Abstract
Fruits, vegetables, spices, and herbs are a potential source of phenolic acids and polyphenols. These compounds are known as natural by-products or secondary metabolites of plants, which are present in the daily diet and provide important benefits to the human body such as antioxidant, anti-inflammatory, anticancer, anti-allergic, antihypertensive and antiviral properties, among others. Plentiful evidence has been provided on the great potential of polyphenols against different viruses that cause widespread health problems. As a result, this review focuses on the potential antiviral properties of some polyphenols and their action mechanism against various types of viruses such as coronaviruses, influenza, herpes simplex, dengue fever, and rotavirus, among others. Also, it is important to highlight the relationship between antiviral and antioxidant activities that can contribute to the protection of cells and tissues of the human body. The wide variety of action mechanisms of antiviral agents, such as polyphenols, against viral infections could be applied as a treatment or prevention strategy; but at the same time, antiviral polyphenols could be used to produce natural antiviral drugs. A recent example of an antiviral polyphenol application deals with the use of hesperidin extracted from Citrus sinensis. The action mechanism of hesperidin relies on its binding to the key entry or spike protein of SARS-CoV-2. Finally, the extraction, purification and recovery of polyphenols with potential antiviral activity, which are essential for virus replication and infection without side-effects, have been critically reviewed.
Keywords: Agri-food residues, Phenolic compounds, Antiviral activity, Antioxidant properties, Viral diseases, Polyphenol recovery
Graphical abstract
1. Introduction
Since ancient times, plants have played an important role for humanity, for example as food, clothing, perfumes and/or medicines (e.g., drugs in traditional medicine). Already in 1978, the World Health Organization (WHO) highlighted the need of scientific research in traditional medicine. Since then it has begun to put in value this type of medicine, as well as to investigate the efficacy of the mechanism of action and chemical bases of traditional herbal medicine for the development of new drugs, and also within the antiviral property that some plants possess (Ruwali et al., 2013; World Health Organization, 1978). Additionally, it is known that plants produce secondary metabolites such as polyphenols to protect themselves from the plethora of biotic and abiotic stresses. Biotic stress can be weeds, insect pests, fungi, and other microorganisms, whereas abiotic stresses can be physical and environmental conditions like salinity, drought, UV radiation, extreme temperatures, and toxic metals (Tuladhar et al., 2021). Polyphenols are not only involved in the defense mechanism of the plant system, but also, they have been found, for example, in the cell division, photosynthetic activity, reproduction, hormonal regulation, and nutrient mineralization mechanisms (Sharma et al., 2019). Thus, for instance, coumarins and tannins reduce stress on plants by repelling herbivores (Lattanzio, 2013).
Infections caused by viruses in humans are a critical and vitally important issue, as has been demonstrated during the last year with the 2019-nCovid caused by SARS-CoV-2 (severe acute respiratory syndrome corona virus-2) (Gorbalenya et al., 2020), in particular to safeguard the health of the population and mitigate its impact on economic and social vectors. Therefore, antiviral agents (e.g., vaccines containing specific virus, or specific antibody with protective therapeutic effect, or plants with antiviral activity) are reported to fight viral diseases, and some cases have been eradicated such as: i) smallpox, poliomyelitis (>99%); and ii) endemic measles, rubella and congenital rubella syndrome practically eliminated from America since 2010 thanks to vaccination (Plotkin et al., 2012). But although immunisation and drug development have been progressing for decades, this has not been enough, as many viruses do not have preventive vaccines and, worse still, traditional antiviral treatments such as drugs with unnatural components (e.g., acyclovir for herpes) may lead to the generation of viral mutants (Lin et al., 2014).
Besides, some drugs used to combat viral diseases can cause human health hazards due to their viral resistance or, in some cases, low efficiency. In addition, viruses are made up of DNA or RNA enclosed in protein capsules (Kamboj et al., 2012), which can invade human body cells and use components from those cells for their replication. This process often damages or destroys infected cells, causing a viral disease (Rouse and Sehrawat, 2010).
On this basis, it is necessary to find alternatives to the traditional treatments for viral diseases (El-Toumy et al., 2018). For example, recently, the use of bioactive compounds like polyphenols has been proposed as an alternative treatment due to their potential health benefits. These substances can be found in vegetables and fruits and can also be recovered from secondary sources, such as agri-food residues (Montenegro-Landívar et al., 2021; Tapia-Quirós et al., 2020). Scientific publications have detailed more than 500 different polyphenols in more than 400 food components (Galanakis, 2018). Additionally, based on epidemiological research and associated meta-analyses, the long-term consumption of diets rich in polyphenols from plants are considered as tools that could offer protection against for example to cardiovascular diseases, neurodegenerative diseases, diabetes development, osteoporosis, and cancers development among others (Pandey and Rizvi, 2009). Therefore, polyphenols are widely promoted to be used as functional foods or in drugs preparations accepted for human consumption around the world (Cheynier, 2012; Shahidi and Ambigaipalan, 2015; Cory et al., 2018).
Recently, Russo et al. (2020), have proposed that alternative treatments to antiviral drugs may also represent a valid approach for the 2019-nCovid disease. It is also stressed that polyphenols, specifically flavonoids, can act satisfactorily in various stages of the coronavirus entry and replication stages. Another example is catechin epigallocatechin-3-gallate (EGCG), present in green tea leaves, which inhibits replication of DNA viruses such as herpes simplex (oral dose of 800 mg), and HIV-1 (concentration ranging from 25 to 250 μmol/L), among others (Steinmann et al., 2013). Moreover, the half-maximal inhibitory concentration (IC50) of 47–73 μM of luteolin, hesperetin, and quercetin, among others flavonoids, can inhibit key proteins (PLpro, 3CLpro) involved in the infectious cycle of SARS coronavirus (Nguyen et al., 2012; Soukhova et al., 2004). More examples can be found as described below, where a list of some types of viruses could be inhibited by phytochemicals such as polyphenols. Of the diseases caused by harmful viruses where the use of polyphenols has been critically reviewed and shown an antiviral activity against them, kaempferol and quercetin, extracted from Broussonetia papyrifera, have shown activity against MERS-CoV and SARS-CoV-1 viruses; and hesperetin and naringenin block the replication of Sindbis virus. Thus, these are examples which, with future developments, could be approved as alternative treatments. In view of above, the main objective of this comprehensive review is to compile and evaluate the studies that have demonstrated the antiviral activity of polyphenols, the postulated mechanisms of action of polyphenols to defeat viruses as well as the synergy effect of antiviral and antioxidant properties against viral diseases. Additionally, the extraction, purification and recovery technologies to produce polyphenols from natural products and/or agri-food waste are also evaluated, since they could be limiting steps for the application of polyphenol molecules as an alternative against viruses on a large scale.
1.1. Therapeutic tools against viral diseases
The type of virus diseases and the specific virus considered in the review are summarized in Table 1 , and includes: i) respiratory infections: influenza virus, coronavirus, rhinovirus, and syncytial virus; ii) gastrointestinal infections: rotavirus; iii) hepatic infections: hepatitis virus, Epstein-Barr virus, human cytomegalovirus, and herpes virus; iv) exanthematous infections: varicella-zoster virus; v) neurologic infections: rabies virus and poliovirus; vi) haemorrhagic fevers: dengue virus and Sindbis virus; vii) immune system infections: human immunodeficiency virus; and viii) multisystem disease: coxsackie virus.
Table 1.
Different families and type of viruses, their specific virus and the role of polyphenols as a possible alternative to treat virus.
| Type of virus | Specific virus | Disease characteristics | Conventional treatment | Alternative treatment with polyphenols | Reference |
|---|---|---|---|---|---|
| Respiratory infections | Influenza virus (A, B and C) | Annually responsible for high mortality in both humans and animals worldwide | NA inhibitors and M2 protein channel blockers after infection, while vaccination is the most effective therapy | 1,2,3,4,6-Penta-O-galloyl-ß-d-glucose (IC50 of 2.36 μg/mL) purified from Echinacea purpurea, Phyllanthus emblica Linn inhibits virus replication | (Fox and Christenson, 2014; Liu et al., 2011; Moscona, 2008) |
| Coronavirus (HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and the novel SARS-CoV-2) | Respiratory tract infections in humans with outbreaks around the world, especially in winter | Currently there are no specific treatments for the CoV infection and preventive vaccines are being developed | Polyphenol extract from Echinacea purpurea against SARS-CoV-2 provided 50% of inhibition Kaempferol and quercetin from Broussonetia papyrifera against MERS-CoV and SARS-CoV effectively inhibited with an IC50 of 27.9 μM and 30.2 μM, respectively |
(Chiow et al., 2016; Liu et al., 2020; Park et al., 2017; Signer et al., 2020) | |
| Rhinovirus | The main cause of the common cold, among other respiratory diseases, also producing shortness of breath in asthmatic people, acute otitis and bronchiolitis | There are no vaccines or antiviral agents for the prevention or treatment of this virus | Resveratrol showed a therapeutic approach to reduce infection when the IC50 was 50 μM Gallic acid, extracted (100 μg/mL) from Woodfordia fruticose flowers, reported 55% virus inhibition |
(Choi et al., 2010; Mastromarino et al., 2015; Ruuskanen et al., 2013) | |
| Syncytial virus | Causes infections in infants and the elderly, causing not only acute morbidity but also recurrent breathing problems | There is no safe and effective treatment. Corticosteroids was treated children of preschool-age who had early bronchitis, but the results were not satisfactory and failed to reduce the infection or breathing problems |
Resveratrol (IC50 189 pg/mL) inhibits 40% virus replication and down-regulates the TIR-domain-containing adapter-inducing interferon-β (TRIF) complex, which sends signals for the activation of innate immune cells | (Beigelman et al., 2014; Tagarro et al., 2014; Xie et al., 2012) | |
| Gastrointestinal infections | Rotavirus | Causes dehydrating gastroenteritis, especially in children under five years of age | There is a vaccine against rotavirus but annually the mortality is around 200,000 deaths worldwide. The treatment focuses on dehydration and not on the use of antiviral agents | Polyphenols (licocoumarone, glycyrin, among others) extracted from Glycyrrhiza uralensis root (EC50 18.7-69.5 μM) can inhibit 50% virus absorption and replication after the cell's entry | (Crawford et al., 2017; Cushnie and Lamb, 2005; Kwon et al., 2010) |
| Hepatic infections | Hepatitis virus (A, B and C) | Cause high morbidity and mortality around the world | Anti-hepatitis virus drugs are members of nucleotides or nucleoside analogs, which inhibit the activity of polymerase or reverse transcriptase, but the prolonged use giving rise to the existence of mutant viruses | Curcumin (150 μM) inhibits hepatitis B virus | (Mouler Rechtman et al., 2010; Sukowati et al., 2016; Yugo et al., 2016) |
| Epstein–Barr virus | Infects human epithelial and lymphoid cells. Infection is associated with a number of human cancers, such as Hodgkin's disease | A vaccine is not yet approved | (−)-Epigallocatechin gallate (EGCG) extracted from green tea (50 μM) blocked the EBV lytic cycle, inhibiting the transcription of immediate-early genes in a range of 40–50% | (Abba et al., 2015; Chang et al., 2003; Cohen, 2018) | |
| Human cytomegalovirus | Not present obvious symptoms, but infection causes morbidity and mortality in transplant recipients or patients with acquired immunodeficiency syndrome (AIDS) | Drugs such as cidofovir, valganciclovir and ganciclovir, which target viral DNA polymerase, but their side-effects include long-term toxicity, low bioavailability, plus drug resistance to the virus | Curcumin, using a low dose of 0.2 μg/mL, inhibits virus protein expression | (Ahmed, 2012; Evers et al., 2005; Lv et al., 2014) | |
| Herpes simplex virus (HSV-1 and HSV-2) | Responsible for orolabial and genital diseases producing, in general, benign lesions but, in some cases, putting the life of patients at risk if the infections are recurrent | There is no vaccine and existing drugs (e.g., acyclovir) do not eradicate the virus infection and cause resistance to drugs | Ent-epiafzelechin-(4 ∝ → 8)-epiafzelechin extracted from Cassia javanica leaves (250 μM of) inhibits more than 90% of HSV-2 penetration to the host cell | (Cheng et al., 2006; Morfin and Thou, 2003; Piret and Boivin, 2011) | |
| Exanthematous infections | Varicella-zoster virus | Causes fever and vesicular rash. Once the disease has disappeared, the virus enters into a state of latency, but it can be reactivated due to stress, causing herpes zoster and acute pain in latently infected lymph nodes | Generally, uses drugs such as acyclovir, valaciclovir, etc., which are often combined with analgesics for pain and corticosteroids for inflammation | Resveratrol (219 μM of) inhibits 100% virus replication | (Docherty et al., 2006; Johnson and Whitton, 2004) |
| Neurologic infections | Rabies virus | Causes an acute and fatal neurological infection in humans and mammals | Disease can be prevented by vaccination | Tannin pentagalloylglucose (PGG) (10 μM) for 24 h possess significant anti-RABV activity; PGG can reverse the expression of miR-455-5p (a microRNA whose excess production regulates host cell signalling pathways and innate immune responses) | (Fisher et al., 2018; Riedel et al., 2019; Tu et al., 2019) |
| Polio virus | The virus drains into the cervical and mesenteric lymph nodes and then into the blood, causing a transient viremia | The incidence has been largely reduced especially by the use of a vaccine, but the disease is still endemic in Africa and Asia | Extract of Avicennia marina leaf, the IC50 was 145.7 μg/mL before and 314.3 μg/mL after attachment stages of virus replication, with a cytopathic effect of 50% in both stages | (Felipe et al., 2006; Racaniello, 2006; Zandi et al., 2009) | |
| Haemorrhagic fevers | Dengue virus | Causing from a mild fever to haemorrhagic fever, nausea, joint pains, etc. | There are no effective vaccines, and the prevention options available for the control of the virus infection are very limited | Baicalein (IC50 was 7.14 μg/mL) potent antiviral agent against adsorption in the host and after entry viral replication, and IC50 of 1.55 μg/mL presents a virucidal effect | (Zandi et al., 2011, Zandi et al., 2012) |
| Sindbis virus | Cause of disease outbreaks in humans in South Africa and Northern Europe | There are no vaccines or therapeutic means | Hesperidin and naringenin with a 50% inhibitory dose (ID50) of 20.5 μg/mL and 14.9 μg/mL respectively, reaching 50% for hesperidin and up to 80% for naringenin of virus replication inhibition | (Ling et al., 2019; Paredes et al., 2003) | |
| Immune system infection | Human immunodeficiency virus (HIV-1 and HIV-2) | Spreads through certain body fluids and attacks the immune system, destroying T lymphocytes. Thus, the body loses its ability to fight infections and diseases | Since HIV was discovered, there has been no preventive vaccine for virus infection, and the applied treatment is antiretroviral therapy drugs which help control the multiplication of HIV in infected patients | Tricyclic coumarin compound from Calophyllum brasiliense stem bark (IC50 was 8.44 μM) inhibits virus replication by suppressing nuclear factor-kappa B (a protein complex that controls DNA transcription) activation | (Bhatti et al., 2016; Häggblom et al., 2016; Kudo et al., 2013; Lin et al., 2014; Sundquist and Kräusslich, 2012) |
| Multisystem diseases | Coxsackie virus | Causes muscle injury, paralysis and death | There is no specific treatment or vaccine available | Apigenin ((EC50 of 9.7 mg/L) and ursolic acid (EC50 of6.6 mg/L) extracted from Ocimum basilicum interfere with virus replication after infection | (Bedard and Semler, 2004; Chiang et al., 2005; Wong et al., 2013) |
In view of the examples described in Table 1, there are a large number of viruses, which can affect human health to different extent. For this reason, it is important to identify new therapeutic and functional strategies using natural sources through bioactive compounds and, more specifically, polyphenols. Furthermore, it is worth noting that polyphenols extracted from plants can efficiently inhibit the different stages of replication of various viruses in a dose-dependent matter.
2. The potential of phenolic acids and polyphenols as antiviral agents
Plants not only have the function of feeding human beings, but have also been used since ancient times as a source of therapeutic agents. According to Naithani et al. (2008), up to 80% of the world population uses plants as alternative medicine for various reasons such as their well-known antiviral features. This claimed activity is due to a wide variety of bioactive compounds present, such as polyphenols, proteins, and terpenoids, among others (Kamboj et al., 2012). Although, polyphenols are common components of the human diet, it has been reported that polyphenols are also toxic due to their biocidal activity at intake concentrations between 1 and 5% of the total daily diet (Galanakis, 2018). Considering the significant amounts of compounds that a person must be consumed, being approximately between 0.025 and 1 g per day (Scalbert and Williamson, 2000), and their multitude activities, it should be noted that they could play an important role in the prevention of numerous diseases, including antivirals. However, it is necessary to consider that despite many promising results obtained in vitro or animal experiments, there is still not enough convincing evidence from human studies, especially with large populations. More research is needed to better understand the value of therapeutic polyphenols, dietary polyphenols, and in the context of their ability to prevent the progression of diseases caused by viruses (Koch, 2019; Martin, 2009; Yang et al., 2020).
The focus of this review is on phenolic compounds and polyphenols, which have a common structural feature consisting of the presence of one or more hydroxyl groups attached to a benzene ring. Polyphenols can be classified into different classes based on their chemical structure, ranging from simple to highly polymerized compounds. Their fundamental physiological functions deal with the growth and reproduction of plants, as well as protection against pathogenic organisms and ultraviolet radiation. In addition, polyphenols strongly influence the organoleptic characteristics of food products, such as color and flavour (Ignat et al., 2011).
Polyphenols are often classified into four main families, namely: phenolic acids, flavonoids, stilbenes and lignans (Saurina and Sentellas, 2015). The basic chemical structure and examples of these polyphenol families are collected in Table 2 .
Table 2.
List of relevant polyphenol classified according to their structure (adapted from Saurina and Sentellas, 2015).
| Class | Structure | Substitutions | Examples |
|---|---|---|---|
| Phenolic acids Hydroxybenzoic acids |
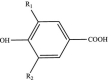 |
R1: H, OH, OCH3 R2: H, OH, OCH3 |
Gallic acid Vanillic acid Procyanidin B1 Theogallin |
| Hydroxycinnamic acids | 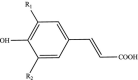 |
R1: H, OH, OCH3 R2: H, OH, OCH3 |
Caffeic acid Ferulic acid p-Coumaric acid Rosmarinic acid |
| Flavonoids Flavonols Flavones Flavanones |
 |
R1: H, OH R2: H, OH R3: H, OH R4: H, OH R5: OH, OCH3 R6: H, OH |
Hesperidin Naringenin Quercetin Kaempferol Luteolin |
| Anthocyanidins |  |
R1: H, OH R2: OH, OCH3 R3: OH R4: H, OH R5: OH R6: H, OH |
Cyanidin Pelargonidin |
| Catechins |  |
R1-R3: OH R4: H, OH R5: OH R6: H, OH |
Catechin Epicatechin Epigallocatechin |
| Isoflavones |  |
R1: OH R2-R3: H, OH |
Genistein Daidzein |
| Chalcones | 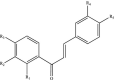 |
R1-R5: H, OH | Xanthohumol Phloretin Isosalipurpurin |
| Lignans | 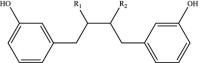 |
R1-R2: H, OH | Enterodiol Matairesinol |
| Stilbenes | 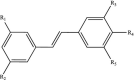 |
R1-R4: H, OH, OCH3 R5: H, OH |
Resveratrol Piceatannol |
As shown in Table 2, polyphenols have a great structural diversity as a function of the number of phenol rings that they contain and the elements that bind these rings. Flavonoids are the largest and most studied group.
Phenolic acids possess a high antioxidant capacity and their medical properties, such as vasodilatory, antibacterial, antiviral, anticarcinogenic and anti-inflammatory, have been reported elsewhere (Oroian and Escriche, 2015).
An important derivation from hydroxybenzoic acids are the so-called hydrolysable tannins, which are mostly present as phenolic polymers with different molecular weights, from 500 to 3000 Da (Andronescu and Grumezescu, 2017). As their principal characteristic, tannins precipitate proteins, thus contributing to regenerate, for example, a burn tissue, besides their antimicrobial, antioxidant and antiviral properties (Haminiuk et al., 2012). Their antiviral activity against Epstein–Barr virus DNA polymerase has been demonstrated, and especially those tannins extracted from mouse-tail plant (Phyllanthus myrtifolius) and chamber bitter (Phyllanthus urinaria) (Naithani et al., 2008).
Regarding flavonoids, they represent the largest amount of polyphenols (up to 60%) consumed in the human diet (Brglez Mojzer et al., 2016). Actually, more than 9000 different flavonoids have been reported, having important benefits on human health because of their antiviral, anti-inflammatory and antidiabetic attributes (Zhang et al., 2015; Krych and Gebicka, 2013; Tian et al., 2013; Ragab et al., 2014). Flavonoids have been studied against the type-1 and type-2 herpes simplex virus, and against the human immunodeficiency virus (HIV-1 and HIV-2) (Naithani et al., 2008).
Finally, stilbenes (including curcuminoids) and lignans have been extensively studied because of their antioxidant properties, but their antiviral activity is not far behind. These compounds and their derivates have been studied against viruses such as herpes simplex (type-1 and type-2), HIV, influenza, and human papilloma, among others (Naithani et al., 2008; Abba et al., 2015).
A list of polyphenols with antiviral activity, the type of virus against which they act, and their plant source is collected in Table 3 .
Table 3.
Summary of relevant polyphenols present in plants with antiviral activity according to the reviewed publications.
| Plant source | Polyphenol | Type of virus | Reference |
|---|---|---|---|
| Berries, tea, almond, beans, tomato, Ficus carica L., capers, caraway, cloves, cumin, Cambuci | Kaempferol | Coronavirus, rotavirus, human cytomegalovirus, HSV-1 and HSV-2, coxsackie B virus | (Naithani et al., 2008; Kamboj et al., 2012; Russo et al., 2020; Watson et al., 2013; Haminiuk et al., 2012) |
| Propolis, Oroxylum indicum | Chrysin | Coronavirus, rotavirus, human cytomegalovirus, HSV-1 and HSV-2, coxsackie B virus | (Cheng and Wong, 1996; Kumar and Pandey, 2013; Cushnie and Lamb, 2005) |
| Euphorbia cooperi, Morus alba, Rhus succedanea | Catechin | HIV, HSV-1 | (Kamboj et al., 2012; Cushnie and Lamb, 2005; El-Toumy et al., 2018) |
| Citrus spp., cocoa, fish mint (H. cordata), Spondias mombin, Spondias tuberosa | Quercetin | Rabies virus, poliovirus, syncytial virus, HSV-2, respiratory syncytial virus, dengue virus, coronavirus | (Suárez et al., 2010; Silva et al., 2011; Zandi et al., 2011; El-Toumy et al., 2018; Chiow et al., 2016) |
| Betula pendula, apple | Quercitrin | Rabies virus, HSV-1, influenza virus | (Kumar and Pandey, 2013; Suárez et al., 2010) |
| Spondias spp., Pavetta owariensis (bark) | Rutin | Rabies virus, influenza virus, dengue virus | (Kamboj et al., 2012; Cushnie and Lamb, 2005) |
| Citrus spp., peppermint, grapefruit | Hesperidin | Influenza virus, HSV, poliovirus, syncytial virus, SARS-CoV-2 | (Mhatre et al., 2020; Bellavite and Donzelli, 2020) |
| Chamomile, parsley, oregano, thyme, grapefruit, orange, onion, mango | Apigenin | HSV-1, HIV | (Kumar and Pandey, 2013; Kamboj et al., 2012) |
| Citrus spp., tomato, aromatic plants | Naringin | Respiratory syncytial virus | (Kumar and Pandey, 2013) |
| Broadleaf plantain (Plantago major), papaya, peach, avocado | Caffeic acid | HIV, HSV | (Pommier et al., 2005; Sytar et al., 2021) |
| Broccoli, rosemary, pistachio, lentils, olive, artichoke, lemon, Aloe vera | Luteolin | HSV-1 and HSV-2 | (Naithani et al., 2008; Lopez-Lazaro, 2008) |
| Berries, pomegranate, walnuts, pecans | Ellagic acid | Dengue virus, hepatitis A and B | (Kang et al., 2006; Kamboj et al., 2012) |
| Grape, berries, peanuts | Resveratrol | Influenza A, hepatitis C virus, respiratory syncytial virus, varicella-zoster virus, Epstein–Barr virus, HSV, HIV | (Docherty et al., 2006; Mastromarino et al., 2015) |
As shown in Table 3, polyphenols with antiviral activity such as quercetin, rutin, hesperidin, apigenin, catechin, and morin are present in abundance in plants like fruits (e.g., berries, citrus fruits, tropical fruits), popular beverages (e.g., green tea, coffee), vegetables (e.g., spinach, beans, onions, olives), spices and herbs (e.g., turmeric, rosemary, ginger), which are consumed in the daily human diet (Brglez Mojzer et al., 2016). Currently, the antiviral activity of various polyphenols makes their study more attractive; for example, 3 mg/kg body weight of curcumin, extracted and purified from turmeric, is sufficient to inhibit HIV (Praditya et al., 2019; Barthelemy et al., 1998; Haslberger et al., 2020).
2.1. Polyphenol recovery from secondary sources
Phenolic compounds can also be obtained from by-products of plant processing, being cheap and easily available to recover them following a circular economy strategy (Tapia-Quirós et al., 2020; Montenegro-Landívar et al., 2021). In addition, the growing interest in polyphenols recovery has led to the study of different technologies that can allow their extraction without losing their antiviral properties (Dzah et al., 2020). Successful cases of extraction and recovery of polyphenols with antiviral activity is summarized in Table 4 .
Table 4.
Applications of extracted polyphenols from different plant sources to treat several target viruses.
| Antiviral polyphenol | Plant source | Target virus | Extraction technique | Purification technique | Study effectiveness against virus | Results | Scaling-up | Reference |
|---|---|---|---|---|---|---|---|---|
| 1,2,3,4,6-Penta-O-galloyl-ß-d-glucose (PGG) | Pomegranate | Influenza A (H1N1) | Maceration | Sephadex LH-20 column | EC50 2.36 ± 0.29 μg/mL of PGG 5 or 8 h upon infection | Significant inhibition virus release | Lab scale | (Liu et al., 2011) |
| Extract rich in polyphenols | Magnolia officinalis bark | Influenza A (H1N1) | PLE | – | In vivo oral administration (10 and 20 mg/kg) for 5 days | Infected mice reduce the production of nitric oxide, pro-inflammatory cytokines, TNF-α and IL-6 | Pilot scale | (Wu et al., 2011) |
| Isoquercetin (quercetin glucoside form) | Hypericum perforatum, Equisetum arvense L. | Influenza A (H1N1) | Maceration | – | In vivo administrated intraperitoneal | Reduce virus titres and pathological changes in lungs of mice infected with influenza A (H1N1) by up to 20-fold at 1:500 (Equisetum arvense L.) or 1:1000 dilutions (Hypericum perforatum) at 24 h post-inoculation | Pilot scale | (Kim et al., 2010) |
| Baicalein | Scutellaria baicalensis root | Influenza H1N1 | Maceration | – | In vivo oral administration | Infected mice showed significant therapeutic activities, including death prevention and lung virus titre reduction | Pilot scale | (Xu et al., 2010) |
| Quercetin, kaempferol, myricetin, quercetin-3-O-galactoside, morin, apigenin, catechin, epicatechin, caffeic acid and rimantadine | Geranium sanguineum aerial roots | Influenza (H3N2) | Maceration | – | Administered in aerosol way (dose 5.4 mg/mL) | Around 70% was the protective index and the survival time was in a range of 2.9–4.9 days, the animal lung infectious virus titre was reduced in comparison with control | Pilot scale | (Serkedjieva et al., 2008) |
| Epigallocatechingallate (EGCG), epigallocatechin (EGC), epicatechingallate (ECG), epicatechin (EC) and catechin gallate | Tea | Influenza | Maceration | – | 76 adult persons around 65 years old gargling 200 mg/mL 3 times daily for 3 months | The catechin-treated group have lower incidence of influenza infection than the control group | Pilot scale | (Yamada et al., 2006) |
Table 4 lists not only the application of polyphenols extracted from different plant sources against numerous target viruses, but also the different extraction techniques integrated, being maceration the most used. Additionally, most of the trials were carried out on a pilot scale.
The polyphenols extraction from plants or from their processing wastes can be achieved by conventional (e.g., mechanical stirring) and enhanced (e.g., ultrasound and microwave assisted) solid-liquid extractions or by combination of both extraction approaches by means of organic and/or aqueous solvents. For their subsequent purification implies a preliminary stage of clean-up and concentration by using sorption on resins, or pressure-driven membrane processes such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO) followed by a final purification by using extraction chromatography (Bottino et al., 2020; Charcosset, 2016).
As mentioned, the commonest technique for polyphenols extraction is maceration (e.g., a solid-liquid process). For example, Edziri et al. (2012) used maceration for polyphenol extraction from Marrubium deserti. The dried product (250 g) was extracted with methanol, butanol, chloroform and ethyl acetate (using a feed to solvent ratio of 1:10) for an extraction time of five days. Results from antiviral activity tests concluded that the extracts with methanol and ethyl acetate showed significant antiviral activity against coxsackie B3 virus with IC50 of 100 and 135 μg/mL, respectively.
Magnetic agitation at 20 °C has been applied to recover active components from apple pomace (10 g) using 100 mL of 70% acetone and 80% methanol in darkness. The results showed that acetonic and methanolic extracts could inhibit the replication of HSV-1 and HSV-2 by more than 50% (Suárez et al., 2010).
The purification of plant extracts is, in general, a complex process and no single method is complete enough. It requires a combination and integration of them to achieve the highest separation and purification factors. A successful example is the study by Zahoor et al. (2020), who compared the separation efficiency of quercetin extracted from Rubus fruticosus by using RO and NF membranes. Quercetin is used against rabies virus, poliovirus, syncytial virus, and HSV-2, among other viruses (Suárez et al., 2010; Silva et al., 2011; Zandi et al., 2011; El-Toumy et al., 2018; Chiow et al., 2016). The results obtained indicated that the RO membrane accomplished a quantitative recovery (e.g., >99%) of the drainage pipe, while NF membranes achieved a 95% of polyphenol recovery. The study shown that the cost of use the RO membranes is higher, due to the higher energy consumption than the use of NF membrane stage followed by a sorption stage of the remaining 5% of the permeate stream by using magnetic carbon nanocomposite.
It should be noted that the phenolic compounds recovered from extracts, such as kaempferol, luteolin, chrysin, gallic acid, ferulic acid, catechin, anthocyanins among others, could be used as a treatment and/or prevention against virus infection (Singh et al., 2020; Kumar and Goel, 2019; Watson et al., 2013; Marín et al., 2015).
2.2. Economic prospects of polyphenol recovery
Taking into account the different antiviral applications of polyphenol extracts, as well as the need to investigate innovative extraction and purification procedures, it is interesting to mention some examples of the economic evaluation of the recovery of polyphenols, which is also applicable to antiviral polyphenols.
The manufacturing cost (COM), expressed as €/kg per year, of the polyphenols extraction from raw material could be estimated using the methodology described by Turton et al. (2009), where five main costs must be taken into account: (i) fixed capital investment (FCI), (ii) cost of operating labour (COL), (iii) cost of utilities (CUT), (iv) cost of waste treatment (CWT), and (v) cost of raw material (CRM). Following this methodology, Vieira et al., 2013, Vieira et al., 2017 compared the COM of extraction of jussara pulp (Euterpe edulis Martius) with strong antioxidant activity by ultrasonic assited extraction (UAE) and agitated bed extraction (ABE) at lab scale. The UAE extracts presented a higher cost (75.2–137.2 €/kg) than the ABE extracts (72.5–139 €/kg). It was reported that the ABE and UAE extracts contained polyphenols such as kaempferol, luteolin, apigenin, quercetin and rutin, which may have medical applications (e.g., antivirals) for human health; and anthocyanidins as natural pigments for feed applications (Favaro et al., 2018; Vieira et al., 2017). Moreover, Osorio-Tobón et al. (2014) also used the COM methodology to evaluate the extraction process of curcumin from turmeric (Curcuma longa Linneo), which possesses a remarkable antiviral effect against dengue virus (Ichsyani et al., 2017). When advanced solid-liquid extractions techniques were evaluated, pressurized liquid extraction (PLE), Soxhlet extraction and low-pressure solvent extraction (LPSE), COM values of 79, 204 and 161 €/kg, respectively were estimated. The results showed that the PLE extraction technique was less expensive due to the short extraction time required (e.g., 30 min) compared with 6 h for Soxhlet and 3 h for LPSE. However, the extraction yields of the three techniques were similar (PLE 11 ± 1, Soxhlet 12 ± 1 and LPSE 12 ± 1).
On the other hand, Ioannou-Ttofa et al. (2017) determined that the total cost of the treatment of olive mill waste water through an integrated process using UF and NF membranes (UFZW-10/NF270) was 9.94 €/m3. The high-added value polyphenols present in this stream, like hydroxytyrosol, can be purified, and the cost can reach 14,900 to 20,900 €/kg.
Besides, it is worth noting that the approximately market value, according to the Sigma-Aldrich website, of 10 mg of kaempferol (≥90% purity) is 113 €, 10 mg of luteolin (≥98%) is 169 €, 10 mg of apigenin (≥97%) is 92 €, 10 g of quercetin (≥95%) is 44 €, 10 g of rutin (≥94%) is 24 € and 10 mg of hydroxytyrosol is 191 € (≥90%), but their purity can make they more expensive.
2.3. Stability, reactivity, synergism and bioavailability of polyphenols
Technically, polyphenols are extracted, purified and concentrated from plants using different techniques as described above, which can be processed into tablets or capsules for human consumption. However, polyphenols are unstable, prone to degrade and/or react with some elements (e.g., oxygen and metal ions during their processing and storage stages), resulting in changing structures and decreasing activities (e.g., antiviral activity) (Galanakis, 2018). Therefore, the stability as well as their reactivity, synergism and bioavailability of polyphenols are the main aspects that must be taken into account in the recovery, processing, storage and consumption of phenolic compounds for their market applications.
2.3.1. Stability
Instability generally due to many polyphenols are sensitive to chemical, enzymatic and physical treatments, which are used in food processing. Chemical and enzymatic instability leads to changes such as oxidation or polymerization, among others, causing alterations on their nutritional and physical-chemical attributes. Physical instability leads to changes like phase separation, flocculation, etc. that can also alter their attributes (Joye and McClements, 2014; Zhang et al., 2020).
2.3.2. Reactivity
Another factor that influences polyphenols is their reactivity, as they can be enzymatically degraded and polymerized during food processing stages. One of the most notable enzymatic reactions is on color, taste and nutritional value of polyphenols, which can even cause significant economic problems due to their impact on quality and shelf life of the products (Galanakis, 2018).
2.3.3. Synergism
The synergism of polyphenols in plant extracts means that a combination of two or more of compounds creates a higher biological activity than when the extracts are analyzed relative to individual polyphenols isolated from the same extracts (Yao et al., 2012; Zhang et al., 2020). However, the commercial application of polyphenols is currently limited due to instability when exposed to light, heat or oxygen as well as low bioavailability. A solution of the limitations mentioned, it could be the encapsulation (Zhang et al., 2020). Long et al. (2015) showed that bioactive food compounds can produce synergistic effects, as they have been reported in traditional Chinese medicine research. Therefore, the synergism between polyphenols must be taken into account for the development of functional foods and thus promote human well-being and prevent diseases such as viral ones.
2.3.4. Bioavailability
Bioavailability plays an important role in terms of the biological properties of polyphenols, which makes it possible to understand the proportion of their absorption, digestion and metabolism after their entry into the circulatory system (Carbonell-Capella et al., 2014). Several epidemiological and experimental studies describe the protective role of polyphenols in diseases such as viral diseases, diabetes, inflammation, among others (Kumar and Goel, 2019). Scalbert and Williamson (2000) reported few human bioavailability studies showing that the amounts of intact polyphenols in urine vary from one to another polyphenols. For example, for quercetin glycoside the percentage found in excretion urine was between 0.3 and 1.4%, while in the case of hesperidin, it was 24.4%. Similar variations were also observed for naringin consumed with grapefruit juice depending on the individual (5 to 57%).
Hong et al. (2014) and Liang et al. (2017) demonstrated that EGCG loading in nanoparticles constructed with zein a protein as zein or with a polysaccharide as chitosan, improved the stability of said polyphenols at the gastrointestinal level. Another study by Xue et al. (2014) using glycosylated casein nanoparticles to encapsulate EGC demonstrated the improvement of its physical stability during storage. Xue et al. (2018) encapsulated curcumin in zein-caseinate nanoparticles, and reported improved stability against UV radiation and heat treatments.
On the other hand, there has also been an interest in encapsulating combinations of polyphenols and taking advantage of their synergistic effects. For example, curcumin and resveratrol have been encapsulated within hyaluronic-coated lipid droplets, as they have similar mechanisms of action to inhibit tumor cell growth and antioxidant and antiviral effects (Nasr, 2016). Encapsulated polyphenols have been shown to have better chemical stability that non-encapsulated ones. However, after encapsulation no improvement in the bioavailability of polyphenols has been observed, as polyphenols can become indigestible. Therefore, the most appropriate administration system for the polyphenols and the food matrix used should be thoroughly evaluated case by case (Dueik and Bouchon, 2016).
3. Antiviral activity of polyphenols
The antiviral activity of different polyphenols has the target of interacting directly with viral particles, but this binding will depend on the nature of the virus (DNA or RNA virus) (Sundararajan et al., 2010; Palamara et al., 2005; Liu et al., 2011). Another characteristic of antiviral polyphenols is that they can exert the activity during intracellular replication, which may be attributed to antioxidant features of phenolic groups, thus inhibiting the oxidation of cells by the replication of some viruses (Sundararajan et al., 2010; Fraternale et al., 2009).
Many natural polyphenols have provided research results and are becoming an important target in the development of some drugs to combat viruses, thanks to their wide availability, inexpensive production and, above all, their low side-effects (Kumar and Goel, 2019; El-Toumy et al., 2018). This is the case with the virus that is currently attacking the entire world, the novel severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), which causes the 2019-nCoV disease transmitted person-to-person (Kampf et al., 2020).
As of June 2021, a total of nearly 171 million confirmed cases have been reported, including almost 4 million deaths worldwide since the start of the outbreak (World Health Organization, 2020). Due to the high infectivity and mortality rate of SARS-CoV-2, there is an opportunity to use the great amount of information on plants used in the Traditional Chinese Medicine (TCM) to be used to treat symptoms related to SARS (homology of SARS-CoV and SARS-CoV-2), considering that natural polyphenols could inhibit SARS-CoV-2 (Mehany et al., 2021). According to Wang et al. (2020) and Chojnacka et al. (2020), the entry of SARS-CoV-2 into the host cells (lung epithelium) is facilitated by a trimeric glycoprotein, called the spike protein (protein S), located in the capsid of the virus (outer envelope). SARS-CoV-2 uses angiotensin converting protein II (ACE2) as a receptor for binding to host cells. The protein S is hydrolysed by endosomal proteases, such as cathepsin or transmembrane cellular serine protease 2 (TMPRSS2), which results in membrane fusion. After the virus enters the host cell, it produces new RNA and the proteins that form its envelope. The binding of SARS-CoV-2 to the receptor ACE2 may depend on several factors, such as variants in the virus protein S that promote the efficiency of their interaction. In the replication and transcription, the main protease 3CLpro and papain-like protease (PLpro) are involved. The therapeutic targets to protect the human body from the entry, replication and transcription of the SARS-CoV-2 virus are the receptor with the proteases cutting spike protein and the proteases. Polyphenols with antiviral activity (e.g., flavonoids such as kaempferol, quercetin, and naringenin, see Table 3) have been developed as protease inhibitors, helping to stop virus infection (e.g., HIV, MERS and SARS) (Paraiso et al., 2020). Current studies show that some extracted polyphenols have antiviral activity, specifically as protease inhibitors. Park et al. (2016) used 95% ethanol to extract chalcones from Angelica keiskei that showed inhibition of protease 3CLpro as well as non-competitive inhibition of protease PLpro of SARS-CoV with IC50 values of 11.4 and 1.2 μM, respectively. Also Park et al. (2017) extracted polyphenols with ethanol from Broussonetia papyrifera with potential anti-coronaviral agents, which inhibit 100% PLpro (the IC50 was 3.7 μM) protease. Recent studies, such as Yudi Utomo and Meiyanto (2020), reported that hesperidin and naringenin, among other citrus flavonoids, and polyphenols from Curcuma spp., such as curcumin, bind strongly to the 3CLpro substrate, the binding domain of SARS-CoV-2, while interacting with the receptor ACE2 and protein S. Khalifa et al. (2020a) demonstrated that anthocyanidins, such as cyanodelphine, phacelianin, techofilin and gentiodelphin, authentically interact with the receptor binding site of SARS-CoV-2-3CLpro. Khalifa et al. (2020b) found that pedunculagin, castalin and tercatain, which are tannins, strongly interact with the SARS-CoV-2-3CLpro receptor binding site. Other polyphenols such as sinigrin, with IC50 of 217 μM, and hesperidin, with IC50 of 8.3 μM, contained in the water extract of the root of Isatis indigotica, have also been shown to be anti-SARS-CoV-2-3CLpro (Xu et al., 2020). These studies triggered that some polyphenols could be used as effective and above all natural anti-2019-nCovid components. Even, the Chinese Health Commission officially confirmed that natural medicine (e.g., TCM) should be used in combination with conventional medicine for the treatment of 2019-nCovid patients, and currently experimental research is focused on the therapeutic potential of polyphenols against SARS-CoV-2 (Yang et al., 2020). Table 5 collects a summary of the antiviral activities of recent studies of natural polyphenols and their mechanism of action against SARS-CoV-2.
Table 5.
Selected polyphenols and their role against SARS-CoV-2.
| Polyphenol | Source | Mechanism of action | Analysis study | Reference |
|---|---|---|---|---|
| Kaempferol, quercetin, luteolin-7-glucoside, demethoxycurcumin, naringenin, apigenin-7-glucoside, oleuropein, curcumin, catechin, epicatechingallate, zingerol, gingerol, and allicin | Medicinal plants | Block the enzymatic activity of SARS-CoV-3CLpro | In silico | (Khaerunnisa et al., 2020) |
| Malvidin, peonidin, petunidin, pelargonidin, cyanidin and malvidin | Pimpinella anisum L. | Binding affinities to 3C-like protease of SARS-CoV-2 (virus replication) | In silico | (Hasan et al., 2020) |
| Hesperetin, myricetin, caflanone, linebacker | Medicinal plants | High affinity to protein S, helicase and protease sites on the CE2 receptor (in silico analysis); in vitro analysis shows potential caflanone to inhibit virus entry | In silico and in vitro | (Ngwa et al., 2020) |
| Baicalein and baicalin | Scutellaria baicalensis and Oroxylum indicum | Down-regulators of the TMPRSS-2 expression. Baicalein (IC50 of 0.94 μM) and baicalin (6.41 μM) promising results to 3Clpro | In silico and in vitro | (Da Silva Antonio et al., 2020) |
| Polyphenol extract | Echinacea purpurea | Virus inactivated upon treatment with 50 μg/mL | In vitro | (Signer et al., 2020) |
Although Table 5 present more in silico experiments that predict promising results, more in vitro and in vivo studies are needed to evaluate the mechanism of action of polyphenols against SARS-CoV-2. Most of the studies were carried out in silico, current technology to predict drug behavior, accelerating the detection rate, since it allows screening many drugs and reduction of the cost of laboratory work, limiting clinical trials to the best candidates.
Recently has been discovered that hesperidin (which is a flavonoid) easily binds to key proteins of the SARS-CoV-2, due to its physicochemical structure (see Table 2) (Adem et al., 2020; Chen et al., 2020; Das et al., 2021; Joshi et al., 2020; Wu et al., 2020; Yudi Utomo and Meiyanto, 2020). These authors investigated if hesperidin is able to bind with a low binding energy. The lower energy required, the stronger and more specific the binding will be in therapeutic terms. Wu et al. (2020) tested hesperidin as a potent antiviral agent. The binding of hesperidin to the spike protein was effective in superimposing the ACE2-receptor binding domain (RBD) on the hesperidin-RBD complex, where a clear overlap of hesperidin with the ACE2 interface was observed. Accordingly, it was concluded that hesperidin can interrupt the ACE2 with RBD. Another low-energy binding site for hesperidin against SARS-CoV-2 is the main protease. This enzyme is called 3CLpro or Mpro and is the target of many chemical antiviral drugs. Das et al. (2021) studied the molecular coupling of the interaction between hesperidin and Mpro. The binding energy of hesperidin with hydrogen bonds to various amino acids (e.g., THR24, THR45, HIS4, SER46, etc.) was estimated as −37.7 kJ/mol. Finally Joshi et al. (2020) identified that hesperidin binds strongly to the main SARS-CoV-2 protease, and also to the ACE2-receptor.
Regarding vitro analysis, Suru et al. (2021) confirmed that pomegranate peel extract and its main polyphenols, such as punicalin and punicalagin, have a great capacity to attenuate the binding of the SARS-CoV-2 glycoprotein S to the ACE2 receptor. The most pronounced in vitro activity was observed in pomegranate peel extract, suggesting a possible synergistic effect of polyphenols, allowing their possible therapeutic application for 2019-nCovid.
On the other hand, there are few in vivo studies investigating the antiviral effect of polyphenols against this novel virus. Deng et al. (2020), studied Pudilan Xiaoyan Oral Liquid (EC50 of 1.078 mg/mL), a traditional Chinese medicine containing four herbs: Indigowoad root (Isatis indigotica), Bunge Corydalis (Corydalis bungeana), Mongolian Dandelion (Taraxacum mongolicum), Scutellaria Amoena (Scutellaria baicalensis) as well as more than 180 compounds (e.g., polyphenols such as chrysin, apigenin, rutin among others), which exhibited potent anti-SARS-CoV-2 activity in infected hACE2 mice. In another study, Schettig et al. (2020) reported that a nebulized formulation of quercetin (20 mg/mL) and N-acetylcysteine (100 mg/mL) greatly alleviated the respiratory symptoms of SARS-CoV-2 in a patient treated with hydroxychloroquine and antibiotics. This demonstrates the importance of conducting further clinical (in vivo) studies to evaluate the potential of polyphenols as an adjuvant or primary therapy for 2019-nCovid.
If polyphenols are analyzed in depth as traditional anti-2019-nCovid therapeutics on humans, they could be innovative and effective, or even against other lethal viral diseases. The use of medicinal plants containing antiviral polyphenols, still has some risks and needs massive and additional experiments.
3.1. Antiviral mechanism
The mechanism of polyphenols deals with the prevention of the entry of the virus into the host cell. This was the case of the proanthocyanidins extracted from Rumex acetosa, which inhibited the entry of the influenza type A virus in its first critical phase (Daglia, 2012). Fig. 1 shows the scheme of the target sites of the antiviral mechanism of some polyphenols (e.g., quercetin, morin, chrysin).
Fig. 1.
Virus replication and polyphenol targets (adapted from Pommier et al., 2005; Kamboj et al., 2012).
The general interest in polyphenols, as antiviral agents, is increasing because of the great advantages of using nature-derived compounds with almost no side-effects on human health (Naithani et al., 2008). Several studies have been done for discovering the antiviral mechanism of different polyphenols; Table 6 gives an overview of some of them.
Table 6.
Examples of antiviral mechanism of polyphenols (adapted from Naithani et al., 2008; Haslberger et al., 2020).
| Antiviral polyphenol | Plant source | Study | Virus type | Main mechanism |
|---|---|---|---|---|
| 4′,5-Dihydroxy 3,3′,7-trimethoxy flavone | Agastache rugosa (Kuntze) | Effect on the replication virus | Rhinovirus coxsackie virus | Replication inhibition, selective inhibition of viral RNA synthesis in the cell culture |
| Quercetin Luteolin | Achyrocline satureioides | Effect on the viral replication cycle of HSV-1 | HSV-1 | Interferes with the events occurring between the third and ninth hour of HSV-1 replication cycle, which includes transcription and translation of viral proteins |
| Salvin | Salvia officinalis | Viral inhibitory before absorption stage | HSV-1, HIV, SARS-CoV | Efficacy before absorption stage, but not in the replication stage |
| Morin Coumarin Quercetin |
Rhus succedanea, Garcinia multiflora, Alnus firma |
Effect on viral replication | HIV | Blockage of RNA synthesis, exhibited HIV-inhibitory activity |
As shown in Table 6, the polyphenols with antiviral properties may have different mechanisms of action, such as inhibiting the entry of the virus, an effect on replication, etc. (Haslberger et al., 2020). New trends in biotechnology and medicine, as well as new processing technologies, could help to optimise the solubility, administration and therapeutic activities to prevent infection by viruses (Patra et al., 2018; Thomford et al., 2018; Lin et al., 2014).
3.2. Relationship between antiviral activity and antioxidant properties
The study of the relationship between antiviral and antioxidant activities has not been explored in depth. Only a few studies report a comparative evaluation. It is worth mentioning that a high formation of free radicals leads to an imbalance in the oxidative metabolism at the mitochondrial level and, as a result, the vitality of the cells of each tissue is affected (Delgado-Roche and Mesta, 2020). This imbalance can be caused by various viruses (e.g., SARS-CoV-2, HIV, influenza virus) and leads to oxidative stress and helps the virus life cycle and eventually causes cell death (Bellavite and Donzelli, 2020).
Viral infection disrupts the defensive antioxidant mechanism of the human body, bringing inflammation and oxidative damage. Experimental animal models have achieved high levels of reactive oxygen species (ROS) and an alteration of innate antioxidant defenses during, for example, a SARS-CoV infection (van den Brand et al., 2014).
Therefore, the use of polyphenols with antiviral and antioxidant activities could be an alternative to prevent the onset of infection or development of viral disease. For example, Lin et al. (2002) reported that HSV-2 infection increases the amount of free radicals, and consequently causes immune response pathology. They used an ethyl acetate extract from Euphorbia thymifolia with antiviral and antioxidant activities (the IC50 was 7.72 ± 0.15 μg/mL) to inhibit HSV-2 growth in the kidney cell line.
As mentioned, many of the pathological effects of the viruses are not only directly related to viral replication, but also to the host response to infection (e.g., inflammation, oxidative stress, etc.) (Mateos-Martín et al., 2014; Bellavite and Donzelli, 2020). Therefore, the combination of antiviral therapy with the antioxidant properties could help favourably to combat the virus infection, reducing toxicity and preventing antiviral resistance.
4. Conclusions
Keeping in mind that diseases caused by viruses remain among the leading causes of morbidity and mortality, in both developed and developing countries, despite having conventional medicine, it is of interest to looking for alternative treatments more biocompatible for humans. As an example of alternative treatments, polyphenols are interesting and promising molecules that could be applied in the pharmaceutical sector. Polyphenols are secondary metabolites from plants which can also be extracted from agri-food residues. The bioactivities of polyphenols, like antioxidant capacity, as well as their mechanisms, such as forming stable radicals, delay and/or prevent oxidative stress-induced cellular damage and disease, are well defined and studied. However, in this comprehensive review, it has specifically shown the potential role of polyphenols with potential targets, such as antiviral activity, in the prevention of diseases caused by viruses. The state of the art indicates that there is not a single mechanism of action of polyphenols against viruses. Indeed, the antiviral mechanism of these bioactive compounds could be by antioxidant activities, viral entry or inhibition of viral reproduction, DNA inhibition among others.
Additionally, due to the complex polyphenol structure, new extraction, purification, formulation and processing technologies could help to improve the stability and bioavailability of antiviral polyphenols, as well as the administration protocols and the therapeutic effects as antiviral treatments. Thus, the use of plant extracts, as polyphenols, is postulated as useful for health, due to their synergistic effects, such as antioxidant, antiviral, anti-inflammatory, among others that must be considered and studied intensively. Therefore, future and more comprehensive studies of the antiviral activity of polyphenols against the SARS-CoV-2 coronavirus could provide an additional strategy, for example as a curative treatment in vaccines, to combat this pandemic that is causing the deadly disease 2019-nCovid.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the R2MIT project (CTM2017-85346-R) financed by the Spanish Ministry of Economy and Competitiveness (MINECO) and by the Catalan Government (ref. 2017-SGR-312), Spain. María Fernanda Montenegro-Landívar thanks MINECO for her predoctoral fellowship (ref. PRE2018-083861). Paulina Tapia-Quirós thanks to The National Council of Science and Technology of Mexico (CONACYT) for her predoctoral fellowship. Xanel Vecino acknowledges Spanish Ministry of Science and Innovation for her financial support under the project PID2019-103873RJ-I00.
Editor: Damià Barceló
References
- Abba Y., Hassim H., Hamzah H., Noordin M.M. Antiviral activity of resveratrol against human and animal viruses. Adv. Virol. 2015;2015 doi: 10.1155/2015/184241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adem S., Eyupoglu V., Sarfraz I., Rasul A., Ali M. 2020. Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors From Natural Polyphenols: An in Silico Strategy Unveils a Hope against CORONA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. Antiviral treatment of cytomegalovirus infection. Infect. Disord. Targets. 2012;11:475–503. doi: 10.1517/14656566.2012.658775. [DOI] [PubMed] [Google Scholar]
- Andronescu E., Grumezescu A.M. 2017. Nanostructures in Therapeutic Medicine Series Nanostructures for Drug Delivery. [DOI] [Google Scholar]
- Barthelemy S., Vergnes L., Moynier M., Guyot D., Labidalle S., Bahraoui E. Curcumin and curcumin derivatives inhibit tat-mediated transactivation of type 1 human immunodeficiency virus long terminal repeat. Res. Virol. 1998;149:43–52. doi: 10.1016/S0923-2516(97)86899-9. [DOI] [PubMed] [Google Scholar]
- Bedard K.M., Semler B.L. Regulation of picornavirus gene expression. Microbes Infect. 2004;6:702–713. doi: 10.1016/j.micinf.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Beigelman A., Zeiger R.S., Mauger D., Strunk R.C., Jackson D.J., Martinez F.D., Morgan W.J., Covar R., Szefler S.J., Taussig L.M., Bacharier L.B. The association between vitamin D status and the rate of exacerbations requiring oral corticosteroids in preschool children with recurrent wheezing. J. Allergy Clin. Immunol. 2014;133 doi: 10.1016/j.jaci.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavite P., Donzelli A. 2020. Hesperidin and SARS-CoV-2: New Light on the Healthy Functions of Citrus Fruit. Preprints. 2020060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti A.B., Usman M., Kandi V. Current scenario of HIV/AIDS, treatment options, and major challenges with compliance to antiretroviral therapy. Cureus. 2016;8:1–12. doi: 10.7759/cureus.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino A., Capannelli G., Comite A., Jezowska A., Pagliero M., Costa C., Firpo R. Treatment of olive mill wastewater through integrated pressure-driven membrane processes. Membranes (Basel) 2020;10:1–16. doi: 10.3390/membranes10110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brglez Mojzer E., Knez Hrncic M., Škerget M., Knez Ž., Bren U. Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21 doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell-Capella J.M., Buniowska M., Barba F.J., Esteve M.J., Frígola A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: a review. Compr. Rev. Food Sci. Food Saf. 2014;13:155–171. doi: 10.1111/1541-4337.12049. [DOI] [PubMed] [Google Scholar]
- Chang L.K., Wei T.T., Chiu Y.F., Tung C.P., Chuang J.Y., Hung S.K., Li C., Liu S.T. Inhibition of epstein-barr virus lytic cycle by (-)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2003;301:1062–1068. doi: 10.1016/S0006-291X(03)00067-6. [DOI] [PubMed] [Google Scholar]
- Charcosset C. 2016. Integrated Membrane Systems and Processes. [DOI] [Google Scholar]
- Chen Y.W., Yiu C.P.B., Wong K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 2020;9:1–17. doi: 10.12688/f1000research.22457.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.C., Wong G. Honey bee propolis: prospects in medicine. Bee World. 1996;77:8–15. doi: 10.1080/0005772X.1996.11099278. [DOI] [Google Scholar]
- Cheng H.Y., Yang C.M., Lin T.C., Shieh D.E., Lin C.C. Ent-epiafzelechin-(4a?8)-epiafzelechin extracted from Cassia javanica inhibits herpes simplex virus type 2 replication. J. Med. Microbiol. 2006;55:201–206. doi: 10.1099/jmm.0.46110-0. [DOI] [PubMed] [Google Scholar]
- Cheynier V. Phenolic compounds: from plants to foods. Phytochem. Rev. 2012;11:153–177. doi: 10.1007/s11101-012-9242-8. [DOI] [Google Scholar]
- Chiang L.C., Ng L.T., Cheng P.W., Chiang W., Lin C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005;32:811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- Chiow K.H., Phoon M.C., Putti T., Tan B.K.H., Chow V.T. Evaluation of antiviral activities of Houttuynia cordata thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med. 2016;9:1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.J., Song J.H., Bhatt L.R., Baek S.H. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phyther. Res. 2010;24:1292–1296. doi: 10.1002/ptr.3101. [DOI] [PubMed] [Google Scholar]
- Chojnacka K., Witek-Krowiak A., Skrzypczak D., Mikula K., Mlynarz P. Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. J. Funct. Foods. 2020;73 doi: 10.1016/j.jff.2020.104146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.I. Human Herpesviruses. Advances in Experimental Medicine and Biology. Springer; Singapore: 2018. Vaccine development for Epstein-Barr virus; pp. 477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:1–9. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S.E., Ramani S., Tate J.E., Parashar U.D., Svensson L., Hagbom M., Franco M.A., Greenberg H.B., O’Ryan M., Kang G., Desselberger U., Estes M.K. Rotavirus infection. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva Antonio A., Moreira Wiedemann L.S., Veiga-Junior V.F. Natural products’ role against COVID-19. RSC Adv. 2020;10:23379–23393. doi: 10.1039/d0ra03774e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2021;39:3347–3357. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Roche L., Mesta F. Med. Res; Arch: 2020. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Xu Y., Kong Q., Xue J., Yu P., Liu J., Lv Q., Li F., Wei Q., Bao L. Therapeutic efficacy of pudilan xiaoyan Oral liquid (PDL) for COVID-19 in vitro and in vivo. Signal Transduct. Target. Ther. 2020;5:2–4. doi: 10.1038/s41392-020-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J.J., Sweet T.J., Bailey E., Faith S.A., Booth T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antivir. Res. 2006;72:171–177. doi: 10.1016/j.antiviral.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Dueik V., Bouchon P. Development of polyphenol-enriched vacuum and atmospheric fried matrices: evaluation of quality parameters and in vitro bioavailability of polyphenols. Food Res. Int. 2016;88:166–172. doi: 10.1016/j.foodres.2016.03.032. [DOI] [PubMed] [Google Scholar]
- Dzah C.S., Duan Y., Zhang H., Wen C., Zhang J., Chen G., Ma H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: a review. Food Biosci. 2020;35 doi: 10.1016/j.fbio.2020.100547. [DOI] [Google Scholar]
- Edziri H., Mastouri M., Aouni M., Verschaeve L. Polyphenols content, antioxidant and antiviral activities of leaf extracts of Marrubium deserti growing in Tunisia. S. Afr. J. Bot. 2012;80:104–109. doi: 10.1016/j.sajb.2012.03.001. [DOI] [Google Scholar]
- El-Toumy S.A., Salib J.Y., El-Kashak W.A., Marty C., Bedoux G., Bourgougnon N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness. 2018;7:91–101. doi: 10.1016/j.fshw.2018.01.001. [DOI] [Google Scholar]
- Evers D.L., Chao C.F., Wang X., Zhang Z., Huong S.M., Huang E.S. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antivir. Res. 2005;68:124–134. doi: 10.1016/j.antiviral.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro L.L., Balcão V.M., Rocha L.K.H., Silva E.C., Oliveira J.M., Jr., Vila M.M.D.C., Tubino M. Physicochemical characterization of a crude anthocyanin extract from the fruits of Jussara (Euterpe edulis Martius): potential for food and pharmaceutical applications. J. Braz. Chem. Soc. 2018;29:1–17. doi: 10.21577/0103-5053.20180082. [DOI] [Google Scholar]
- Felipe A.M.M., Rincão V.P., Benati F.J., Linhares R.E.C., Galina K.J., De Toledo C.E.M., Lopes G.C., De Mello J.C.P., Nozawa C. Antiviral effect of Guazuma ulmifolia and Stryphnodendron adstringens on poliovirus and bovine herpesvirus. Biol. Pharm. Bull. 2006;29:1092–1095. doi: 10.1248/bpb.29.1092. [DOI] [PubMed] [Google Scholar]
- Fisher C.R., Streicker D.G., Schnell M.J. The spread and evolution of rabies virus: conquering new frontiers. Nat. Rev. Microbiol. 2018;16:241–255. doi: 10.1038/nrmicro.2018.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T.G., Christenson J.C. Influenza and parainfluenza viral infections in children. Pediatr. Rev. 2014;35:217–228. doi: 10.1542/pir.35-6-217. [DOI] [PubMed] [Google Scholar]
- Fraternale A., Paoletti M.F., Casabianca A., Nencioni L., Garaci E., Palamara A.T., Magnani M. GSH and analogs in antiviral therapy. Mol. Asp. Med. 2009;30:99–110. doi: 10.1016/j.mam.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Galanakis C.M. 1st ed. Woodhead Publishing; Vienna: 2018. Polyphenols: Properties, Recovery, and Applications. [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom A., Svedhem V., Singh K., Sönnerborg A., Neogi U. Virological failure in patients with HIV-1 subtype C receiving antiretroviral therapy: an analysis of a prospective national cohort in Sweden. Lancet HIV. 2016;3:e166–e174. doi: 10.1016/S2352-3018(16)00023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haminiuk C.W.I., Maciel G.M., Plata-Oviedo M.S.V., Peralta R.M. Phenolic compounds in fruits - an overview. Int. J. Food Sci. Technol. 2012;47:2023–2044. doi: 10.1111/j.1365-2621.2012.03067.x. [DOI] [Google Scholar]
- Hasan A., Nahar N., Jannat K., Afroze T., Jahan R., Rahmatullah M. 2020. In Silico Studies on Phytochemicals of Pimpinella Anisum L. (Apiaceae) as Potential Inhibitors of SARS-CoV-2 3C-like Protease 6640. [Google Scholar]
- Haslberger A.G., Jacob U., Hippe B., Karlic H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: mini review. Funct. Foods Heal. Dis. 2020;10:195–209. doi: 10.31989/ffhd.v10i5.707. [DOI] [Google Scholar]
- Hong Z., Xu Y., Yin J.F., Jin J., Jiang Y., Du Q. Improving the effectiveness of (-)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J. Agric. Food Chem. 2014;62:12603–12609. doi: 10.1021/jf504603n. [DOI] [PubMed] [Google Scholar]
- Ichsyani M., Ridhanya A., Risanti M., Desti H., Ceria R., Putri D.H., Sudiro T.M., Dewi B.E. Antiviral effects of Curcuma longa L. against dengue virus in vitro and in vivo. IOP Conf. Ser. Earth Environ. Sci. 2017;101 doi: 10.1088/1755-1315/101/1/012005. [DOI] [Google Scholar]
- Ignat I., Volf I., Popa V. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;1821–1835 doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Ioannou-Ttofa L., Michael-Kordatou I., Fattas S.C., Eusebio A., Ribeiro B., Rusan M., Amer A.R.B., Zuraiqi S., Waismand M., Linder C., Wiesman Z., Gilron J., Fatta- Kassinos D. Treatment efficiency and economic feasibility of biological oxidation, membrane filtration and separation processes, and advanced oxidation for the purification and valorization of olive mill wastewater. Water Res. 2017;114:1–13. doi: 10.1016/j.watres.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Johnson R.W., Whitton T.L. Management of herpes zoster (shingles) and postherpetic neuralgia. Expert. Opin. Pharmacother. 2004;551–559 doi: 10.1517/14656566.5.3.551. [DOI] [PubMed] [Google Scholar]
- Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joye I.J., McClements D.J. Biopolymer-based nanoparticles and microparticles: fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014;19:417–427. doi: 10.1016/j.cocis.2014.07.002. [DOI] [Google Scholar]
- Kamboj A., Saluja A.K., Kumar M., Atri P. Antiviral activity of plant polyphenols. J. Pharm. Res. 2012;5:2402–2412. [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E.H., Kown T.Y., Oh G.T., Park W.F., Park S.Il, Park S.K., Lee Y.I. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antivir. Res. 2006;72:100–106. doi: 10.1016/j.antiviral.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S. Potential inhibitor of COVID-19 Main protease (M pro) from several medicinal plant compounds by molecular docking study. Preprints. 2020;1–14 doi: 10.20944/preprints202003.0226.v1. [DOI] [Google Scholar]
- Khalifa I., Nawaz A., Sobhy R., Althawb S.A., Barakat H. Polyacylated anthocyanins constructively network with catalytic dyad residues of 3CLpro of 2019-nCoV than monomeric anthocyanins: a structural-relationship activity study with 10 anthocyanins using in-silico approaches. J. Mol. Graph. Model. 2020;100 doi: 10.1016/j.jmgm.2020.107690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa I., Zhu W., Mohammed H.H.H., Dutta K., Li C. Tannins inhibit SARS-CoV-2 through binding with catalytic dyad residues of 3CLpro: an in silico approach with 19 structural different hydrolysable tannins. J. Food Biochem. 2020;44:1–19. doi: 10.1111/jfbc.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Narayanan S., Chang K.O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antivir. Res. 2010;88:227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Koch W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients. 2019;11:1–35. doi: 10.3390/nu11051039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krych J., Gebicka L. Catalase is inhibited by flavonoids. Int. J. Biol. Macromol. 2013;58:148–153. doi: 10.1016/j.ijbiomac.2013.03.070. [DOI] [PubMed] [Google Scholar]
- Kudo E., Taura M., Matsuda K., Shimamoto M., Kariya R., Goto H., Hattori S., Kimura S., Okada S. Inhibition of HIV-1 replication by a tricyclic coumarin GUT-70 in acutely and chronically infected cells. Bioorganic Med. Chem. Lett. 2013;23:606–609. doi: 10.1016/j.bmcl.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Kumar N., Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Reports. 2019;24 doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H.J., Kim H.H., Ryu Y.B., Kim J.H., Jeong H.J., Lee S.W., Chang J.S., Cho K.O., Rho M.C., Park S.J., Lee W.S. In vitro anti-rotavirus activity of polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorganic Med. Chem. 2010;18:7668–7674. doi: 10.1016/j.bmc.2010.07.073. [DOI] [PubMed] [Google Scholar]
- Lattanzio V. Phenolic compounds: introduction. Nat. Prod. 2013;1–4242 doi: 10.1007/978-3-642-22144-6_57. [DOI] [Google Scholar]
- Liang J., Yan H., Wang X., Zhou Y., Gao X., Puligundla P., Wan X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017;231:19–24. doi: 10.1016/j.foodchem.2017.02.106. [DOI] [PubMed] [Google Scholar]
- Lin C.C., Cheng H.Y., Yang C.M., Lin T.C. Antioxidant and antiviral activities of Euphorbia thymifolia L. J. Biomed. Sci. 2002;9:656–664. doi: 10.1159/000067281. [DOI] [PubMed] [Google Scholar]
- Lin L.T., Hsu W.C., Lin C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J., Smura T., Lundström J.O., Pettersson J.H.-O., Sironen T., Vapalahti O., Lundkvist Å., Hesson J.C. Introduction and dispersal of sindbis virus from Central Africa to Europe. J. Virol. 2019;93 doi: 10.1128/jvi.00620-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Xiong S., Xiang Y.F., Guo C.W., Ge F., Yang C.R., Zhang Y.J., Wang Y.F., Kitazato K. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza a virus. Arch. Virol. 2011;156:1359–1369. doi: 10.1007/s00705-011-0989-9. [DOI] [PubMed] [Google Scholar]
- Liu D.X., Liang J.Q., Fung T.S. Human coronavirus-229E, -OC43, -NL63, and -HKU1. Ref. Modul. Life Sci. 2020;43:1–13. doi: 10.1016/b978-0-12-809633-8.21501-x. [DOI] [Google Scholar]
- Long F., Yang H., Xu Y., Hao H., Li P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2008;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- Lv Y., An Z., Chen H., Wang Z., Liu L. Mechanism of curcumin resistance to human cytomegalovirus in HELF cells. BMC Complement. Altern. Med. 2014;14:1–7. doi: 10.1186/1472-6882-14-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín L., Miguélez E.M., Villar C.J., Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.R. Polyphenols as dietary supplements: a double-edged sword. Nutr. Diet. Suppl. 2009;1 doi: 10.2147/nds.s6422. [DOI] [Google Scholar]
- Mastromarino P., Capobianco D., Cannata F., Nardis C., Mattia E., De Leo A., Restignoli R., Francioso A., Mosca L. Resveratrol inhibits rhinovirus replication and expression of inflammatory mediators in nasal epithelia. Antivir. Res. 2015;123:15–21. doi: 10.1016/j.antiviral.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Mateos-Martín M.L., Fuguet E., Jiménez-Ardón A., Herrero-Uribe L., Tamayo-Castillo G., Torres J.L. Identification of polyphenols from antiviral Chamaecrista nictitans extract using high-resolution LC-ESI-MS/MS. Anal. Bioanal. Chem. 2014;406:5501–5506. doi: 10.1007/s00216-014-7982-6. [DOI] [PubMed] [Google Scholar]
- Mehany T., Khalifa I., Barakat H., Althwab S.A., Alharbi Y.M., El-Sohaimy S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: a review with research evidence and underlying mechanisms. Food Biosci. 2021;40 doi: 10.1016/j.fbio.2021.100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre S., Srivastava T., Naik S., Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine. 2020:153286. doi: 10.1016/j.phymed.2020.153286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-Landívar M.F., Tapia-Quirós P., Vecino X., Reig M., Valderrama C., Granados M., Cortina J.L., Saurina J. Fruit and vegetable processing wastes as natural sources of antioxidant-rich extracts: evaluation of advanced extraction technologies by surface response methodology. J. Environ. Chem. Eng. 2021 doi: 10.1016/j.jece.2021.105330. [DOI] [Google Scholar]
- Morfin F., Thou D. Herpes Simplex Virus Resistance to Anti Viral Drugs. Vol. 26. 2003. pp. 29–37. [DOI] [PubMed] [Google Scholar]
- Moscona A. Medical management of influenza infection. Annu. Rev. Med. 2008;59:397–413. doi: 10.1146/annurev.med.59.061506.213121. [DOI] [PubMed] [Google Scholar]
- Mouler Rechtman M., Har-Noy O., Bar-Yishay I., Fishman S., Adamovich Y., Shaul Y., Halpern Z., Shlomai A. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1a. FEBS Lett. 2010;584:2485–2490. doi: 10.1016/j.febslet.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Naithani R., Huma L., Holland L., Shukla D., McCormick D., Mehta R., Moriarty R. Antiviral activity of phytochemicals: a comprehensive review. Mini-Rev. Med. Chem. 2008;8:1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- Nasr M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016;23:1444–1452. doi: 10.3109/10717544.2015.1092619. [DOI] [PubMed] [Google Scholar]
- Nguyen T.T.H., Woo H.J., Kang H.K., Nguyen V.D., Kim Y.M., Kim D.W., Ahn S.A., Xia Y., Kim D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa W., Kumar R., Thompson D., Lyerly W., Moore R., Reid T.-E., Lowe H., Toyang N. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules. 2020;1–10 doi: 10.3390/molecules25112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroian M., Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Osorio-Tobón J.F., Carvalho P.I.N., Rostagno M.A., Petenate A.J., Meireles M.A.A. Extraction of curcuminoids from deflavored turmeric (Curcuma longa L.) using pressurized liquids: process integration and economic evaluation. J. Supercrit. Fluids. 2014;95:167–174. doi: 10.1016/j.supflu.2014.08.012. [DOI] [Google Scholar]
- Palamara A.T., Nencioni L., Aquilano K., De Chiara G., Hernandez L., Cozzolino F., Ciriolo M.R., Garaci E. Inhibition of influenza a virus replication by resveratrol. J. Infect. Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso I.L., Revel J.S., Stevens J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020;32:149–155. doi: 10.1016/j.cofs.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A., Alzuru M., Mendez J., Rodríguez-Ortega M. Anti-sindbis activity of flavanones hesperetin and naringenin. Biol. Pharm. Bull. 2003;26:108–109. doi: 10.1248/bpb.26.108. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Ko J.A., Kim D.W., Kim Y.M., Kwon H.J., Jeong H.J., Kim C.Y., Park K.H., Lee W.S., Ryu Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem. 2016;31:23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Ryu Y.B., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzyme Inhib. Med. Chem. 2017;32:504–512. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.-S., Rodriguez-Torres M.del P. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16:1–33. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret J., Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S., Orenstein W., Offit P. 6th ed. Saunders; 2012. Vaccines: expert consult. [Google Scholar]
- Pommier Y., Johnson A.A., Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat. Rev. Drug Discov. 2005;4:236–248. doi: 10.1038/nrd1660. [DOI] [PubMed] [Google Scholar]
- Praditya D., Kirchhoff L., Brüning J., Rachmawati H., Steinmann J., Steinmann E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019;10:1–16. doi: 10.3389/fmicb.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V.R. One hundred years of poliovirus pathogenesis. Virology. 2006;344:9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ragab F.A., Yahya T.A.A., El-Naa M.M., Arafa R.K. Design, synthesis and structure-activity relationship of novel semi-synthetic flavonoids as antiproliferative agents. Eur. J. Med. Chem. 2014;82:506–520. doi: 10.1016/j.ejmech.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Riedel C., Vasishtan D., Pražák V., Ghanem A., Conzelmann K.K., Rümenapf T. Cryo EM structure of the rabies virus ribonucleoprotein complex. Sci. Rep. 2019;9:1–6. doi: 10.1038/s41598-019-46126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M., Moccia S., Spagnuolo C., Tedesco I., Russo G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020;328 doi: 10.1016/j.cbi.2020.109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuskanen O., Waris M., Ramilo O. New aspects on human rhinovirus infections. Pediatr. Infect. Dis. J. 2013;32:553–555. doi: 10.1097/INF.0b013e3182833c90. [DOI] [PubMed] [Google Scholar]
- Ruwali P., Rai N., Kumar N., Gautam P. Antiviral potential of medicinal plants: an overview. Int. Res. J. Pharm. 2013;4:8–16. doi: 10.7897/2230-8407.04603. [DOI] [Google Scholar]
- Saurina J., Sentellas S. Determination of phenolic compounds in food matrices: application to characterization and authentication. Fast Liq. Chromatogr. Spectrom. Methods Food Environ. Anal. 2015:517–547. doi: 10.1142/9781783264940_0013. [DOI] [Google Scholar]
- Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073–2085. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Schettig R., Sears T., Klein M., Tan-Lim R., Matthias R., Jr., Aussems C., Hummel M., Sears R., Poteet Z., Warren D., Oertle J., Prato D. COVID-19 patient with multifocal pneumonia and respiratory difficulty resolved quickly: possible antiviral and anti-inflammatory benefits of quercinex (Nebulized quercetin-NAC) as adjuvant. Adv. Infect. Dis. 2020;10:45–55. doi: 10.4236/aid.2020.103006. [DOI] [Google Scholar]
- Serkedjieva J., Gegova G., Mladenov K. Protective efficacy of an aerosol preparation, obtained from Geranium sanguineum L., in experimental influenza infection. Pharmazie. 2008;63:160–163. doi: 10.1691/ph.2008.7617. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects - a review. J. Funct. Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Sharma A., Shahzad B., Rehman A., Bhardwaj R., Landi M., Zheng B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24:1–22. doi: 10.3390/molecules24132452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer J., Jonsdottir H.R., Albrich W.C., Strasser M., Züst R., Ryter S., Ackermann-Gäumann R., Lenz N., Siegrist D., Suter A., Schoop R., Engler O.B. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020;17:1–11. doi: 10.1186/s12985-020-01439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.R.A., Morais S.M., Marques M.M.M., Lima D.M., Santos S.C.C., Almeida R.R., Vieira I.G.P., Guedes M.I.F. Antiviral activities of extracts and phenolic components of two spondias species against dengue virus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011;17:406–413. doi: 10.1590/S1678-91992011000400007. [DOI] [Google Scholar]
- Singh S., Sk M.F., Sonawane A., Kar P., Sadhukhan S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA-dependent RNA polymerase (RdRp) inhibition: an in-silico analysis. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1796810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukhova N., Soldin O.P., Soldin S.J. Isotope dilution tandem mass spectrometric method for T4/T3. Clin. Chim. Acta. 2004;343:185–190. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann J., Buer J., Pietschmann T., Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013;168:1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez B., Álvarez Á.L., García Y.D., del Barrio G., Lobo A.P., Parra F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010;120:339–342. doi: 10.1016/j.foodchem.2009.09.073. [DOI] [Google Scholar]
- Sukowati C.H.C., El-Khobar K.E., Ie S.I., Anfuso B., Muljono D.H., Tiribelli C. Significance of hepatitis virus infection in the oncogenic initiation of hepatocellular carcinoma. World J. Gastroenterol. 2016;22:1497–1512. doi: 10.3748/wjg.v22.i4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan A., Ganapathy R., Huan L., Dunlap J.R., Webby R.J., Kotwal G.J., Sangster M.Y. Influenza virus variation in susceptibility to inactivation by pomegranate polyphenols is determined by envelope glycoproteins. Antivir. Res. 2010;88:1–9. doi: 10.1016/j.antiviral.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist W.I., Kräusslich H.G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suru R., Stojiljkovi P., Savikin K., Zduni G., Skrbi R. Vol. 114. 2021. Bioorganic Chemistry Pomegranate Peel Extract Polyphenols Attenuate the SARS-CoV-2 S-glycoprotein Binding Ability to ACE2 Receptor: In Silico and in Vitro Studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytar O., Brestic M., Hajihashemi S., Skalicky M., Kubeš J., Lamilla-Tamayo L., Ibrahimova U., Ibadullayeva S., Landi M. Covid-19 prophylaxis efforts based on natural antiviral plant extracts and their compounds. Molecules. 2021 doi: 10.3390/molecules26030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagarro A., Pérez L., Quintero V.M., Cañete A. Dexamethasone Does Not Reduce Length of Hospitalization or Recurrent Wheezing 1 Year After Early Bronchiolitis. Vol. 66. 2014. pp. 1–2. [PubMed] [Google Scholar]
- Tapia-Quirós P., Montenegro-Landívar M.F., Reig M., Vecino X., Alvarino T., Cortina J.L., Saurina J., Granados M. Olive mill and winery wastes as viable sources of bioactive compounds: a study on polyphenols recovery. Antioxidants. 2020;9:1074. doi: 10.3390/antiox9111074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomford N.E., Dzobo K., Chimusa E., Andrae-Marobela K., Chirikure S., Wonkam A., Dandara C. Personalized herbal Medicine? A roadmap for convergence of herbal and precision medicine biomarker innovations. Omi. A J. Integr. Biol. 2018;22:375–391. doi: 10.1089/omi.2018.0074. [DOI] [PubMed] [Google Scholar]
- Tian S.S., Jiang F.S., Zhang K., Zhu X.X., Jin B., Lu J.J., Ding Z.S. Flavonoids from the leaves of Carya cathayensis sarg. inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia. 2013;92:34–40. doi: 10.1016/j.fitote.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Tu Z., Xu M., Zhang J., Feng Y., Hao Z., Tu C., Liu Y. Pentagalloylglucose inhibits the replication of rabies virus via mediation of the miR-455/SOCS3/STAT3/IL-6 pathway. J. Virol. 2019;93:1–17. doi: 10.1128/JVI.00539-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar P., Sasidharan S., Saudagar P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. Biocontrol Agents Second. Metab. 2021;419–441 doi: 10.1016/b978-0-12-822919-4.00017-x. [DOI] [Google Scholar]
- Turton R., Bailie R.C., Whiting W.B., Shaeiwitz J.A. Third ed. 2009. Analysis, Synthesis and Design of Chemical Process. [Google Scholar]
- van den Brand J.M.A., Haagmans B.L., van Riel D., Osterhaus A.D.M.E., Kuiken T. The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. J. Comp. Pathol. 2014;151:83–112. doi: 10.1016/j.jcpa.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira G.S., Cavalcanti R.N., Meireles M.A.A., Hubinger M.D. Chemical and economic evaluation of natural antioxidant extracts obtained by ultrasound-assisted and agitated bed extraction from Jussara pulp (Euterpe edulis) J. Food Eng. 2013;119:196–204. doi: 10.1016/j.jfoodeng.2013.05.030. [DOI] [Google Scholar]
- Vieira G.S., Marques A.S.F., Machado M.T.C., Silva V.M., Hubinger M.D. Determination of anthocyanins and non-anthocyanin polyphenols by ultra performance liquid chromatography/electrospray ionization mass spectrometry (UPLC/ESI–MS) in Jussara (Euterpe edulis) extracts. J. Food Sci. Technol. 2017;54:2135–2144. doi: 10.1007/s13197-017-2653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Dhindsa R., Povysil G., Zoghbi A., Motelow J., Hostyk J., Nickols N., Rettig M., Goldstein D.B. 2020. TMPRSS2 Transcriptional Inhibition as a Therapeutic Strategy for COVID-19. [DOI] [Google Scholar]
- Watson R.R., Preedy V.R., Zibadi S. 2013. Polyphenols in Human Health and Disease. [DOI] [Google Scholar]
- Wong J., Si X., Angeles A., Zhang J., Shi J., Fung G., Jagdeo J., Wang T., Zhong Z., Jan E., Luo H. Cytoplasmic redistribution and cleavage of AUF1 during coxsackievirus infection enhance the stability of its viral genome. FASEB J. 2013;27:2777–2787. doi: 10.1096/fj.12-226498. [DOI] [PubMed] [Google Scholar]
- World Health Organization The promotion and development of traditional medicine [WWW Document] 1978. https://apps.who.int/iris/handle/10665/40995 [PubMed]
- World Health Organization . 2020. Coronavirus Disease COVID-2019, Novel Coronavirus (2019-nCoV): Situation Reports. [Google Scholar]
- Wu X.N., Yu C.H., Cai W., Hua J., Li S.Q., Wang W. Protective effect of a polyphenolic rich extract from Magnolia officinalis bark on influenza virus-induced pneumonia in mice. J. Ethnopharmacol. 2011;134:191–194. doi: 10.1016/j.jep.2010.11.074. [DOI] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.H., Zang N., Li S.Min, Wang L.Jia, Deng Y., He Y., Yang X.Qiang, Liu E.Mei. Resveratrol Inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation. 2012;35:1392–1401. doi: 10.1007/s10753-012-9452-7. [DOI] [PubMed] [Google Scholar]
- Xu G., Dou J., Zhang L., Guo Q., Zhou C. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol. Pharm. Bull. 2010;33:238–243. doi: 10.1248/bpb.33.238. [DOI] [PubMed] [Google Scholar]
- Xu Z., Peng C., Shi Y., Zhu Z., Mu K., Wang X., Zhu W. Nelfinavir Was Predicted to be a Potential Inhibitor of 2019-nCov Main Protease by an Integrative Approach Combining Homology Modelling, Molecular Docking and Binding Free Energy Calculation. Vol. 1201. 2020. pp. 0–2. [DOI] [Google Scholar]
- Xue J., Tan C., Zhang X., Feng B., Xia S. Fabrication of epigallocatechin-3-gallate nanocarrier based on glycosylated casein: stability and interaction mechanism. J. Agric. Food Chem. 2014;62:4677–4684. doi: 10.1021/jf405157x. [DOI] [PubMed] [Google Scholar]
- Xue J., Zhang Y., Huang G., Liu J., Slavin M., Yu L., (Lucy) Zein-caseinate composite nanoparticles for bioactive delivery using curcumin as a probe compound. Food Hydrocoll. 2018;83:25–35. doi: 10.1016/j.foodhyd.2018.04.037. [DOI] [Google Scholar]
- Yamada H., Takuma N., Daimon T., Hara Y. Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: a prospective clinical study. J. Altern. Complement. Med. 2006;12:669–672. doi: 10.1089/acm.2006.12.669. [DOI] [PubMed] [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Luong T.N., Lepik M., Aftab N., Fong V.H., Vieira A. Synergism of antioxidant phytochemicals: comparisons among purified polyphenols and dietary-plant extracts. Acta Hortic. 2012;939:121–127. doi: 10.17660/ActaHortic.2012.939.15. [DOI] [Google Scholar]
- Yudi Utomo R., Meiyanto E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2. Infection. 2020;2:1–8. doi: 10.20944/preprints202003.0214.v1. [DOI] [Google Scholar]
- Yugo D.M., Hauck R., Shivaprasad H.L., Meng X.J. Hepatitis virus infections in poultry. Avian Dis. 2016;60:576–588. doi: 10.1637/11229-070515-Review.1. [DOI] [PubMed] [Google Scholar]
- Zahoor M., Shah A.B., Naz S., Ullah R., Bari A., Mahmood H.M. Isolation of quercetin from Rubus fruticosus, their concentration through NF/RO membranes, and recovery through carbon nanocomposite. A pilot plant study. Biomed Res. Int. 2020 doi: 10.1155/2020/8216435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi K., Taherzadeh M., Yaghoubi R., Tajbakhsh S., Rastian Z., Fouladvand M., Sartavi K. Antiviral activity of Avicennia marina against herpes simplex virus type 1 and vaccine strain of poliovirus (an in vitro study) J. Med. Plants Res. 2009;3:771–775. [Google Scholar]
- Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M., Abubakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011;8:1–11. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M.R., AbuBakar S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012;12 doi: 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huang H., Zhao X., Lv Q., Sun C., Li X., Chen K. Effects of flavonoids-rich chinese bayberry (Myrica rubra sieb. et zucc.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Foods. 2015;14:144–153. doi: 10.1016/j.jff.2015.01.030. [DOI] [Google Scholar]
- Zhang L., McClements D.J., Wei Z., Wang G., Liu X., Liu F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020;60:2083–2097. doi: 10.1080/10408398.2019.1630358. [DOI] [PubMed] [Google Scholar]




