Abstract
Background
After major lower limb amputation, persistent pain is common, with up to 85% of patients reporting recurring phantom or residual-limb pain. Although pain management is an important factor of quality of life in patients with lower limb amputations, there are few long-term data regarding the frequency of persistent pain and how it impacts prosthesis use.
Questions/purposes
(1) How prevalent are different types of pain at long-term follow-up after amputation for malignant tumors? (2) What association do different pain types have with daily prosthesis use?
Methods
Between 1961 and 1995, 124 major amputations for malignant tumors were performed at one center in Austria in patients (1) who spoke German and (2) whose surgical date resulted in the possibility of a minimum follow-up time of 20 years at the time of this survey; those patients were considered potentially eligible for this retrospective study. The indications for major amputation were to achieve local tumor control in limbs that the surgeon deemed unsalvageable without amputation. Of those 124 patients, 71% (88) had died, 9% (11) could not be reached, and 3% (4) declined to participate. Thus, 58% (21 of 36) of those living at the time of this study and who underwent lower limb amputation between 1961 and 1993 with a median (range) follow-up duration of 41 years (23 to 55) completed a standardized questionnaire, including an assessment of pain and daily prosthesis use during the year before the survey. Phantom pain, residual limb pain, and back pain were each further subclassified into pain frequency, intensity, and restrictions in activities of daily living (ADL) due to the specific pain form and rated on a 5- (pain frequency) and 10-point (pain intensity, restrictions in ADL) numerical rating scale. Before multivariate regression analysis, daily prosthesis use was correlated with pain parameters using Spearman correlation testing.
Results
Seventeen of 21 patients reported phantom limb and back pain, and 15 patients reported residual limb pain in the past year. Median (range) phantom pain intensity was 7 (1 to 10) points, median residual limb pain intensity was 4 (1 to 9) points, and median back pain intensity was 5 (1 to 10) points. After controlling for relevant confounding variables such as age at amputation, age at survey, and stump length, we found that less intense residual limb pain (defined on a 10-point scale with 1 representing no pain at all and 10 representing extremely strong pain [95% CI 0.3 to 1.0]; r = 0.8; p = 0.003) was associated with greater daily prosthesis use. Higher amputation levels showed a decreased daily prosthesis use compared with patients with lower amputation levels (defined as transfemoral amputation versus knee disarticulation versus transtibial amputation [95% CI 0.3 to 5.1]; r = 0.5; p = 0.03).
Conclusion
Decades after surgery, many patients with lower limb amputations experience pain that restricts them in terms of ADLs and decreases their daily prosthesis use. This information supports the need for regular residual limb inspections and careful prosthesis fitting even at long-term follow-up, as effective prosthesis fitting is a modifiable cause of residual limb pain. Future studies evaluating long-term treatment effects of pain relief surgery and therapeutic alternatives to conservative pain treatments should be performed, as these approaches may help alleviate pain in patients with refractory postamputation pain.
Level of Evidence
Level IV, therapeutic study.
Introduction
Postamputation pain is common and often disabling [11]. Although the ability to perform activities of daily living (ADL) is known to be the most important predictor of self-perceived health and quality of life [26, 28], adequate pain management is fundamental to a patient’s adjustment to his or her limitations, as well as to esthetic and functional satisfaction [4]. Patients who undergo lower limb amputation may experience different forms of pain. Phantom pain is described as painful sensations in the area of the missing limb, whereas residual limb or stump pain is defined as pain originating from the residual limb [6, 27]. Residual limb pain is considered a multifactorial condition, and there is a need for cooperation between postoperative residual limb care teams and patient-mobilization experts, as a poorly fitted prosthesis may lead to pressure marks, ulcerations, and the inability to perform ADLs [5]. Addressing chronic pain may be especially important for patients who undergo major amputation because of malignant tumors when surviving the disease because these patients tend to be younger and thus may have more recreational or occupational demands in terms of ADLs than patients who are older or who undergo amputations for diabetes or vascular insufficiency [20, 21].
Although phantom limb pain is a known complication, relatively little is known at very long-term follow-up, and it is not known whether pain fades or persists many decades after amputation [6, 9, 15, 25]. Similarly, little is known regarding the degree to which different types of pain may influence daily prosthesis use, especially at long-term follow-up. A more nuanced understanding of these themes may help guide specific prophylactic and therapeutic pain treatments and may determine the degree to which continued inspections of the residual limb and prosthesis re-fittings are appropriate, as these are preventable sources of residual limb pain.
We therefore asked: (1) How prevalent are different types of pain at long-term follow-up after amputation for malignant tumors? (2) What association do different pain types have with daily prosthesis use?
Patients and Methods
Study Design and Setting
This is a retrospective study based on the Vienna Bone and Soft Tissue Tumor Registry for German-speaking patients at the orthopaedic tumor department of the Medical University of Vienna who were treated with a unilateral major amputation of the lower limb for a malignant tumor from 1961 to 1995.
Patients
Between 1961 and 1995, 124 major amputations for malignant tumors were performed in patients at one center in Austria who (1) spoke German and (2) whose surgical date resulted in the possibility of a minimum follow-up time of 20 years at time of this survey; those patients were considered potentially eligible for this retrospective study. Of those 124 patients, 71% (88) were excluded because they died before data and survey collection, which occurred between October 2015 and November 2016, another 9% (11) could not be reached because no current telephone numbers and addresses were available and we could not obtain this information from the Austrian registration offices, and another 3% (4) declined to participate, leaving 17% (21) for analysis here, representing 58% (21 of 36) of patients who were alive at the time of the survey. The median (range) age at the time of amputation was 16 years (9 to 41), the median follow-up after amputation was 41 years (23 to 55).
Because a high proportion of patients were missing at long-term follow-up, we wished to determine whether those who were available for follow-up differed in important ways from those who were missing. For that reason, we compared the groups. In general, patients who were included in this study were younger at the time of amputation (16 years [range 9 to 41] versus 40 years [range 5 to 85]; p = 0.001), more likely to have osteosarcoma (76% [16 of 21] versus 40% [41 of 103]; p = 0.002), and more likely to receive chemotherapy (95% [20 of 21] versus 64% [66 of 103]; p = 0.005), but no other differences were observed with the numbers available (Table 1).
Table 1.
Demographics and clinical characteristics of patients included and lost to follow-up
| Parameter | Study participants (n = 21) | Lost to follow-up (n = 103) | p value |
| Age at the time of the study in yearsa | 60 (33-74) | 59 (5-104) | 0.27b |
| Follow-up time after amputation in yearsa | 41 (23-55) | 3 (0-45) | 0.001b |
| Age at amputation in years | 16 (9-41) | 40 (5-85) | 0.001b |
| Men | 67 (14) | 48 (49) | 0.11 |
| Primary tumor type | |||
| Osteosarcoma | 76 (16) | 40 (41) | 0.002c |
| Ewing sarcoma | 5 (1) | 3 (3) | 0.66c |
| Chondrosarcoma | 5 (1) | 3 (3) | 0.66c |
| Soft tissue sarcoma | 14 (3) | 38 (39) | 0.04c |
| Melanoma | 0 (0) | 1 (1) | 0.65c |
| Carcinoma | 0 (0) | 10 (10) | 0.17c |
| Adamantinoma | 0 (0) | 1 (1) | 0.65c |
| PNET | 0 (0) | 3 (3) | 0.43c |
| Malignant tumor not further specified | 0 (0) | 2 (2) | 0.52c |
| Amputation level | |||
| Transfemoral | 67 (14) | 53 (55) | 0.26c |
| Knee disarticulation | 19 (4) | 22 (23) | 0.74c |
| Transtibial | 14 (3) | 24 (25) | 0.32c |
| Oncological therapies | |||
| Chemotherapy | 95 (20) | 64 (66) | 0.005 c |
| Radiotherapy | 19 (4) | 17 (17) | 0.78 c |
Time of death or fictitious follow-up date of January 1, 2016.
t-test.
chi-square-test.
Data presented as median (range) or % (n); regarding oncological therapies, only patients receiving those therapies are listed; PNET = primitive neuroectodermal tumor.
Fourteen of 21 included patients were men and seven were women. Fourteen of 21 patients underwent transfemoral amputation, four patients underwent knee disarticulation, and three patients underwent transtibial amputation (Table 1). Twenty patients received chemotherapy after amputation, and four received postoperative radiotherapy. Regarding patients’ occupations, six were retired due to age, five were retired due to disability, eight had an active occupation, one was studying, and one declined to answer this question.
General Indications for Amputation During the Study Period
During the study period, the surgeon generally used amputation to achieve local tumor control in limbs he or she classified as unsalvageable. Typically, amputations were performed in large tumors with substantial loss of muscular leg function or in tumors infiltrating critical limb structures, leading to function loss because of impaired limb perfusion or sensibility. With a large patient inclusion period, surgical methods for limb reconstruction and resection techniques changed, however, and it must be assumed that a larger percentage of patients received limb salvage in the latter period than in the earlier part, even for similar indications.
Outcome Measures
We used a questionnaire to collect demographic information (age, gender, education, weight, and height) and amputation information (time and level of amputation).
Primary and Secondary Study Goals
Our primary study goal was to assess the prevalence and quality of residual limb, phantom, and back pain in long-term patients with lower limb amputations. To achieve this, we asked standardized questions related to the intensity and frequency of phantom, residual limb, and back pain as well as restriction to ADL due to the specific pain form. Patient answers regarding the intensity or degree of each of those types of pain were recorded on a numerical rating scale ranging from 1 to 10, with 1 representing no pain at all and 10 representing extremely strong pain. The frequency of pain episodes was represented by a 5-point scale, with 0 defined as never and 5 defined as always. Restrictions to ADLs were measured with a 10-point scale, with 1 representing no restrictions and 10 defined as inability to execute ADL due to pain.
Our secondary study goal was to find associations of different pain types on daily prosthesis use. We asked patients how many hours per day they usually wore their prosthesis and used multivariate linear regression to find associations to pain intensity, frequency, and restrictions of ADL due to specific pain forms.
Ethical Approval
Ethical approval for this study was obtained from the Medical University of Vienna.
Statistical Analysis
We used Spearman correlation coefficient testing to identify correlations between parameters. Afterward, we performed multivariate linear regression analysis to identify relationships between residual limb pain intensity and prosthesis daily use. Linear regression was used to determine differences in daily prosthesis use depending on amputation levels. The statistical analysis was performed with IBM SPSS version 26 (IBM). A p value < 0.05 was considered significant.
Results
Prevalence of Pain at Long-term Follow-up
Pain was extremely common among patients who underwent amputation for malignant musculoskeletal tumors more than 20 years ago; 17 of 21 patients reported phantom limb and back pain, and 15 patients reported residual limb pain in the past year. Phantom pain intensity was high, with a median (range) score of 7 (1 to 10) points on the 10-point numerical rating scale for pain (Fig. 1). Seventeen of 21 patients reported phantom pain in the past year, with eight patients grading their phantom pain as rare and seven patients rating their pain as occasional (Fig. 2). After giving the option to further describe their pain, 9 of 15 patients felt phantom pain especially in their toes, 4 of 15 patients felt most of their phantom pain in the foot, and 2 of 15 described more proximal localizations.
Fig. 1.
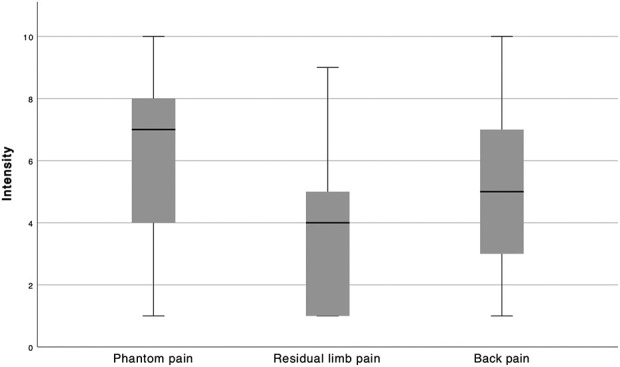
This graph shows the intensities of different forms of pain (phantom pain, residual limb pain, and back pain on the x axis) on a 10-point numerical rating scale (1 = no pain, 10 = most intense pain imaginable on the y axis) at the time of the survey,
Fig. 2.

This graph shows the frequencies of different forms of pain (phantom pain, residual limb pain, and back pain on the x axis) on a 5-point scale (0 = never, 5 = always on the y axis) at the time of the survey.
The median (range) score for the intensity of residual limb pain was 4 (1 to 9) points on the 10-point numerical rating scale for pain (Fig. 1). Fifteen of 21 patients reported residual limb pain, which was classified as rare by six patients, occasional by five, regular by three, and always by one (Fig. 2). Patients with more frequent residual limb pain also had more intense residual limb pain than those who had less frequent residual limb pain (95% CI 0.1 to 0.9; r = 0.6; p = 0.002). After being asked to further specify their residual limb pain, 3 of 8 patients stated they had pain in their whole residual limb, while the pain originated from the end of the residual limb in 2 of 8 patients and especially from pressure marks in 2 of 8 patients with lower limb amputation.
The mean score for back pain intensity was 4.6 ± 2.5 points on the 10-point numerical rating scale for pain (Fig. 1), with 17 of 21 patients reporting back pain in the past year. Back pain was described as rare by three patients, occasional by five patients, regular by five patients, most of the time by two patients, and always by two patients. No patients reported having severe restrictions to ADLs caused by back pain (Fig. 3).
Fig. 3.

This graph represents restrictions in activities of daily living (ADL) regarding different forms of pain (phantom pain, residual limb pain, and back pain on the x axis) on a 10-point scale (1 = no restrictions, 10 = inability to perform activities of daily living on the y axis) at the time of the survey.
Factors Associated with Prosthesis Use
After controlling for relevant confounding variables like age at amputation, age at survey, and length of the residual limb, we found that residual limb pain intensity (defined on a numeric rating scale with 1 = no pain, 10 = extremely strong pain [95% CI 0.3 to 1.0]; r = 0.8; p = 0.003) was associated with decreased daily prosthesis use. Higher amputation levels showed a decreased daily prosthesis use compared with patients who had lower amputation levels (transfemoral amputation versus knee disarticulation versus transtibial amputation [95% CI 0.3 to 5.1]; r = 0.5; p = 0.03). Patients wore their prosthesis for a median of 14 (range, 0 to 18) hours per day. Median (range) daily prosthesis use for the 14 patients with transfemoral amputations was 10 (0 to 17) hours, it was 15 (14 to 16) hours for the four patients after knee disarticulation, and 16 (14 to 18) hours for the three patients with transtibial amputations (Fig. 4). The median number of years that patients in this series wore prostheses was 38 years (14 to 55). One patient with 54 years of follow-up stopped wearing his prosthesis 40 years ago because he felt more comfortable using crutches. Nineteen of 21 patients had pressure marks on the residual limb. Patients owned a median of 6 (1 to 20) limb prostheses in the follow-up period, which indicates a prosthesis change at a median of every 5 years (1.5 to 54).
Fig. 4.

This graph represents the daily prosthesis use structured by amputation levels (transfemoral amputation, knee disarticulation, and transtibial amputation).
Discussion
Postamputation pain is a condition of varying influence and commonly limits patients’ abilities to perform ADLs. However, relatively little is known at very long-term follow-up after amputations for malignancy, in particular with respect to prosthesis use and subsidence or persistence of chronic pain. We found that phantom, residual limb, and back pain were very common after lower limb amputation for malignant tumors at long-term follow-up. We further found that residual limb pain and more proximal amputation levels led to decreased prosthesis use. This information should promote interdisciplinary collaboration, and it supports the need for regular residual limb inspections in patients with amputations even at long term, as regular prosthesis adjustment is needed to avoid skin breakdown and other painful prosthesis-related complications. This may also lead to higher daily prosthesis use.
Limitations
The most important limitation in a study of this design is selection bias. Indications for amputation during the study period were not standardized, and during that time, particularly during the latter portion, many patients underwent limb salvage. We believe that in general, amputation was used when the extremity was not regarded as salvageable, such as in large tumors with substantial loss of muscular function after resection, or in tumors surrounding critical structures. By contrast, limb salvage generally was used when surgeons believed functional results could be achieved with that approach. The definition of an unsalvageable limb evolved as methods of limb reconstruction developed, as closer resections could be performed, and as chemotherapeutic approaches advanced, especially in the latter part of the inclusion period [7].
Compared with patients lost to follow-up, included patients were younger, more likely to have had osteosarcoma, and were more likely to have received chemotherapy. This is a logical consequence of the inclusion criteria, as many older patients with amputations might not have survived 20 years after the index procedure. The fact that the demographics here skewed younger tended to result in a higher proportion of malignant tumors common to a younger patient population (such as osteosarcoma) being represented in this series; those tumors commonly are treated with chemotherapy. Readers should consider these confounding variables as they interpret the results of this report
With 21 patients, this study is fairly small. Because the mean time of amputation was 40 years before participants took the survey, most patients were young at the time of amputation. Although the study size limits its generalizability, we hope that the long follow-up duration answers questions that larger but shorter studies cannot. In addition, we performed a worst-case analysis, which assumed all 36 patients were alive and further assumed that none of the missing patients had residual pain; even in this stringent analysis, postamputation pain would be a serious problem, with 47% (17 of 36) of patients in this analysis reporting phantom pain and back pain and 42% (15 of 36) reporting residual limb pain. Insofar as missing patients often do worse than patients who are accounted for, this further strengthens the main message of our report. Regarding surgical procedures, a high number of transfemoral amputations and a low number of knee disarticulations and transtibial amputations were reported in this study population. Because there were only three recorded transtibial amputations, we cannot provide detailed information about correlations between mobility and the amputation level.
Because of the heterogeneity in chemotherapeutic drugs and radiotherapy protocols used in this series (a function of differences in the tumors treated as well as their sizes and locations), we could not analyze the potential impact of other treatments on postamputation pain. To observe these influences, a large case series limited to specific medication or radiotherapies would have been needed. However, many chemotherapeutic agents have pain-inducing neurotoxic effects, and this may contribute to chronic pain in these patients [3].
Prevalence of Pain at Long-term Follow-up
Pain was extremely common at long-term follow-up in this series among patients who underwent amputation for malignant musculoskeletal tumors; based on this, we think surgeons should exhaust available measures to prevent or mitigate postamputation pain, even in patients many years after amputation. These measures might range from conservative procedures, such as oral pain relievers, acupuncture, or mirror box training, to injection therapies or pain relief surgery, such as targeted muscle reinnervation and regenerative peripheral nerve interfaces [13, 16]. Although phantom pain was the most intense form of pain, the intensity and frequency of phantom pain varied widely. This result is similar to that of Morgan et al. [18], who reported that phantom pain, although common and often severe, had only a small impact on pain-related disability. In our study, the total proportions of patients experiencing phantom pain (81%), residual limb pain (71%), and back pain (81%) were comparable with those in other studies [8, 24, 27]. High pain levels even in the long-term have been reported before, with Esfandiari et al. [9] showing phantom pain in 63% and back pain in 69% of 587 patients at a mean of 22 years after amputation. Additionally, Smith et al. [27] reported phantom pain in 63%, residual limb pain in 76%, and back pain in 71% of 92 patients at a mean of 18 years after amputation. There are large differences in the definition and estimation of the prevalence and incidence of back pain [10]. In a cross-sectional study, Husky et al. [12] found that 38% of 17,249 individuals in a general population had back pain. In our study, with a prevalence of 81% and a mean pain frequency score of 2.2 points on a 5-point scale, back pain was the most frequently observed type of pain in patients undergoing lower limb amputation (Fig. 2). An increase in the prevalence of back pain compared with in a general population thus might be attributed to the amputation procedure. This increase in back pain is a known phenomenon in patients who undergo this procedure; Murray et al. [19] showed that patients accommodated their prosthesis by using alternate gait patterns as well as unphysiologic forms of joint stress and lower back compensation. Considering the high prevalence of back pain in patients who undergo lower limb amputation, prophylactic measures such as physiotherapy, weight control, and physical training should be regularly recommended postoperatively.
Recently, surgical techniques have been established to address postamputation pain [16, 17, 22]. Techniques such as targeted muscle reinnervation and regenerative peripheral nerve interfaces are supplemental surgical options for prophylactic and therapeutic pain treatment [13, 14]. Although long-term observations have not been performed, current studies of these newly implemented techniques have provided promising results for pain management [1, 16]. We found that even 20 years after lower limb amputation, chronic pain did not subside. Conservative treatments, which the patients presumably received during the long follow-up period, did not ease their pain to a low level. Thus, indications for pain relief surgery may be found during primary amputation and as a secondary procedure, even many decades later, but future studies will need to evaluate the efficacy of these approaches in this patient population.
Factors Associated with Prosthesis Use
Intense residual limb pain and more proximal amputation levels were associated with decreased daily prosthesis use at long-term follow-up. As residual limb pain is multifactorial and heavily influenced by prosthesis fitting and residual limb care, the high frequency of residual limb pain we observed should result in increased emphasis on regular interdisciplinary collaboration, including careful inspections of patients’ residual limbs by orthopaedic technicians and surgeons. Expert prosthesis refitting is especially recommended if a patient feels restrictions in daily prosthesis use are caused by pain. Although more proximal amputations were associated with less prosthesis use in our series, phantom limb and residual limb pain may not have been the reason because we did not find a correlation between the amputation level and phantom and residual limb pain. Associations between amputation levels and pain on daily prosthesis use were also found by Behr et al. [2], who reported decreased levels of prosthesis use in patients after knee disarticulation compared with those who had lower amputation levels, and Raichle et al. [23], who saw a connection between residual and phantom limb pain and daily prosthesis use.
Conclusion
Most patients with lower limb amputations experience pain that restricts them in terms of ADLs and decreases their daily prosthesis use, even many years after amputation. This information supports the need for regular residual limb inspections and careful prosthesis fitting even at long-term follow-up, as effective prosthesis fitting is a modifiable cause of residual limb pain. Future studies evaluating long-term treatment effects of pain-relief surgery should be performed and may help alleviate pain in patients with refractory postamputation pain.
Acknowledgments
We thank Ms. Manuela Gangl and her predecessor, Mag. Teresa Zettl, as register coordinators of the Medical University of Vienna’s Orthopaedic Department.
Footnotes
One of the authors (RW) receives personal fees in an amount more than USD 10,000 from Johnson & Johnson Medical Limited and personal fees in an amount more than USD 10,000 from Stryker European Operations Limited.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research®editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the Medical University of Vienna, Vienna, Austria (approval number 1041/2016).
This work was performed at the Medical University of Vienna, Vienna, Austria.
Contributor Information
Kevin Döring, Email: kevin.doering@meduniwien.ac.at.
Carmen Trost, Email: carmen.trost@meduniwien.ac.at.
Christoph Hofer, Email: hofer-christoph@aon.at.
Martin Salzer, Email: m.salzer@doctorsfordisabled.at.
Tryphon Kelaridis, Email: tryphon.kelaridis@gmail.com.
Reinhard Windhager, Email: reinhard.windhager@meduniwien.ac.at.
References
- 1.Alexander JH, Jordan SW, West JM, et al. Targeted muscle reinnervation in oncologic amputees: early experience of a novel institutional protocol. J Surg Oncol. 2019;120:348-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behr J, Friedly J, Molton I, et al. Pain and pain-related interference in adults with lower-limb amputation: comparison of knee-disarticulation, transtibial, and transfemoral surgical sites. J Rehabil Res Dev. 2009;46:963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffeen U, Sotomayor-Sobrino MA, Jiménez-González A, et al. Chemotherapy-induced neuropathic pain characteristics in Mexico's National Cancer Center pain clinic. J Pain Res. 2019;12:1331-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmond D, Gallagher P, Henderson-Slater D, et al. Pain and psychosocial adjustment to lower limb amputation amongst prosthesis users. Prosthet Orthot Int. 2008;32:244-252. [DOI] [PubMed] [Google Scholar]
- 5.Dwornik G, Weiß T, Hofmann GO, et al. Residual limb and phantom pain: causes and therapeutic approaches [in German]. Orthopade. 2015;44:435-444. [DOI] [PubMed] [Google Scholar]
- 6.Ehde DM, Czerniecki JM, Smith DG, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81:1039-1044. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF. An abbreviated history of orthopaedic oncology in North America. Clin Orthop Relat Res. 2000;374:115-124. [DOI] [PubMed] [Google Scholar]
- 8.Ephraim PL, Wegener ST, MacKenzie EJ, et al. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86:1910-1919. [DOI] [PubMed] [Google Scholar]
- 9.Esfandiari E, Yavari A, Karimi A, et al. Long-term symptoms and function after war-related lower limb amputation: a national cross-sectional study. Acta Orthop Traumatol Turc. 2018;52:348-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatoye F, Gebrye T, Odeyemi I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol Int. 2019;39:619-626. [DOI] [PubMed] [Google Scholar]
- 11.Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husky MM, Ferdous Farin F, Compagnone P, et al. Chronic back pain and its association with quality of life in a large French population survey. Health Qual Life Outcomes. 2018;16:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubiak CA, Kemp SWP, Cederna PS. Regenerative peripheral nerve interface for management of postamputation neuroma. JAMA Surg. 2018;153:681-682. [DOI] [PubMed] [Google Scholar]
- 14.Kubiak CA, Kemp SWP, Cederna PS, et al. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast Reconstr Surg. 2019;144:421e-430e. [DOI] [PubMed] [Google Scholar]
- 15.Larbig W, Andoh J, Huse E, et al. Pre- and postoperative predictors of phantom limb pain. Neurosci Lett. 2019;702:44-50. [DOI] [PubMed] [Google Scholar]
- 16.McNamara CT, Iorio ML. Targeted muscle reinnervation: outcomes in treating chronic pain secondary to extremity amputation and phantom limb syndrome. J Reconstr Microsurg. 2020;36:235-240. [DOI] [PubMed] [Google Scholar]
- 17.Mioton LM, Dumanian GA. Targeted muscle reinnervation and prosthetic rehabilitation after limb loss. J Surg Oncol. 2018;118:807-814. [DOI] [PubMed] [Google Scholar]
- 18.Morgan SJ, Friedly JL, Amtmann D, et al. Cross-sectional assessment of factors related to pain intensity and pain interference in lower limb prosthesis users. Arch Phys Med Rehabil. 2017;98:105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray AM, Gaffney BM, Davidson BS, et al. Biomechanical compensations of the trunk and lower extremities during stepping tasks after unilateral transtibial amputation. Clin Biomech (Bristol, Avon). 2017;49:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagarajan R, Neglia JP, Clohisy DR, et al. Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: what are the long-term implications? J Clin Oncol. 2002;20:4493-4501. [DOI] [PubMed] [Google Scholar]
- 21.Narres M, Kvitkina T, Claessen H, et al. Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: a systematic review. PLoS One. 2017;12:e0182081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prologo JD, Gilliland CA, Miller M, et al. Percutaneous image-guided cryoablation for the treatment of phantom limb pain in amputees: a pilot study. J Vasc Interv Radiol. 2017;28:24-34.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raichle KA, Hanley MA, Molton I, et al. Prosthesis use in persons with lower- and upper-limb amputation. J Rehabil Res Dev. 2008;45:961-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnik L, Ekerholm S, Borgia M, et al. A national study of veterans with major upper limb amputation: survey methods, participants, and summary findings. PLoS One. 2019;14:e0213578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schley MT, Wilms P, Toepfner S, et al. Painful and nonpainful phantom and stump sensations in acute traumatic amputees. J Trauma. 2008;65:858-864. [DOI] [PubMed] [Google Scholar]
- 26.Sinha R, Van Den Heuvel WJ. A systematic literature review of quality of life in lower limb amputees. Disabil Rehabil. 2011;33:883-899. [DOI] [PubMed] [Google Scholar]
- 27.Smith DG, Ehde DM, Legro MW, et al. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. 1999;361:29-38. [DOI] [PubMed] [Google Scholar]
- 28.Weiss GN, Gorton TA, Read RC, et al. Outcomes of lower extremity amputations. J Am Geriatr Soc. 1990;38:877-83. [DOI] [PubMed] [Google Scholar]


