Abstract
The COVID-19 pandemic has caused acute lung injury in millions of individuals worldwide. Some patients develop COVID-related acute respiratory distress syndrome (CARDS) and cannot be liberated from mechanical ventilation. Others may develop post-COVID fibrosis, resulting in substantial disability and need for long-term supplemental oxygen. In both of these situations, treatment teams often inquire about the possibility of lung transplantation. In fact, lung transplantation has been successfully employed for both CARDS and post-COVID fibrosis in a limited number of patients worldwide. Lung transplantation after COVID infection presents a number of unique challenges that transplant programs must consider. In those with severe CARDS, the inability to conduct proper psychosocial evaluation and pretransplantation education, marked deconditioning from critical illness, and infectious concerns regarding viral reactivation are major hurdles. In those with post-COVID fibrosis, our limited knowledge about the natural history of recovery after COVID-19 infection is problematic. Increased knowledge of the likelihood and degree of recovery after COVID-19 acute lung injury is essential for appropriate decision-making with regard to transplantation. Transplant physicians must weigh the risks and benefits of lung transplantation differently in a post-COVID fibrosis patient who is likely to remain stable or gradually improve in comparison with a patient with a known progressive fibrosing interstitial lung disease (fILD). Clearly lung transplantation can be a life-saving therapeutic option for some patients with severe lung injury from COVID-19 infection. In this review, we discuss how lung transplant providers from a number of experienced centers approach lung transplantation for CARDS or post-COVID fibrosis.
Key Words: ARDS, COVID-19, lung transplantation, pulmonary fibrosis
Abbreviations: 6MWT, 6-min walk test; AKI, acute kidney injury; CARDS, COVID-related acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; fILD, fibrosing interstitial lung disease; ILD, interstitial lung disease; LTx, lung transplantation; PFT, pulmonary function testing; rt-PCR, real-time polymerase chain reaction
COVID-19 has infected over 150 million people worldwide since the start of the pandemic.1 Critical disease, characterized by respiratory failure, shock, and multi-organ system failure, occurs in approximately 5% of infections, which equates to approximately 7.5 million individuals struck down by critical disease thus far.2 Death rates amongst patients with critical COVID-19 infections are high, at more than 30% in most series.3 A proportion of survivors from COVID-19 acute lung injury are left with residual lung disease, resulting in the need for supplemental oxygen and impaired mobility.4 The massive influx of critically ill patients has had profound impacts on health care systems throughout the world. The field of lung transplantation (LTx) has not been spared from this and has been affected in a myriad of ways as well. Many of the victims of critical COVID-19 lung injury are relatively young and previously healthy individuals with single-organ dysfunction, so LTx is often considered as a salvage therapeutic option. Transplant centers have seen a large uptick in the number of requests for evaluation for LTx; often involving emotionally and intellectually challenging situations in which patients do not meet traditional criteria for acceptable LTx recipients but have no other path forward to recovery. In this review, we present two very different cases of patients affected by COVID who were referred for LTx evaluation. We will then discuss some common scenarios that can lead to referral for LTx evaluation and discuss issues that must be considered when performing transplantation on patients with COVID-19.
Case 1
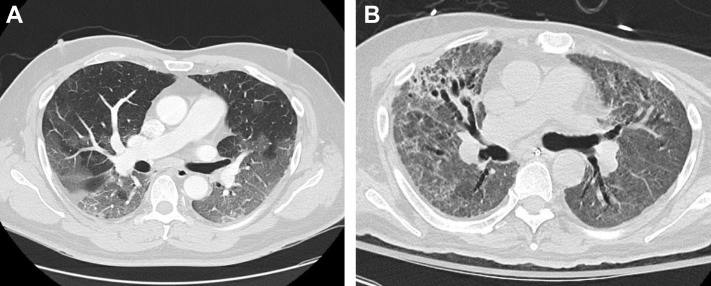
A 62-year-old man presented to the clinic for an LTx evaluation in October 2020, approximately 5 months after initially developing COVID-19. The patient was previously healthy, specifically with no known lung disease, until he was infected with SARS-CoV-2 and required hospitalization. He was treated with remdesivir, steroids, and tocilizumab but developed COVID-19 acute respiratory distress syndrome (CARDS). Although intubation was avoided, he remained reliant on high-flow nasal cannula and noninvasive ventilation, with marked desaturations that limited his mobility. Given dysphagia and marked desaturation, the patient had a tracheostomy and percutaneous gastrostomy tube placed and was discharged to a long-term acute care facility after a 3-month hospitalization. The patient had been decannulated before returning to clinic but remained quite debilitated, having difficulty with activities of daily living and requiring 4 L supplemental oxygen at rest and 6 L with ambulation. CT of the lungs (Fig 1 B) obtained at the time of the clinic visit revealed diffuse ground-glass opacities, upper lung peripheral consolidation, and traction bronchiectasis that had progressed from a CT obtained at the time of admission (Fig 1A). Pulmonary function testing showed a moderately severe restrictive defect (FVC, 1.82 L [45%]; FEV1, 1.55L [50%]). He was unable to tolerate the diffusion capacity of the lung for carbon monoxide maneuver. The patient was referred to pulmonary rehabilitation and scheduled for follow-up in clinic in several months to assess for clinical improvement.
Figure 1.
Case 1: A, Diffuse ground-glass opacities on CT obtained at the time of admission; B, CT chest obtained at initial clinic follow-up 5 months after developing COVID-19 demonstrates diffuse ground-glass opacities, upper lung peripheral consolidation and traction bronchiectasis.
Case 2
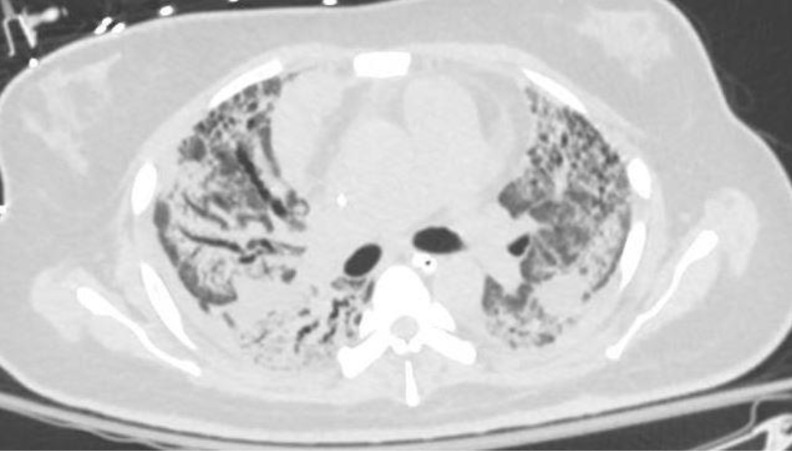
A 37-year-old woman with no significant medical history developed COVID-19 pneumonia with progressive respiratory failure. She was treated with remdesivir, dexamethasone, diuretics, and empiric antibiotics, with no significant improvement. She was intubated and subsequently placed on venous-venous extracorporeal membrane oxygenation (ECMO) approximately 20 days after her initial symptoms. Her hospital course was complicated by ventilator-associated Stenotrophomonas and methicillin-resistant Staphylococcus aureus pneumonia, for which she received appropriate antibiotics. Over time she was weaned off sedation to a point at which she could be awake, interactive, and able to participate in physical therapy. However, her lung mechanics showed no significant improvement, and after 8 weeks she remained on full support from both the ventilator and the ECMO circuit. Her chest CT (Fig 2 ) showed upper lobe predominant pulmonary fibrosis and traction bronchiectasis along with areas of ground-glass opacities throughout.
Figure 2.
Case 2: CT chest with upper lobe predominant pulmonary fibrosis and traction bronchiectasis along with areas of ground-glass opacities.
What Is the Current Status of Lung Transplantation for COVID-19 Lung Injury?
Despite the COVID pandemic generating a tremendous number of potential candidates for LTx, as well as significant interest and enthusiasm for use of LTx as a salvage option for residual COVID-19 lung disease, the actual number of transplants performed worldwide is fairly small. Although the exact number of transplants is not known, the available data give us some sense of the scope of LTx for COVID-19. A query of the United Network for Organ Sharing showed that as of April 30, 2021, only 78 LTxs carrying a recipient diagnosis of COVID-19 had been performed in the United States, 50 for CARDS and 28 for COVID fibrosis.4 This number is likely lower than the true number of transplants, as the United Network for Organ Sharing implemented COVID diagnoses on October 28, 2020, and therefore LTx performed before that date would not be captured unless centers retroactively re-coded prior transplants.4 The European experience seems similar. As of April 23, 2021, the Eurotransplant consortium (responsible for organ allocation in Austria, Belgium, Croatia, Germany, Hungary, Luxemburg, the Netherlands, and Slovenia) reported only 21 patients undergoing transplantation for a diagnosis of COVID-19 (Personal communication, Juergen Behr, April 2021). The relatively small number of LTxs proportional to such a high number of potential recipients is likely multifactorial. Health care systems overwhelmed by the pandemic may not have had adequate resources to provide the intensive support required in recipients. In fact, many transplant programs were placed on hold during the peak of the pandemic. Clinical uncertainty regarding best practices surrounding this new indication for LTx likely also contributed. Finally, many of the referred patients likely had significant relative contraindications to transplantation that precluded their candidacy. Moving forward, it will be essential to review outcomes from the cohort of patients with COVID-19 who underwent transplantation to ensure their outcomes are comparable to other indications for LTx and to identify predictors of success.
How Should One Approach the Outpatient Evaluation for Transplantation for a Patient With COVID Fibrosis?
Should COVID-19 Fibrosis Be Approached Differently From Other Forms of Fibrotic Interstitial Lung Disease?
Likely the COVID-19 pandemic will affect the management of fibrotic interstitial lung disease (fILD) for years to come. COVID-19 acute lung injury will be added to the differential diagnosis or contributory exposure for all fILD, and assessing for a history of COVID-19 pneumonia will become a requisite standard during history taking. Indeed, we posit that any COVID-19 infection might emerge as a risk factor for the subsequent development of interstitial lung disease (ILD), akin to burn-pit exposures and World Trade Center exposures, which only became evident years later. These patients, particularly those more severely affected, are already being referred to ILD and LTx programs. However, the optimal approach in the evaluation and treatment of these patients is yet to be determined. An essential element in the decision to list a patient for LTx is weighing the risk of transplantation vs that of their underlying lung disease. Transplant pulmonologists and surgeons take into consideration their knowledge of the natural history of the patient’s lung disease and only advise listing for LTx when doing so is likely to improve longevity and the patient’s quality of life. The International Society of Heart and Lung Transplantation provided criteria for both referral and listing for LTx in a consensus guideline released in 2014.5 Many of the criteria for ILD could be applied to COVID-19 fibrosis, because they are meant to apply to progressive fILD. However, the rate of progression and the potential for improvement in COVID-19 fibrosis are largely unknown yet and render application of those criteria questionable.
What Is the Natural History of, Risk Factors for, and Pathogenesis of COVID-19 Fibrosis?
Decisions regarding the appropriateness of LTx for COVID-19 in the outpatient setting hinge on knowledge and understanding of the natural history of the disorder, and therefore better insight into the probability of progression or improvement in COVID-19 fibrosis is essential. Unfortunately, data regarding this are limited. The Swiss Covid-19 lung study reported on pulmonary function testing (PFT) and radiographic features 4 months after initial symptoms in 113 patients representing the spectrum of COVID-19 disease, including patients with mild or moderate as well as those with severe disease.6 Patients with prior severe or critical disease had lower lung volumes than patients with mild or moderate disease and had abnormally reduced diffusion capacity, reduced functional capacity, and demonstrated exertional oxygen desaturation. Over 50% of patients had mosaic attenuation, reticulations, or architectural distortion on CT scan after severe or critical disease.6 Fibrotic changes on chest CT were demonstrated in 35% of patients from a prospective cohort of 114 patients who survived severe COVID-19 pneumonia, an additional 27% had interstitial thickening or ground-glass opacification, and 38% had complete radiographic resolution.7 Another series reported 3-month follow-up data on a cohort of 62 patients who required ICU care for CARDS.8 Of these post-CARDS patients, 49% of patients had evidence of reticular lesions, and a further 21% had more distinctive fibrotic patterns. Risk factors for the development of post-COVID fibrosis identified thus far include advanced age, greater severity of illness and longer ICU stay, need for mechanical ventilation, and history of smoking or alcoholism.9 The pathogenesis of pulmonary fibrosis after COVID is incompletely understood. It is believed that the virus activates profibrotic pathways through alteration of the renin-angiotensin system balance and activation of growth factors, including fibroblast growth factor, epithelial growth factor, and transforming growth factor beta. Additionally, direct cellular injury of alveolar epithelial and endothelial cells and macrophages, inflammation, and damage from mechanical forces can lead to fibroblast/myofibroblast activation with resultant fibrosis.10 Likely some patients have a genetic predilection to fibrosis formation after COVID-19 as well, akin to the purported mechanisms in other fILD.
Based on the available data and extrapolating from other cause of ARDS, likely the vast majority of patients with COVID-19 fibrosis will improve or remain stable.11 The duration of time that one can expect ongoing recovery remains unclear. Anecdotally, the authors have observed ongoing improvement over the course of many months. However, patients should be closely followed-up because there may be a minority who develop progressive fibrosis, either from post-COVID fibrosis alone or from exacerbation of a previously unrecognized fibrotic lung disease. For example, an estimated 2% to 7% of nonsmokers and 4% to 9% of smokers have interstitial lung abnormalities, with most of these likely going undiagnosed or without consequence in the absence of CT imaging of the chest.12 How many of such cases are uncovered by an intercurrent COVID-19 infection and whether the existence of these lesions represent a risk factor for a more fibrotic response is uncertain. In such cases, whether COVID is the cause or simply uncovers occult ILD is open to speculation.
How Do the Authors Approach the Evaluation and Management of Post-COVID Fibrosis?
The authors’ approach to post-COVID fibrosis is very similar to that taken for ILD in general. It starts with a careful assessment for previously unrecognized fibrotic lung disease (Table 1 ). The history should assess for dyspnea before the development of COVID-19. Patients should be queried about exposures both occupational and otherwise known to be associated with ILD, family history of ILD, and signs, symptoms, or history of connective tissue disease. Chest imaging obtained before COVID infection, if available, should be carefully reviewed for signs of ILD. Baseline PFT, chest CT, and 6-minute walk test (6MWT) should be obtained. Consideration can be given to obtaining connective-tissue disease serologies, particularly if signs or symptoms exist. Some centers advocate reviewing post-COVID fibrosis cases at a multidisciplinary pulmonary meeting to get input regarding the optimal diagnostic and therapeutic strategy. Patients with any residual pulmonary sequelae should be referred to pulmonary rehabilitation, especially if significant debility exists. Given mounting data on potential benefit, a course of corticosteroids should be considered in patients with radiographic evidence of organizing pneumonia.8 , 13 , 14 Patients should have repeat PFT and 6MWT performed on serial follow-up. Licensed anti-fibrotic therapy, either pirfenidone or nintedanib, can be considered in patients demonstrating evidence of progression.15 Clinical trials to define the role of antifbrotic therapy in CARDS and post-COVID fibrosis are ongoing.16, 17, 18, 19 LTx should be reserved for patients with progressive disease or static disease with substantial disability directly attributable to lung disease. Patients should be screened for anxiety, depression, and posttraumatic stress disorder after their illness, and if identified be referred for proper medical and psychological treatment. In patients with static disease, efforts at medical treatment and rehabilitation should be undertaken, and adequate time for recovery should be allowed before entertaining LTx. Where there is uncertainty regarding eventual recovery, evaluation and education about LTx can proceed with the hope that the patient will recover to the point of not requiring a LTx.
Table 1.
Considerations Prior to Transplant in Outpatients With Post-COVID Fibrosis
| Assess for evidence of preexisting ILD |
|
|
|
| Obtain baseline PFTs, 6MWT, and imaging, and monitor serially |
| Consider a trial of corticosteroids |
| Consider anti-fibrotic (pirfenidone or nintedanib) if evidence of progression |
| Refer for pulmonary rehabilitation |
| Transplantation is reserved for severe debility failing to improve with time, medical therapy, and rehabilitation or progressive disease |
6MWT = 6-min walk test; ILD = interstitial lung disease; PFT = pulmonary function testing
How Should One Approach the Inpatient Evaluation for Transplant of a COVID Fibrosis Patient?
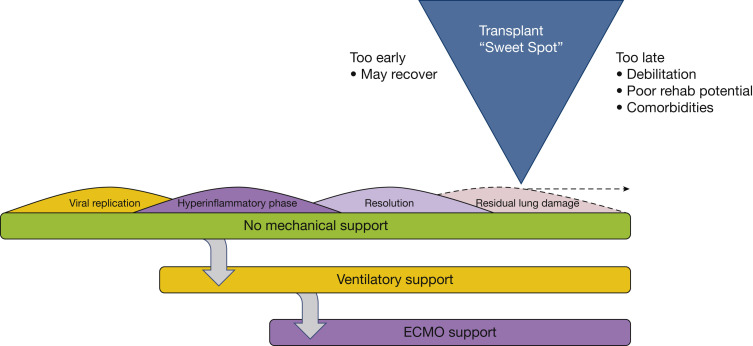
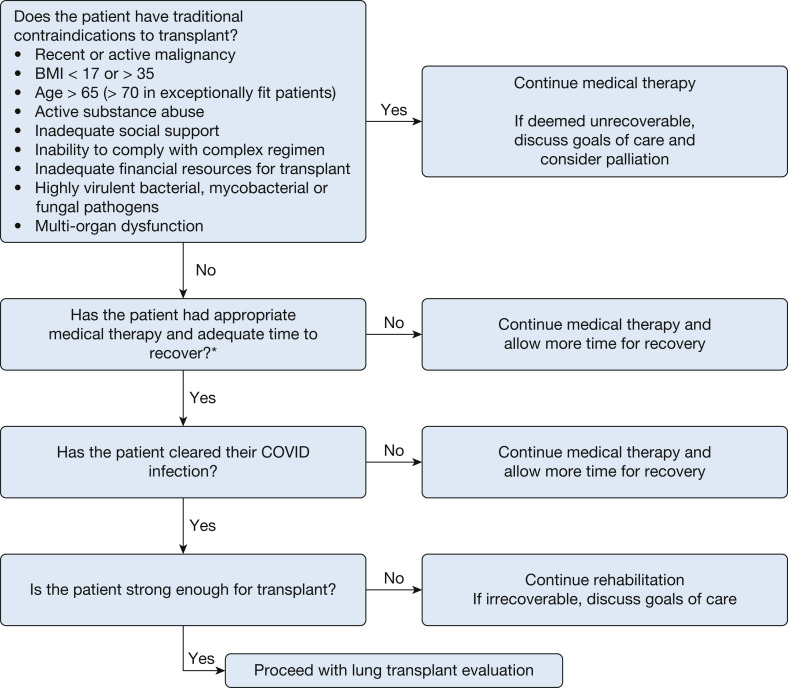
Inpatient evaluation of COVID-19 patients for LTx presents a unique set of challenges from that for outpatients and requires different considerations. In the authors’ collective experience, two general phenotypes of patients are referred for LTx evaluation for COVID. The first are patients with CARDS on either invasive mechanical ventilation or ECMO and failing to improve. The second phenotype are patients that survived their initial COVID infection, but remain dependent on a significant amount of supplemental oxygen, which precludes safe discharge and also limits activity because of exertional desaturation. Clinicians face the difficult dilemma of not performing transplants in patients who are likely to recover from their illness, but also not waiting so long that the patient develops complications or severe deconditioning that precludes transplantation (Fig 3 ). In the following section, we walk through an algorithm of how the authors approach inpatients with CARDS or fibrosis regarding LTx (Fig 4 ). Although we provide a general roadmap to LTx, it should be recognized that not only is each potential recipient unique, but so too are individual lung transplant programs. Every LTx program must take into consideration their own experience and expertise in undertaking high-risk transplants, while factoring in the adequacy of hospital resources, especially at times of a COVID-19 surge. Lung transplantation is by nature triage medicine in which the likelihood of a successful outcome for individual patients needs to be weighed against the societal need in light of an ongoing donor shortage.
Figure 3.
Figure illustrating the clinical course of COVID-19 acute lung injury and the optimal timing of lung transplantation.
Figure 4.
Algorithm for potential diagnostic approach to evaluation of inpatient lung transplant candidates with COVID-19. ∗Adequate time to recovery should consider the individual clinical situation of the patient and must weigh the likelihood of recovery against the risk of development of complications that may be fatal without transplantation.
Does the Patient Have Traditional Contraindications for Transplantation?
This question should be considered first, because if patients have a well-established contraindication to transplantation, then the question of lung transplantation as a salvage option can be quickly laid to rest. Absolute medical contraindications include active or recent malignancy (minimum 2-year disease-free interval in cancer with a low likelihood of recurrence, although a 5-year interval is preferred), significant chest wall deformity, uncorrectable bleeding diathesis, BMI <17 or >35, or untreatable major organ dysfunction (cardiac, liver disease).5 Irreversible neurologic dysfunction represents an absolute contraindication to transplantation. Establishing neurologic status can be difficult in unstable patients who develop marked hypoxemia or hemodynamic instability when not sedated. Other contraindications to transplantation include inability to follow a complex medical regimen, active substance abuse before illness (alcohol, illicit or prescription drugs, nicotine), and inadequate social (no postoperative caregiver) or financial support. Although patients may have apparent single-organ failure, it should not be forgotten that COVID-19 can be a multisystem disease. Much of the multiorgan involvement can be explained by endothelial dysfunction, excessive inflammation, and coagulation abnormalities.20 Although most of these extrapulmonary sequelae manifest during the acute phase of the disease, any residual end-organ dysfunction needs to be ruled out as part of the standard transplant evaluation, because these need to be factored into the patient’s overall transplant candidacy. In general in the United States, insurance coverage is required for adequate financial support to proceed with transplantation. Advanced age also represents a contraindication for transplantation. In general, patients being evaluated for LTx who require advanced life support and are in the midst of a prolonged hospitalization should be younger than age 65, although exceptionally robust individuals older than this can be considered on a case-by-case basis. The age criteria provided here are somewhat arbitrary, but are generally agreed on for post-COVID LTx given the relative lack of experience with this indication. As the experience with post-COVID transplantation grows, perhaps the acceptable age range will grow as well.
In general, all efforts should be made to wake patients up before transplantation to obtain consent for the procedure, provide education, assess interest, and engage them in active rehabilitation. If mechanical ventilation and sedation requirements preclude mobilization and rehabilitation, then ECMO support should be strongly considered. All patients and their caregivers should undergo rigorous LTx education and evaluation by a multidisciplinary care team before transplantation.
Is the Patient’s Lung Injury Irreversible?
This is often a particularly difficult question to answer and requires the best judgment of the lung transplant team. Patients should receive appropriate standard-of-care medical therapy for their COVID-19 infection to optimize the chances for recovery with adequate time allowed for lung recovery. Best clinical practices regarding lung protective ventilation and negative fluid balance are essential to prevent potentiation of lung injury. Although arbitrary, a minimum of 4 weeks’ time for recovery has been suggested in the medical literature, unless a life-threatening complication that cannot be managed without LTx arises earlier.21 , 22 The authors agree that 4 weeks is considered an absolute minimum, and more often wait for 8+ weeks before seriously considering transplantation. Review of CT imaging may be helpful as well. Findings suggestive of irreversible change include traction bronchiectasis and subpleural fibrosis. Anecdotally, we have seen cases with CT evidence of “fibrosis” that has subsequently improved. On the other end of the spectrum, ground-glass infiltrates are commonly encountered early on and are typically due to an alveolar-filling process and hence regarded as potentially reversible. However, this radiographic pattern can also be attributable to early “fine fibrosis,” which should be suspected in patients who are further out, especially if seen in the context of traction bronchiectasis or bronchiolectasis. If evidence of organizing pneumonia is present on CT scan, a trial of corticosteroids and possibly azithromycin is reasonable. CT scanning is also useful in assessing for other potentially treatable causes, including pulmonary edema, pleural disease, and bacterial pneumonia. Nosocomial infection is a potential cause of ongoing lung dysfunction as well and should be assessed for and treated before making a determination of irreversible lung disease. Multidrug-resistant bacterial infections were noted to complicate the course of many reported patients who underwent transplantation for CARDS, and they likely contributed to the irreversible lung damage they developed.22
Has the Patient Cleared Their COVID-19 Infection?
One major concern with transplantation for patients with COVID-19 is the potential impact of lingering active virus. Even a small inoculum of residual viable virus could have potentially devastating consequences, especially in the context of profound immunosuppression typically employed in the early posttransplantation period. With unbridled viral proliferation, COVID-19 could result in acute lung injury, thereby mimicking and perhaps being misdiagnosed as primary graft dysfunction, and thus jeopardizing the patient’s outcome. To our knowledge, this concern has not been realized since transplant programs have taken a conservative approach for the patients receiving transplants thus far.21 , 22 The diagnosis of COVID-19 is most commonly established with real-time polymerase chain reaction (rt-PCR) testing to detect COVID-19 RNA.23 Testing is generally performed on upper respiratory tract samples, although lower respiratory tract samples have a higher viral load and are less likely to yield a false negative result.23 Having a positive rt-PCR result does not necessarily translate into having actively replicating virus, because RNA from viral fragments may still yield a positive rt-PCR. Unfortunately no test aside from viral culture can establish the presence of active virus; however, performance of viral culture is not widely available and presents infection control issues.24 Data suggest that immunocompetent patients affected with severe or critical disease do not have replication-competent virus 20 days after symptom onset; however, severely immunocompromised patients may continue to harbor active virus for significantly longer periods.25 , 26 The prolonged harboring of active virus has potential implications in patients with preexisting ILD exacerbated by COVID-19 who have been managed with chronic immunosuppression. It is also conceivable that persistent virus may be fostered by therapy with corticosteroids or immunomodulators such as tocilizumab. As such, a cautious approach to confirming clearance of COVID-19 is warranted. Bharat and colleagues21 advocated for two negative rt-PCR tests, obtained at least 24 hours apart, from BAL samples in intubated patients before proceeding with LTx. For patients with no tracheostomy or endotracheal tube, two negative upper respiratory tract rt-PCR tests obtained at least 24 hours apart would be the minimum threshold the authors would require to proceed with LTx.22 Because of persistent positive testing, this approach may result in delays in transplantation, but it seems to be a reasonable albeit conservative approach to adopt until further data are available on this issue.
Is the Patient Physically Conditioned Enough for Transplantation?
Most patients with critical COVID-19 will have endured prolonged hospitalization and immobilization, compromised nutritional status from critical illness, and treatment with corticosteroids and neuromuscular blockade, all of which predispose to critical illness polyneuropathy/myopathy and marked deconditioning. Before transplantation, every effort should be made to optimize nutritional status and achieve a wakeful, interactive state in which patients can participate meaningfully in the transplantation process and rehabilitation. ECMO support may be required to achieve these goals. In exceptional circumstances, a patient with a normal baseline functional status and good potential for recovery post-LTx whose pulmonary status precludes rehabilitation before transplantation could be considered. Whether rehabilitation potential and frailty present a contraindication to LTx must be interpreted in the context of the patient’s global clinical picture and must rely on the clinical judgment of the multidisciplinary transplant team. In addition to their physical functional ability, their mental resilience is equally important in withstanding the acute psychological stress of transplantation, as well as the long-term commitment to a strict medical regimen. This is especially difficult for patients who were well before their COVID-19 infection and who have not had the time to accept or adapt psychologically to their new reality.
Should Dual Organ Transplantation Be Considered?
The experience with dual organ transplantation for patients with COVID-19 is limited. Acute kidney injury (AKI) is estimated to occur in approximately 35% of patients hospitalized with COVID-19, with 12% to 15% requiring renal replacement therapy.27 , 28 Mechanical ventilation is a risk factor for severe AKI. Given the potential reversibility of AKI in COVID-19, the appropriateness of proceeding with renal transplantation in this setting is questionable. Some centers have elected to pursue LTX in patients with COVID-19 complicated by AKI requiring renal replacement therapy who were deemed to have a high likelihood of renal recovery. A lung-kidney transplantation has been performed in a patient with lung and renal failure deemed irreversible.29 One heart-lung transplant for COVID-19 in a patient with preexisting cardiomyopathy has been reported.30 To our knowledge, no lung-liver transplantations for COVID-19 have been performed as of yet. Although it is possible that dual organ transplantation could be entertained in the future for highly select candidates, at this time, the authors believe that multi-organ dysfunction should preclude candidacy for LTx in most candidates.
Are There Other Issues Specific to COVID-19 to Consider When Performing a Transplantation?
Given that the LTx recipient will be tested for and proven clear of COVID-19 infection before transplantation, the operation need not be performed in a negative-pressure environment. Surgical teams may consider wearing N-95 or equivalent masks and eye protection in addition to standard gown and gloves. Bilateral lung transplantation for COVID-19 has been recommended, because many patients develop significant pulmonary hypertension.23 , 24 Additionally, explants from COVID-19 LTX recipients revealed cavitary areas of pneumonia that could serve as a nidus of infection if a single-lung transplantation was performed.23 Single-lung transplantation can be considered on a case-by-case basis, even in the presence of pulmonary hypertension, especially in patients who are in dire straits with a short window to receive a transplant. There may be added theoretic attraction to single-lung transplants, because in some patients this could serve as a “bridge to recovery” of the remaining native lung. Intraoperatively, surgical teams should be prepared for bleeding given the likelihood of pleural adhesions and platelets dysfunction in patients managed with preoperative ECMO support.24 Transplant centers undertaking these cases should be experienced in high-acuity transplantation, with robust resources for extracorporeal support and postoperative rehabilitation. Our collective experience in performing transplantation in these patients is that their course and risk of specific posttransplantation complications, pulmonary or extrapulmonary, such as acute kidney injury, is no different from that of a general transplant population. This is likely because of these patients being closely vetted for end-organ dysfunction before acceptance.
Case 1 Follow-up
The patient was treated with a course of corticosteroids and completed pulmonary rehabilitation. On follow-up 6 months after his initial hospitalization, his FVC had increased by approximately 100 mL to 1.9 L (50% predicted), and his 6MWT had increased by 75 m to 316 m, with decreased need for supplemental oxygen. He felt less dyspneic with activities of daily living. The decision was made to continue rehabilitation and follow-up in several months, but to defer initiation of a lung transplant evaluation and assess for ongoing improvement.
Case 2 Follow-up
In the setting of no significant clinical improvement despite maximum respiratory support, the patient underwent an expedited lung transplant evaluation. After 10 weeks in the hospital, 7 of which were on venous-venous ECMO and mechanical ventilation, she received a bilateral lung transplant. Of note, she had two negative COVID swabs and cleared COVID precautions per the hospital epidemiology team before being listed for transplantation. She had a full recovery with minimal complications, and on postoperative day 16 was discharged to an acute rehabilitation facility without any subsequent oxygen needs.
Conclusion
COVID-19 can result in severe, irreversible lung injury. In these cases, LTx may represent the only viable therapeutic option, albeit in a very small, highly select group of patients. This patient population presents a number of unique challenges for providers that require careful consideration. Likely COVID-19-associated lung disease will impact the field of ILD and LTx for years to come. Further study is required to determine the natural history of COVID-19-related lung disease. Questions to be addressed through future research include which patients are likely to fully recover, who will be left with residual lung injury, and who will progress to develop persistent or progressive fibrosis, requiring transplant consideration. Further study is also required to determine whether outcomes from LTx for COVID are equivalent to other indications and whether these patients are at risk for unique post-LTx complications, including VTE and neurocognitive issues. An International Registry of COVID-related lung transplants could provide a foundation for expediting the answers to these and other emerging questions in this nascent area.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
References
- 1.World Health Organization WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from The Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong R.A., Kane A.D., Kursumovic E., Oglesby F.C., Cook T.M. Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies. Anaesthesia. 2021;76:537–548. doi: 10.1111/anae.15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health & Human Services Organ Procurement and Transplantation Network. https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced/
- 5.Weill D., Benden C., Corris P.A., et al. A consensus document for the selection of lung transplant candidate: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Trans. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Guler S.A., Ebner L., Aubry-Beigelman C., et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57:2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X., Fan Y., Alwalid O., et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez J., Benitez I.D., Carmona P., et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies [Published online ahead of print August 20, 2020] Pulm Med. 2020 doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald L.T. Healing after COVID-19: are survivors at risk for pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2021;320(2):L257–L265. doi: 10.1152/ajplung.00238.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udwadia Z.F., Koul P.A., Richeldi L. Post-COVID lung fibrosis: the tsunami that will follow the earthquake. Lung India. 2021;38:S41–S47. doi: 10.4103/lungindia.lungindia_818_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatabu H., Hunninghake G.M., Richeldi L., et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med. 2020;8(7):726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myall K.J., Mukherjee B., Castanheira A.M., et al. Persistent post-COVID-19 interstitial lung disease: an observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadasz I., Husain-Syed F., Dorfmuller P., Roller F.C., Tello K. Severe organizing pneumonia following COVID-19. Thorax. 2021;76(2):201–204. doi: 10.1136/thoraxjnl-2020-216088. [DOI] [PubMed] [Google Scholar]
- 15.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for anti-fibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health Clinical Center Treatment With Pirfenidone for COVID-19 Related Severe ARDS. NCT04653831. ClinicalTrials.gov. National Institutes of Health; 2020. https://clinicaltrials.gov/ct2/show/NCT04653831?term=pirfenidone&cond=Covid19&draw=2&rank=1 Updated December 4, 2020.
- 17.National Institutes of Health Clinical Center Pirfenidone Compared to Placebo in Post-COVID19 Pulmonary Fibrosis COVID-19 (FIBRO-COVID). NCT04607928. ClinicalTrials.gov. National Institutes of Health; 2020. https://clinicaltrials.gov/ct2/show/NCT04607928?term=pirfenidone&cond=Covid19&draw=2&rank=2 Updated November 5, 2020.
- 18.National Institutes of Health Clinical Center Pirfenidone vs. Nintedanib for Fibrotic Lung Disease After Coronavirus Disease-19 Pneumonia (PINCER). NCT04856111. ClinicalTrials.gov. National Institutes of Health; 2021. https://clinicaltrials.gov/ct2/show/NCT04856111?term=pirfenidone&cond=Covid19&draw=2&rank=3 Updated April 28, 2021.
- 19.National Institutes of Health Clinical Center The Study Will Evaluate the Use of Nintedanib in Slowing Lung Fibrosis in Patients With Pulmonary Infiltrates Related to COVID-19 (ENDCOV-I). NCT04619680. ClinicalTrials.gov. National Institutes of Health; 2020. https://clinicaltrials.gov/ct2/show/NCT04619680?term=nintedanib&cond=Covid19&draw=2&rank=4 Updated April 1, 2021.
- 20.Matsuishi Y., Mathis B.J., Shimojo N., Subrina J., Okubo N., Inoue Y. Severe COVID-19 infection associated with endothelial dysfunction induces multiple organ dysfunction: a review of therapeutic interventions. Biomedicines. 2021;9(3):279. doi: 10.3390/biomedicines9030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12(574):eabe4282. doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharat A., Machuca T.N., Querrey M., et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9:487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai C.K.C., Lam W. Laboratory testing for the diagnosis of COVID-19. Biochem Biophys Res Commun. 2021;538:226–230. doi: 10.1016/j.bbrc.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jefferson T., Spencer E.A., Brassey J., Heneghan C. Viral cultures for COVID-19 infectious potential assessment: a systematic review [Published online ahead of print December 3, 2020] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Interim Guidance on Ending Isolation and Precautions for Adults with COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
- 26.Aydillo T., Gonzalez-Reiche A.S., Aslam S., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch J.S., NG J.H., Ross D.W., et al. Acute kidney injured in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowe B., Cai M., Xie Y., Gibson A.K., Maddukuri G., Al-Azy Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanford Medicine News Center Double transplant at Stanford saves life of critically ill COVID-19 patient. https://med.stanford.edu/news/all-news/2021/03/double-transplant-saves-life-of-critically-ill-covid-19-patient.html
- 30.Vanderbilt University Medical Center COVID patient’s heart-lung transplant is world’s first. https://news.vumc.org/2020/10/08/covid-patients-heart-lung-transplant-is-worlds-first/