lesions in the CRC screening. Despite the proven efficacy, using colonoscopy as a primary screening tool for all eligible subjects incurs heavy burdens for workload and costs in medical care systems. Because the overall prevalence reported was 0.4% (95% CI, 0.3%–0.5%) for CRC and 4.6% (95% CI, 3.8%–5.5%) for AA, respectively, substantial colonoscopies would be conducted unnecessarily if offered to the whole population (23,24). Our simulated analyses suggested that the risk-adapted screening strategy reduced 17.2%–84.0% colonoscopy load when compared with using colonoscopy as the primary method. Thus, the risk-adapted screening strategy may be a desirable method to save colonoscopy resources, especially for regions where healthcare resources are constrained. However, to balance the effectiveness and cost of CRC screening, an optimal threshold still needs to be determined for the risk-adapted screening strategy, provided the large variation in yielding for AN with threshold changing.
In a simulated screening setting, our results show that the effectiveness and cost of the risk-adapted screening strategy were more profoundly affected by the positivity threshold for APCS but for FIT. Among all alternative scenarios, the risk-adapted screening scenarios with a positivity threshold of 4 for the APCS and at each of the 3 thresholds for FIT were acceptable with saving 48.7%–50.1% colonoscopy load at a cost of 12.5%–15.0% missed AN. By contrast, it would be inefficacy for the risk-adapted screening with APCS threshold presetting at 3 or 5 points. The former led to an 18% reduction of colonoscopy load and costs, although achieving around 13% increase in AN detection, and the latter resulted in missing more than one half of AN, although saving more than 65% colonoscopy load. As reported by previous studies, a similar level of threshold for APCS of 4 points is also commonly used in many Asian countries currently (17,25,26).
The superiority of the risk-adapted screening strategy may be partly attributed to the introduction of a risk assessment questionnaire to CRC screening. Given the impact of these risk factors on CRC development and death, individuals at high risk for CRC are more prone to develop CRC and its precancerous lesions, when compared with those at low CRC risk (27,28). It is thus understandable that screening with colonoscopy among individuals with higher risk scores may confer a more efficient use of colonoscopy resources with higher AN detection. In addition, a previous study conducted by Wang demonstrated that according to individuals' risk profiles, the absolute benefit of colonoscopy screening was more than twice higher for individuals with the highest CRC risk score compared with the lowest score from a long-term perspective (29). However, previous studies showed that the existing risk prediction models based on environmental risk factors had modest discriminatory capabilities, which may reduce the cost-effectiveness of a risk-adapted screening strategy (30). Hence, further research is still needed to explore potential technology that enables improving the discriminatory capabilities of the risk prediction models, such as polygenic risk scores, circulating microRNA, and fecal DNA (31–33).
The main strength of our study was that we conducted a comprehensive comparison between risk-adapted screening and FIT-only according to test accuracy, effectiveness, and cost in a screening setting. Such analyses would provide a potential reference for designing personalized CRC screening strategies in the future. However, some limitations still need to be considered. First, although the long-term effectiveness and cost-effectiveness are crucial for CRC screening, our study only considered the yield and cost in one single round of screening among colonoscopy, FIT, and risk-adapted screening. It could be attributed to the short time from the APCS system being established on. Therefore, the long-term evaluation could not be conducted, and the respective parameters that used in the model cohort are not available, although the APCS-based risk-adapted screening strategy has been applied in many Asian countries (17,25,26). Given the long-term effectiveness and cost-effectiveness counts, the ongoing Target-C study will further make a long-term comparison among the 3 screening strategies in the future. Second, separate analyses on CRC were not conducted because of the limited number of CRC cases. Because our CRC cases were derived from the population-based screening, the distribution of CRC in our study reflected the CRC prevalence in a real population to some extent. Third, the generalizability of the current findings based on the hypothetical cohort might be limited. Therefore, further investigations are needed based on a real population-based screening.
In summary, this study demonstrated that risk-adapted screening using the APCS combined with FIT guaranteed the yield of screening with saving healthcare resources and cost, which could be a promising alternative strategy to the primary colonoscopy-based screening strategy, especially for regions where there were medical resources limited. However, the positivity thresholds of this strategy, especially for APCS, need to be tailored considering colonoscopy resources to ensure effectiveness and cost savings.
CONFLICTS OF INTEREST
Guarantors of the article: Ming Lu, MBBS, Hongda Chen, PhD, and Min Dai, PhD.
Specific author contributions: Hongda Chen, PhD, and Min Dai, PhD, MD, are co-corresponding authors. H.C.: conceptualized and designed the study. M.L., H.C., W.L., Y.Z., C.L., B.L., L.D., X.L., D.D., D.W., Y.G., J.S., J.R., and M.D.: participated in the acquisition of data and analysis and interpretation of data. M.L. and H.C.: participated in the statistical analysis and drafted the article. All authors critically revised the article and approved the final version.
Financial support: This work was supported by the Beijing Nova Program of Science and Technology (Z191100001119065), the Natural Science Foundation of Beijing Municipality (7202169), and the CAMS Innovation Fund for Medical Sciences (2017-I2M-1-006). The funders had no role in the study design and conduct; data collection, management, analysis, and interpretation; article preparation, review, or approval; and the decision to submit the article for publication.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Risk-adapted screening integrating risk stratification score and established screening modalities might improve effectiveness and cost-effectiveness in colorectal cancer screening.
✓ A risk-adapted screening approach including Asia-Pacific Colorectal Screening (APCS) score, fecal immunochemical test, and colonoscopy was developed and showed promising screening efficacy in previous studies.
✓ Optimization of the positivity thresholds of such risk-adapted screening warrants further investigation but with sparse evidence.
WHAT IS NEW HERE
✓ Adjusting the APCS threshold from 3 to 5 points for the risk-adapted screening method varied from an increase of 12.6%–14.1% to a decrease of 55.6%–60.1% in advanced neoplasm detection, with the reduction of cost from 4.2%–5.3% rising to 66.4%–68.5%.
✓ The positivity threshold of APCS rather than the fecal immunochemical test strongly affected the yield and efficiency of the risk-adapted screening approach.
✓ Risk stratification is the core of risk-adapted screening, and the positivity threshold of which needs to be tailored considering colonoscopy resources to ensure effectiveness and cost savings.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A666
Contributor Information
Ming Lu, Email: luming2018@hotmail.com.
Le Wang, Email: wangle@zjcc.org.cn.
Yuhan Zhang, Email: zyhmsf426@163.com.
Chengcheng Liu, Email: liucc_2011@126.com.
Bin Lu, Email: lb838744529@126.com.
Lingbin Du, Email: dulb@zjcc.org.cn.
Xianzhen Liao, Email: 125844068@qq.com.
Dong Dong, Email: ddong2002@163.com.
Donghua Wei, Email: 870826630@qq.com.
Yi Gao, Email: 1094186227@qq.com.
Jufang Shi, Email: shijf@cicams.ac.cn.
Jiansong Ren, Email: renjiansong@sina.com.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–24. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin TR, Corley DA, Jensen CD, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology 2018;155(5):1383–91.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buskermolen M, Cenin DR, Helsingen LM, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A microsimulation modelling study. BMJ 2019;367:l5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: A randomized clinical trial of competing strategies. Arch Intern Med 2012;172(7):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut 2015;64(6):982–90. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Luo X, Li N, et al. Diagnostic accuracy of fecal occult blood tests for detecting proximal versus distal colorectal neoplasia: A systematic review and meta-analysis. Clin Epidemiol 2019;11:943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imperiale TF, Gruber RN, Stump TE, et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: A systematic review and meta-analysis. Ann Intern Med 2019;170(5):319–29. [DOI] [PubMed] [Google Scholar]
- 9.Robertson DJ, Ladabaum U. Opportunities and challenges in moving from current guidelines to personalized colorectal cancer screening. Gastroenterology 2019;156(4):904–17. [DOI] [PubMed] [Google Scholar]
- 10.Tikk K, Weigl K, Hoffmeister M, et al. Study protocol of the RaPS study: Novel risk adapted prevention strategies for people with a family history of colorectal cancer. BMC cancer 2018;18(1):720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Li N, Shi J, et al. Comparative evaluation of novel screening strategies for colorectal cancer screening in China (TARGET-C): A study protocol for a multicentre randomised controlled trial. BMJ Open 2019;9(4):e025935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng L, Balavarca Y, Weigl K, et al. Head-to-head comparison of the performance of 17 risk models for predicting presence of advanced neoplasms in colorectal cancer screening. Am J Gastroenterol 2019;114(9):1520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usher-Smith JA, Walter FM, Emery JD, et al. Risk prediction models for colorectal cancer: A systematic review. Cancer Prev Res (Phila) 2016;9(1):13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu HM, Ching JY, Wu KC, et al. A risk-scoring system combined with a fecal immunochemical test is effective in screening high-risk subjects for early colonoscopy to detect advanced colorectal neoplasms. Gastroenterology 2016;150(3):617–25.e3. [DOI] [PubMed] [Google Scholar]
- 15.Yeoh KG, Ho KY, Chiu HM, et al. The Asia-Pacific colorectal screening score: A validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut 2011;60(9):1236–41. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Lu M, Liu C, et al. Comparative evaluation of participation and diagnostic yield of colonoscopy vs fecal immunochemical test vs risk-adapted screening in colorectal cancer screening: Interim analysis of a multicenter randomized controlled trial (TARGET-C). Am J Gastroenterol 2020;115(8):1264–74. [DOI] [PubMed] [Google Scholar]
- 17.Sung JJY, Wong MCS, Lam TYT, et al. A modified colorectal screening score for prediction of advanced neoplasia: A prospective study of 5744 subjects. J Gastroenterol Hepatol 2018;33(1):187–94. [DOI] [PubMed] [Google Scholar]
- 18.Venables WN, Smith DM; the R Core Team. An Introduction to R 2020. R Foundation: Vienna, Austria, 2020. (https://www.R-project.org/). Accessed June 24, 2020. [Google Scholar]
- 19.Gies A, Cuk K, Schrotz-King P, et al. Direct comparison of diagnostic performance of 9 quantitative fecal immunochemical tests for colorectal cancer screening. Gastroenterology 2018;154(1):93–104. [DOI] [PubMed] [Google Scholar]
- 20.Gies A, Bhardwaj M, Stock C, et al. Quantitative fecal immunochemical tests for colorectal cancer screening. Int J Cancer 2018;143(2):234–44. [DOI] [PubMed] [Google Scholar]
- 21.Katsoula A, Paschos P, Haidich AB, et al. Diagnostic accuracy of fecal immunochemical test in patients at increased risk for colorectal cancer: A meta-analysis. JAMA Intern Med 2017;177(8):1110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He XX, Yuan SY, Li WB, et al. Improvement of Asia-Pacific colorectal screening score and evaluation of its use combined with fecal immunochemical test. BMC Gastroenterol 2019;19(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong MCS, Huang J, Huang JLW, et al. Global prevalence of colorectal neoplasia: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18(3):553–61.e10. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Li N, Ren J, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut 2019;68(8):1450–7. [DOI] [PubMed] [Google Scholar]
- 25.Aniwan S, Rerknimitr R, Kongkam P, et al. A combination of clinical risk stratification and fecal immunochemical test results to prioritize colonoscopy screening in asymptomatic participants. Gastrointest Endosc 2015;81(3):719–27. [DOI] [PubMed] [Google Scholar]
- 26.Sekiguchi M, Kakugawa Y, Matsumoto M, et al. A scoring model for predicting advanced colorectal neoplasia in a screened population of asymptomatic Japanese individuals. J Gastroenterol 2018;53(10):1109–19. [DOI] [PubMed] [Google Scholar]
- 27.Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol 2016;2(9):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X, Wu K, Ogino S, et al. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology 2018;155(2):355–73.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Ma W, Wu K, et al. Long-term colorectal cancer incidence and mortality after colonoscopy screening according to individuals' risk profiles. J Natl Cancer Inst 2021;2021:djab01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladabaum U, Mannalithara A, Mitani A, et al. Clinical and economic impact of tailoring screening to predicted colorectal cancer risk: A decision analytic modeling study. Cancer Epidemiol Biomarkers Prev 2020;29(2):318–28. [DOI] [PubMed] [Google Scholar]
- 31.Marcuello M, Vymetalkova V, Neves RPL, et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med 2019;69:107–22. [DOI] [PubMed] [Google Scholar]
- 32.Bosch LJW, Melotte V, Mongera S, et al. Multitarget stool DNA test performance in an average-risk colorectal cancer screening population. Am J Gastroenterol 2019;114(12):1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weigl K, Thomsen H, Balavarca Y, et al. Genetic risk score is associated with prevalence of advanced neoplasms in a colorectal cancer screening population. Gastroenterology 2018;155(1):88–98.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.