Abstract
Background
The role of antibodies in coronavirus disease 2019 (COVID-19) in patients with X-linked agammaglobulinaemia (XLA) has yet to be characterised and clinical courses observed in this cohort of patients have been heterogeneous. Whilst some exhibit spontaneous recovery, others have experienced a more protracted disease length. Previous reports have described successful use of convalescent plasma, however there is a paucity of information around the use of the REGN-COV2 antibody cocktail in these patients.
Case report
A patient with XLA was admitted to hospital with COVID-19 and remained persistently symptomatic with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) swab positivity despite treatment with Remdesivir and dexamethasone. Attempts at modulating the immune response with anakinra were unsuccessful. Consent for compassionate use of REGN-COV2 was obtained with administration taking place on day 87 of his illness. This was followed by a period of convalescence and SARS-CoV-2 nasopharyngeal swab negativity. As a consequence of prolonged immunosuppression, the patient developed pneumocystis pneumonia.
Conclusion
This case highlights the role of antibodies in clearing SARS-CoV-2 in a hypogammaglobulinaemic host and demonstrates the consequences of prolonged immunosuppression and delayed treatment. We propose that this may be of particular significance given the capacity of SARS-CoV-2 to develop advantageous mutations in a chronically infected host.
Keywords: SARS-CoV2, COVID-19, Agammaglobulinemia, REGN-COV2, Remdesivir
Introduction
Despite large-scale efforts, few antiviral therapies have been identified to target severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This has far-reaching implications for those with impaired humoral (antibody-dependent) immunity. X-linked agammaglobulinemia (XLA), or Bruton’s agammaglobulinemia, is a primary immunodeficiency characterized by mutations in the Bruton tyrosine kinase gene leading to lack of production of mature B cells and hence lack of immunoglobulins. So far, clinical courses described in patients with XLA and coronavirus disease 2019 (COVID-19) have been heterogeneous. Case reports observing recovery in this cohort of patients (Soresina et al., 2020) suggest that a normal T-cell response may be sufficient to defeat the virus in some cases, however, others have observed a more protracted disease length. Accounts of successful use of convalescent plasma in the latter have highlighted the role of antibodies in inducing viral clearance (Buckland et al., 2020, Mira et al., 2020, Hovey et al., 2020, Jin et al., 2020).
Regeneron’s REGN-COV2 is a cocktail of two monoclonal neutralizing antibodies (REGN10987 and REGN10933) that target the SARS-CoV-2 spike protein. Previous human studies have demonstrated a greater reduction in SARS-CoV-2 viral load in non-hospitalized patients with COVID-19 treated with REGN-COV2 compared to placebo(Weinreich et al., 2021) and it is currently being evaluated in phase 3 trials in the United Kingdom (RECOVERY), where its use has not yet been authorized.
We report the case of a patient in London with XLA who experienced a prolonged disease course and recurrent nasopharyngeal SARS-CoV-2 RT-PCR swab positivity in which viral clearance was achieved following co-administration of REGN-COV2 and remdesivir which has not been documented in the case literature before.
Case report
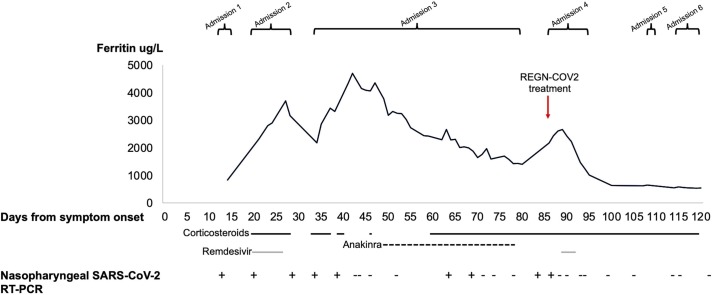
A 29-year-old man with XLA receiving monthly subcutaneous immunoglobulin injections was admitted to hospital with a 13-day history of cough and fever which had not improved on oral antibiotics. He had been well prior to this and had not had any complications related to his immunodeficiency for several years. On presentation, he was clinically well with normal vital signs and an unremarkable physical examination. Nasopharyngeal SARS-CoV-2 RT-PCR swab was positive on admission. He was not lymphopenic but inflammatory markers were elevated with a raised C-reactive protein (CRP) and serum ferritin (Fig. 1 ). A chest X-ray showed bilateral upper-mid zone shadowing. No treatment other than prophylactic dalteparin was administered and he was discharged home the following day (day 14 of symptoms).
Fig. 1.
Timeline of disease course and treatment from symptom onset. Abbreviations: Nasopharyngeal SARS-CoV-2 RT-PCR positive (+) or negative (−).
On day 20, he re-presented to hospital with worsening lethargy, fever of 39.6 °C and breathlessness on exertion. Blood tests at this point showed further increases in CRP and ferritin alongside a new rise in alanine aminotransferase (ALT). A repeated chest radiograph showed increased interstitial shadowing. He developed an oxygen requirement of 2 L/min and was started on a 10-day course of remdesivir alongside high-dose dexamethasone as per the hospital guidelines. We observed rapid clinical improvement with a reduction in CRP to within normal ranges. Remdesivir was ceased after 7 doses due to derangement in liver function tests and he was discharged on day 30. In spite of the use of remdesivir to enhance viral clearance, nasopharyngeal SARS-CoV-2 RT-PCR testing was positive at the beginning and end of admission with comparable cycle threshold values.
He clinically deteriorated following discharge, prompting readmission on day 34 with a return in oxygen requirement, fever and a CRP rise. SARS-CoV-2 RT-PCR testing remained positive. Blood cultures, urine cultures and respiratory virus PCR testing were negative. He received broad-spectrum antibiotics and a 5-day course of dexamethasone once again resulting in rapid improvement in oxygenation and defervescence, accompanied by a fall in CRP from 150 to 12 mg/L.
The recurrence of fevers and increasing ferritin levels upon withdrawal of dexamethasone prompted us to consider a COVID hyper-inflammation state driven by a dysregulated host response to ongoing SARS-CoV-2 infection (Mehta et al., 2020). A trial of the interleukin-1 (IL-1) antagonist anakinra was initiated on day 48 and, though titrated according to response, failed to achieve equivalent improvements in clinical and laboratory parameters to dexamethasone. Hence, he was weaned onto dexamethasone monotherapy which resulted in the termination of fevers. During this admission, the decision was made to source experimental treatment to promote viral clearance and thus a request was made to Regeneron for compassionate use of REGN-COV2 which was subsequently granted on day 73. Treatment was held due to consecutive nasopharyngeal SARS-CoV-2 RT-PCR swab negativity on days 72, 74 and 78, which, at the time, was thought to represent viral clearance. After a period of observation, he was discharged on day 80 to continue high-dose dexamethasone with outpatient follow-up.
Despite favourable clinical progression before his discharge, there was deterioration in the community with the reappearance of fevers, limb pain, and worsening shortness of breath on exertion. SARS-CoV-2 RT-PCR testing on day 83 returned positive and ferritin once again began to increase rapidly. Consent for experimental treatment with REGN-COV2 (dosed at 1200 mg of REGN10933 and 1200 mg of RGN10987) in conjunction with remdesivir was taken and administration took place on day 87. We continued remdesivir for 3 days at which point a rise in ALT to 155 IU/L prompted its cessation. The patient’s SARS-CoV-2 antibody titres had been undetectable in the months prior to REGN-COV2 treatment but were detected with comparable titres both 7- and 13-days post-infusion. There was an improvement in symptomatology following treatment reflected by a reduction in serum ferritin to near-normal levels. Four negative SARS-COV-2 RT-PCR swabs were obtained before discharge on day 95 to complete a steroid weaning regime.
Against hopes that this admission would be followed by a period of convalescence, the patient soon developed complications secondary to prolonged immunosuppression. He had two further hospitalisations in the following month with symptoms of fevers, cough and shortness of breath. We noted exertional dyspnoea accompanied by a significant oxygen desaturation. A high-resolution computed tomography chest scan showed ground-glass opacifications consistent with pneumocystis jirovecii pneumonia (PCP), and this diagnosis was confirmed on bronchoalveolar lavage sampling on day 119. All successive nasopharyngeal SARS-CoV-2 RT-PCR swabs (a total of 9) taken over the 34 days following REGN-COV2 infusion have not detected SARS-CoV-2 RNA. The patient underwent treatment with high dose clindamycin and primaquine due to an allergy to sulfa-containing drugs. There was resolution of the PCP infection and, at the time of writing, the patient remains COVID-19 free.
Discussion
There have been several case reports of young patients with XLA who have developed SARS-CoV-2 infection with an atypical course. Some have recovered spontaneously with no need for specific treatment(Buckland et al., 2020), whilst other case reports have discussed the successful use of remdesivir(Mira et al., 2020) and convalescent plasma(Mira et al., 2020, Hovey et al., 2020, Jin et al., 2020) in treating these patients. As yet, there is no data published on the use of REGN-COV2 or anakinra in patients with XLA. Although the clinical course of disease in patients with XLA has been heterogeneous, the severity has generally been mild to moderate without the need for admission to intensive care.
In this case, virological response to the first course of the antiviral agent, remdesivir, was limited, as exhibited by viral persistence on SARS-CoV-2 RT-PCR. We acknowledge that the efficacy of remdesivir treatment in this case may have been limited due to early discontinuation in response to liver injury. However, the profound improvements in clinical and laboratory markers observed after REGN-COV2 and remdesivir co-administration following such a prolonged duration of illness supports the notion that antibodies are fundamental in inducing viral clearance in certain patients. This is in keeping with recent cases where convalescent plasma has precipitated recovery in patients with XLA. We acknowledge that there are other factors present that may have contributed to this clinical outcome; however, we feel that these cases highlight the pressing need for further investigations to explore the utility of monoclonal antibodies in patients with congenital agammaglobulinemia. This is of particular significance given the capacity of SARS-CoV-2 to undergo mutation in the chronically infected immunocompromised host, with the potential to give rise to variants with increased virulence and transmissibility.
As a consequence of persistent SARS-CoV-2 infection, our patient experienced a continuous dysregulated host response to the virus. Corticosteroids, as used in this case, have been the mainstay of anti-inflammatory treatment in COVID-19. Due to static clinical progression, we proposed that an increase in pro-inflammatory cytokine release was contributing to this patient’s ongoing hyperinflammatory state. An attempt at immunomodulation with the IL-1 antagonist anakinra, however, did not appear to produce as effective a result as steroid treatment. Indeed, a recent randomized control trial found that it failed to improve outcomes in patients with mild to moderate COVID-19 disease (CORIMUNO-19 Collaborative group, 2021). Significantly, our patient went on to develop PCP as a result of prolonged immunosuppression. In such patients with impaired humoral immunity, concomitant use of an antibody treatment alongside an antiviral treatment should be considered early in the course of the disease to address the underlying cause of infection and obviate the need for prolonged and unnecessary immunosuppression. Advice should be sought from a doctor with expertise in primary immunodeficiency. If immunosuppression is required, additional considerations should include rigorous monitoring for opportunistic infection and use of antibiotic prophylaxis.
CRediT authorship contribution statement
Hanna Nguyen: Conceptualization, Writing – original draft, Visualization. Jo Salkeld: Conceptualization, Writing – original draft. Sangita Agarwal: Writing – review & editing, Supervision. Anna Goodman: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank David Stein at Regeneron USA and Martin Bradley (Pharmacist, Guy’s and St. Thomas’ NHS Foundation Trust) for coordinating the activities that led to the procurement of REGN-COV2. Many other doctors were involved in the patient's care including (but not limited to) Dr Geraldine O'Hara, Dr Boris Lams, Dr William Newsholme, Dr Jennifer Roe and we received helpful advice from Dr James Galloway and Dr David Lowe. We thank the patient for enabling the sharing of his case.
References
- Buckland M.S., Galloway J.B., Fhogartaigh C.N., et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey, J.G., Tolbert, D., Howell, D., 2020. Burton’s Agammaglobulinemia and COVID-19. Cureus; Available at: https://www.cureus.com/articles/40472-burtons-agammaglobulinemia-and-covid-19 (accessed 4 February 2021). [DOI] [PMC free article] [PubMed]
- CORIMUNO-19 Collaborative group Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet. Respir. Med. 2021;9(3):295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Reed J.C., Liu S.T.H., et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J. Allergy Clin. Immunol. Practice. 2020;8(10):3594–3596.e3. doi: 10.1016/j.jaip.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira E., Yarce O.A., Ortega C., et al. Rapid recovery of a SARS-CoV-2–infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J. Allergy Clin. Immunol. Practice. 2020;8(8):2793–2795. doi: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soresina A., Moratto D., Chiarini M., et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020;31(5):565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl. J. Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]