Abstract
Exclusive breastfeeding (EBF)—giving infants only breast-milk for the first 6 months of life—is a component of optimal breastfeeding practices effective in preventing child morbidity and mortality. EBF practices are known to vary by population and comparable subnational estimates of prevalence and progress across low- and middle-income countries (LMICs) are required for planning policy and interventions. Here we present a geospatial analysis of EBF prevalence estimates from 2000 to 2018 across 94 LMICs mapped to policy-relevant administrative units (for example, districts), quantify subnational inequalities and their changes over time, and estimate probabilities of meeting the World Health Organization’s Global Nutrition Target (WHO GNT) of ≥70% EBF prevalence by 2030. While six LMICs are projected to meet the WHO GNT of ≥70% EBF prevalence at a national scale, only three are predicted to meet the target in all their district-level units by 2030.
Subject terms: Nutrition disorders, Risk factors, Developing world
Bhattacharjee and Schaeffer et al. map exclusive breastfeeding (EBF) in 94 low- and middle-income countries (LMICs), finding increased EBF practice and reduced subnational variation across the majority of LMICs from 2000 to 2018. However, only six LMICs will meet WHO’s target of ≥70% EBF by 2030 nationally, and only three will achieve this in all districts.
Main
Exclusive breastfeeding (EBF)—giving infants only breast-milk (and medications, vitamins or oral rehydration solution (ORS) as needed) for the first 6 months of life—is effective in preventing deaths from diarrhoea, pneumonia and other leading causes of child mortality1–4. Breast-milk has been characterized as a ‘personalized medicine’ for infants2 due to its nutritional properties, natural growth stimulators and tailored immune-protective properties, which collectively contribute to infant growth, development and survival5–8. Furthermore, evidence suggests long-term health benefits of breastfeeding, including reduced risks of cardiovascular diseases and increased benefits to human capital in adulthood2,9,10. The introduction of supplementary food and water during the first 6 months of life, particularly in settings lacking reliable access to clean water, can expose infants to infections from a range of pathogens1,3. Along with the initiation of breastfeeding within the first hour after birth and continued breastfeeding to 2 years, the World Health Organization (WHO) considers EBF to be an optimal breastfeeding practice11 and included it as a proven protective intervention in the Global Action Plan for Pneumonia and Diarrhoea (GAPPD)1. Despite the benefits, the proportion of exclusively breastfed children remains low in many low- and middle-income countries (LMICs), where most child deaths attributed to suboptimal breastfeeding occur12. Accelerated uptake in EBF is required to successfully achieve the World Health Organization’s Global Nutrition Target (WHO GNT) of at least 50% EBF prevalence by 202511 and the recently updated WHO GNT of at least 70% EBF prevalence by 203013.
This study is a part of a body of work mapping high-spatial-resolution estimates to track progress toward the WHO GNTs14–17. Building on our previous geospatial analysis of EBF prevalence in sub-Saharan Africa14, we synthesized data from 349 geo-referenced household surveys from years 1998 to 2018 representing 302,435 infants under 6 months to produce annual 2000–2018 subnational estimates for the proportion and absolute number of exclusively breastfed infants for 94 LMICs. We used 14 geographically distinct modelling regions which were determined on the basis of epidemiological homogeneity and geographical contiguity by the Global Burden of Disease (GBD) study18 (Supplementary Table 4 and Supplementary Fig. 7). We first mapped estimates on a 5 × 5-km grid to align with the resolution of many of the covariates used in this study and then aggregated to more policy-relevant second- and first-administrative-level units for each country in our analysis. Here we provide mapped annual estimates of EBF prevalence and trends at policy-relevant administrative and national levels from 2000 to 2018, as well as the estimated number of infants not receiving EBF. On the basis of trends in the most recent years, we project these estimates to the years 2025 and 2030, and determine the probability of meeting the WHO GNTs of ≥50% and ≥70% EBF prevalence in the respective target years. Furthermore, we examine relative and absolute subnational inequalities of EBF prevalence within LMICs and compare areas with low EBF prevalence to areas with high disease burden and low coverage of mitigating interventions. The full array of our model outputs—at various spatial levels and aggregations—is available through an online visualization tool (https://vizhub.healthdata.org/lbd/ebf), with additional results in the Supplementary Information.
Results
Regional, national and subnational trends in EBF prevalence
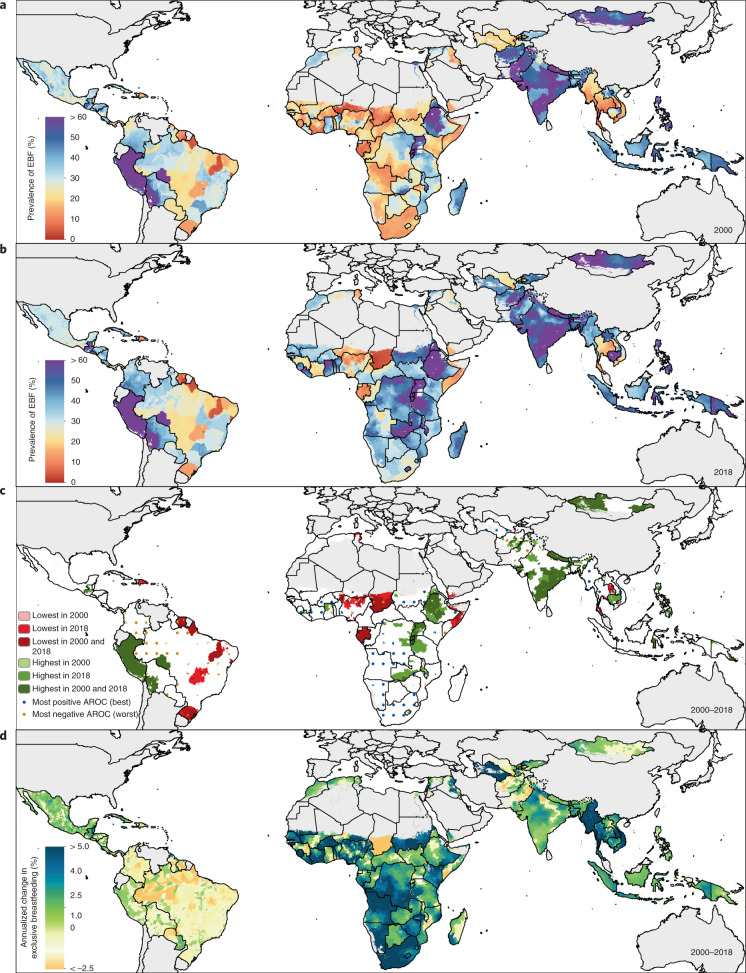
EBF prevalence varied widely between and within LMICs from 2000 to 2018 (Fig. 1a,b). General increases in mean EBF prevalence occurred across LMICs over the study period, from 28.6% (95% uncertainty interval: 22.9–35.4%) in 2000 to 38.7% (28.3–49.9%) in 2018. Regionally, most LMICs in Andean South America, South Asia and East Asia had relatively high EBF levels throughout the study period; for example, Peru (63.6% (60.9–66.4%) in 2000; 69.2% (57.6–79.1%) in 2018), Nepal (64.2% (49.1–76.9%) in 2000; 64.5% (53.6–74.3%) in 2018) and Mongolia (51.9% (49.3–54.4%) in 2000; 55.1% (52.1–58.1%) in 2018) all maintained high national EBF prevalence. Several countries in other regions maintained low EBF prevalence throughout the study, including the Dominican Republic (13.2% (9.7–17.8%) in 2000; 8.2% (4.7–14.3%) in 2018), Suriname (6.4% (4.3–9.2%); 5.7% (3.2–9.6%)), Tunisia (10.9% (6.7–17.0%); 12.2% (7.7–18.0%)), Yemen (11.7% (4.7–22.3%); 12.5% (7.3–20.2%)) and Thailand (7.5% (4.9–11.1%); 13.9% (9.8–19.0%)). National 2018 EBF levels varied broadly between countries in the regions of Central America and the Caribbean (8.2% (4.7–14.3%) in the Dominican Republic; 50.7% (40.3–61.7%) in Guatemala), Tropical South America (5.7% (3.2–9.6%) in Suriname; 32.4% (29.1–35.8%) in Paraguay), Central Asia (18.7% (13.9–24.7%) in Uzbekistan; 51.8% (44.9–58.8%) in Afghanistan), Southeast Asia (13.9% (9.8–19.0%) in Thailand; 62.0% (50.4–72.9%) in Cambodia), North Africa (12.2% (7.7–18.0%) in Tunisia; 51.3% (44.7–57.6%) in Sudan) and throughout sub-Saharan Africa. Overall, in 2018, national EBF prevalence varied by as much as 39.2 times across all LMICs, ranging from 2.2% (1.1–4.0%) in Chad (Western sub-Saharan Africa) to 87.7% (76.9–94.2%) in Rwanda (Eastern sub-Saharan Africa).
Fig. 1. EBF prevalence and progress (2000–2018) among infants under 6 months across LMICs.
a,b, Prevalence of EBF practices at the district level in 2000 (a) and 2018 (b). c, Overlapping population-weighted highest and lowest deciles of prevalence and weighted AROC between 2000 and 2018, at the district level. d, Weighted annualized percentage change in EBF prevalence between 2000 and 2018. Maps reflect administrative boundaries, land cover, lakes and population; grey-coloured grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’ or were not included in this analysis50–55.
Select LMICs made notable progress in the study period. In 2000, 57 LMICs had <30% estimated mean EBF prevalence in at least half of their first-administrative-level units (henceforth ‘provinces’); by 2018, eight of these countries had increased mean EBF prevalence to come close to the original WHO GNT of 50% EBF prevalence, with at least 45% EBF in most provinces: Cambodia (88.2%; 30 of 34 provinces), Democratic Republic of the Congo (DRC; 69.2%; 18 of 26), Guinea-Bissau (77.8%; 7 of 9), Lesotho (100.0%; 10 of 10), Liberia (80.0%; 12 of 15), Sudan (88.9%; 16 of 18) and Turkmenistan (66.7%; 4 of 6). For example, Kâmpóng Chhnang province in central Cambodia (19.5% (14.0–26.3%) in 2000; 63.4% (47.5–77.8%) in 2018) and West Kurdufan state in southern Sudan (13.4% (10.4–17.2%) in 2000; 51.9% (40.6–63.0%) in 2018) both experienced large gains. Overall, 34 LMICs had at least one province that made similar gains from <30% to ≥45% EBF prevalence (45.1%; 296 of 656 provinces across these 34 LMICs).
To compare trends and prevalence levels, we overlaid the highest and lowest population-weighted deciles of EBF at the second-administrative level (henceforth ‘district’) to the highest and lowest deciles of annualized rates of change (AROC) (Fig. 1c). Along with having some of the lowest levels of EBF practice in 2000 and 2018, Chad, Suriname, Somalia and Brazil also had among the highest rates of annualized decline in EBF during the study period. Districts in Niger, Nigeria, Gabon, Yemen, Tunisia, the Dominican Republic, southern Thailand and central Philippines also had among the lowest EBF prevalence levels in both 2000 and 2018; even despite some of the highest rates of EBF increase in southern Vietnam and northeastern Thailand, EBF remained among the lowest levels in these districts in both years (Fig. 1c). Districts throughout much of Peru, southeastern Bolivia, eastern Brazil, Ethiopia, Uganda, Rwanda, Burundi, India, Nepal, Mongolia and the Philippines had among the highest prevalence levels in both years; as did select districts in Guatemala, Zambia, Malawi, Eritrea, Afghanistan, Pakistan and Indonesia. Districts scattered throughout Guatemala, the DRC, northern Liberia, northern Ghana, Eritrea, western Tanzania, Zambia, Malawi, Lesotho, Bangladesh and Cambodia had among the highest levels for the year 2018, as did select districts in western Honduras, eastern and western Sudan and northern Laos. Districts with some of the highest rates of annualized increase in EBF were located in southern Sierra Leone, central Côte d’Ivoire, southern Burkina Faso, central Niger, central Nigeria, Sudan, eastern Ethiopia, DRC, Angola, Namibia, South Africa, northern Mozambique, central Kenya, Turkmenistan, western Kyrgyzstan, Myanmar, northern Thailand, southern Laos and southern Vietnam. In contrast, the highest rates of annualized decline in EBF were seen in eastern Honduras, Colombia, Brazil, eastern Bolivia, eastern Zambia, eastern Ghana, eastern Niger, central Nigeria, central Mozambique, central Madagascar, central Afghanistan and Pakistan. The Philippines and Brazil both had among the best-performing and worst-performing districts for both years in regard to prevalence and Niger, Nigeria and Mozambique had districts among the highest and lowest rates of annualized change.
By mapping AROC from 2000 to 2018, we show where and to what degree EBF practices have increased or decreased on average over the study period (Fig. 1d). Most district-level units across LMICs experienced increases in estimated mean prevalence of EBF over the study period (62.6%; 15,379 of 24,556 districts), while over a third experienced decreases 37.2% (9,137 of 24,556 districts). Overall, 28 LMICs experienced annualized increases in mean EBF prevalence in all districts; 25 LMICs had >2.5% annualized increase in all districts, including Bangladesh, Cambodia, Botswana, Liberia and Lesotho (Supplementary Table 8a). Sudan, Zimbabwe, South Africa, Kenya, Myanmar and Turkmenistan were among 14 LMICs that experienced among the highest annualized EBF increases (>5% AROC) in all of their districts’ mean estimates (Supplementary Table 8a). In 13 LMICs, most districts had decreasing annualized trends in EBF practice (<0% AROC); Chad was the only LMIC that experienced EBF declines in all of its districts (Supplementary Table 8b). A large proportion (69.1%; 65 of 94) of LMICs had both annualized increases and decreases in EBF across their districts; 7 (7.4%) LMICs had districts that had experienced both extremes of the mapped annualized increases (>5%) and decreases (≤−2.5%): Nigeria, Somalia, Mozambique, Niger, Thailand, the Philippines and India (Supplementary Table 8c).
Comparison of units with low EBF and other health conditions
To identify some of the highest-need provinces across LMICs, we compared the lowest decile of EBF prevalence in this study to the highest decile levels of our previously published geospatial estimates of stunting16, childhood diarrhoea19 and under-5-yr mortality20 and the lowest decile of coverage of ORS21 and access to piped water22 (Supplementary Information section 5.5 and Supplementary Figs. 19–23). Several provinces in Chad had among the lowest levels of EBF, as well as some of the highest levels of under-5-yr mortality, stunting and diarrhoea and some of the lowest coverage levels of ORS and access to piped water. Also among the lowest levels of EBF, select provinces in Nigeria had among the lowest ORS coverage and both Niger and Nigeria had provinces with low EBF prevalence and some of the highest child stunting and mortality rates. Yemen had provinces with codistribution of low EBF prevalence and high levels of child diarrhoea and stunting. Somalia had several provinces with low EBF and high under-5-yr mortality rates, while Gabon had one province with among the highest childhood diarrhoea rates and lowest EBF prevalence. One province in Comoros and several provinces in Thailand had among the lowest levels of EBF as well as access to piped water (Supplementary Table 12).
Geographic inequalities in EBF prevalence
We calculated Gini coefficients as a measure of geographic inequality at the country level23. Our results suggest that geographic inequality in EBF prevalence decreased in most of the countries from 2000 to 2018 (77 of 94) on the basis of Gini coefficients; while there were 11 countries in 2000 whose Gini coefficient was >0.25, only Nigeria and the Philippines had coefficients above 0.25 in 2018.
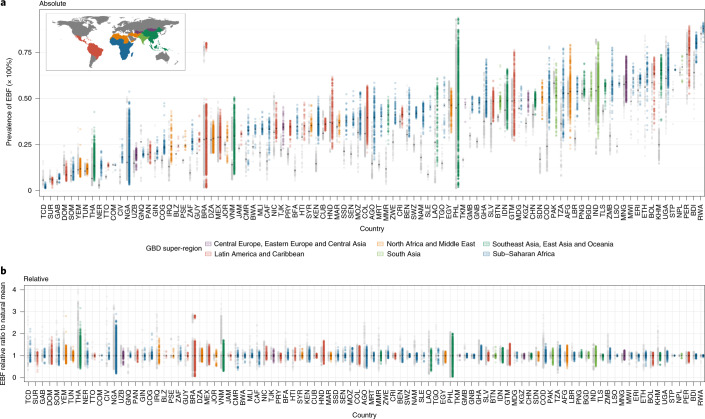
We quantified absolute geographic inequalities in EBF prevalence by calculating the absolute differences between district-level units with the lowest and highest prevalence in each country (method details in Supplementary Information section 4.4.3). Between 2000 and 2018, absolute geographic inequalities had increased in over a third (38.3%; 36 of 94) of LMICs, at least doubling in eight countries, including Afghanistan, Jamaica, Jordan, Nepal, Niger, Republic of the Congo, Sierra Leone and Turkmenistan (Fig. 2). Of the 92.6% (88 of 94) of LMICs which had increased in EBF national prevalence, almost half (42.1% (37 of 88)) had also increased in absolute inequalities—including in Afghanistan and Republic of the Congo—indicating areas left behind in overall national progress. While 39.3% (37 of 94) of LMICs had increased absolute inequalities between districts, 12.6% (12 of 94) of LMICs decreased their absolute inequalities; absolute inequalities in the other 45 LMICs in the analysis remained relatively the same. Several countries had reduced absolute inequalities by at least one-third while also increasing their EBF prevalence, including Burundi, Cuba, Eritrea, Gabon, Guinea, Malawi, Mali, Rwanda, Trinidad and Tobago and Uganda. Absolute inequalities in EBF were at least halved in eight LMICs: Burundi, Chad, Cuba, Eritrea, Gambia, Guinea, Mali and Rwanda. Along with substantial reductions in absolute inequalities, Gambia also substantially increased its national EBF prevalence, while Guinea, Mali and Rwanda experienced marginal increases in national prevalence; Chad, however, had decreased EBF prevalence across all its district-level units. In 2018, absolute differences in EBF between the highest- and lowest-prevalent districts within countries ranged from 1.1 to 45.3 times; São Tomé and Príncipe had the least variation, ranging from 66.0% (29.8–90.9%) in Me-Zochi (São Tomé) to 67.8% (31.3–93.0%) in Pague (Príncipe), while the Philippines ranged from 1.5% (0.9–2.3%) in San Jose (Antique) to 92.8% (88.6–95.9%) in Bagamanoc (Catanduanes). Most LMICs (60.0% (57 of 94)) had twofold or more difference in EBF between districts in 2000; 36.8% (35 of 94) had this difference in 2018. A threefold or greater difference between units was experienced in 34 (35.8%) and 15 (15.8%) LMICs in 2000 and 2018, respectively. A sixfold or greater difference was experienced by 14 (14.7%) LMICs in 2000 and 4 (4.2%) LMICs in 2018—Brazil, Nigeria, the Philippines and Thailand.
Fig. 2. Geographic inequalities in EBF prevalence across 94 countries for 2000 and 2018.
a, Absolute inequalities: range of EBF estimates in district-level units within 94 LMICs. b, Relative inequalities: range of ratios of EBF estimates in district-level units relative to country means. Each dot represents a district-level unit. The lower bound of each bar represents the district-level unit with the lowest EBF in each country. The upper end of each bar represents the district-level unit with the highest EBF in each country. Thus, each bar represents the extent of geographic inequality in EBF estimated for each country. Bars indicating the range in 2018 are coloured according to their GBD super-region. Grey bars indicate the range in EBF in 2000. The black diamond in each bar represents the median EBF estimated across district-level units in each country and year. A coloured bar that is shorter than its grey counterpart indicates that geographic inequality has narrowed. Countries are labeled by their ISO 3 codes (full country names are listed in Supplementary Table 4).
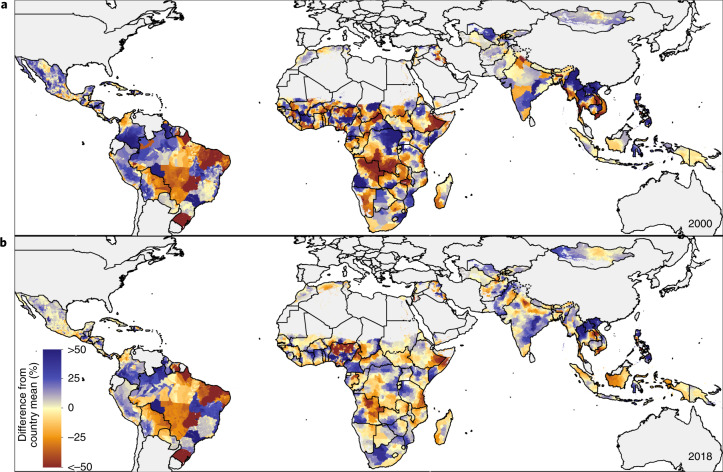
We quantified relative inequalities by calculating the relative differences between each district-level unit and its country’s average for 2000 and 2018 (Supplementary Information section 4.4.3). Overall, within-country relative inequalities in EBF coverage declined; 48 LMICs in 2000 and 25 LMICs in 2018 had district-level units that deviated by >50% from the country mean (Fig. 2). Throughout the study period, Belize, Egypt, Eritrea and Papua New Guinea demonstrated low within-country relative differences in EBF, whereas Myanmar, Cambodia, Laos, Ghana and Peru had reduced relative geographic inequalities over time (Fig. 3). As an example, northern districts of Myanmar positively deviated and southwestern districts negatively deviated by ≥30% from the national mean in 2000 but these within-country relative differences decreased to <10% from the national mean in either direction by 2018. Within-country relative inequalities remained high, however, in Comoros, Brazil, the Philippines and Guyana in both 2000 and 2018. In 2018, the largest relative inequalities were in Nigeria, Brazil, Thailand and the Philippines. In particular, Nigeria’s most negatively deviating district-level units were concentrated in the north and southeast, while central districts loomed largely above the mean in 2018 (20.1% (18.8–21.4%) national mean; 3.4% (1.7–5.9%) in Baure (Osun); 53.7% (41.3–62.7%) in Ife Central (Osun)). Additionally, in Brazil, deviating patterns were scattered, with districts throughout much of the Amazon Basin in the west positively deviating from the national mean (for example, 80.1% (80.8–83.7) in Machadinho municipality (Rondônia) in 2018) and many districts in the Brazilian Highlands negatively deviating from the mean (for example, 10.7% (9.9–11.7%) in Abadia de Goiás municipality (Goiás); national mean 27.1% (25.6–28.6%)).
Fig. 3. Relative geographic inequalities within countries in EBF prevalence in 2000 and 2018: comparing district-level units to the country-level means.
a,b, Relative deviation of EBF prevalence in district-level units from the country-level EBF mean in 2000 (a) and 2018 (b). Blue indicates a positive deviation from the EBF country-level mean, indicating a higher EBF prevalence level. Red indicates a negative deviation from the EBF country-level mean, indicating a lower EBF prevalence level. Maps reflect administrative boundaries, land cover, lakes and population; grey-coloured grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’ or were not included in this analysis50–55.
Absolute number of children not exclusively breastfed
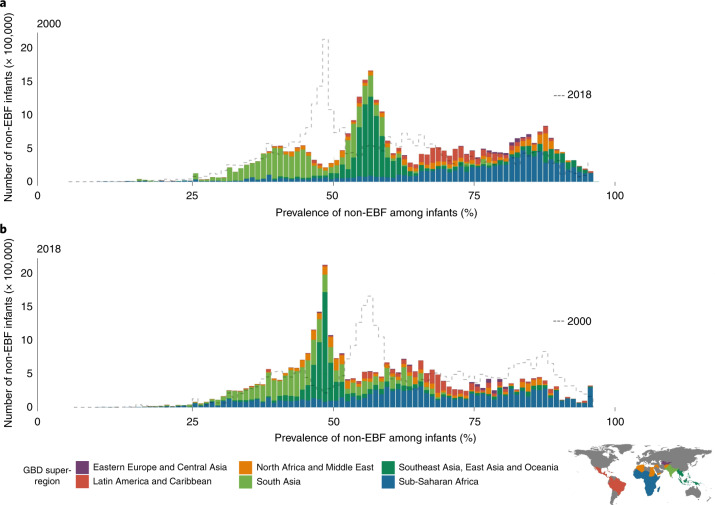
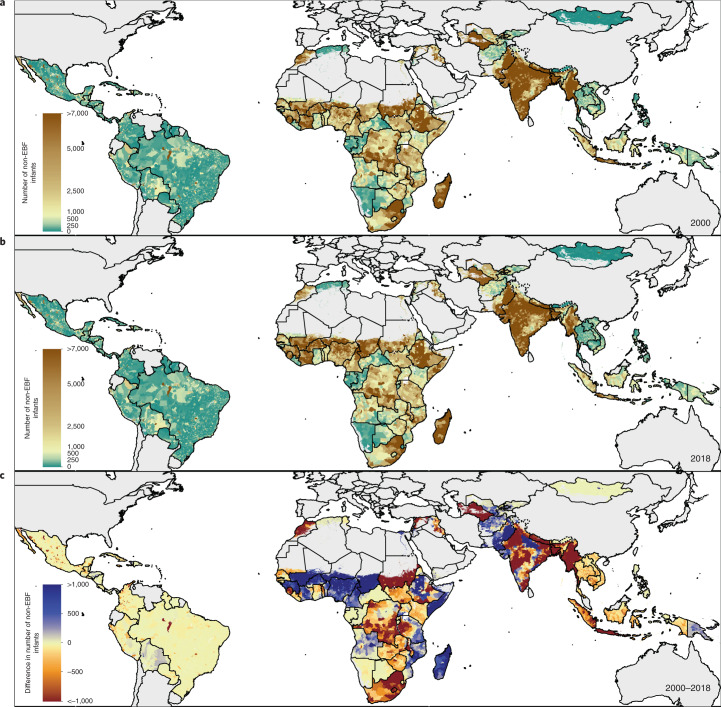
In 2000, of the ~56,039,700 (51,145,700–60,940,400) infants under 6 months in the populations across the 94 countries in our analysis (according to the 2019 GBD Study24), an estimated 33,489,000 (31,867,900–35,031,200) infants were not exclusively breastfed. In 2018, among a population of ~57,787,200 (51,016,200–64,661,000) infants under 6 months in 94 LMICs24, an estimated 31,878,600 (28,721,500–34,999,000) children were not exclusively breastfed, representing a 4.8% (0.1–9.9%) decrease since 2000. A comparison of shifts in prevalence and numbers of non-EBF children over the past two decades suggests that, despite some of the largest increases in EBF prevalence in Asia and Oceania, the bulk of the total number of infants not benefiting from EBF still comes from these regions (Fig. 4).
Fig. 4. Number of infants under 6 months who are not being exclusively breastfed, distributed across non-EBF prevalence in 2000 and 2018, across 94 countries.
a, Non-EBF infants under 6 months in 2000. b, Non-EBF infants under 6 months in 2018. The dotted line in the 2000 plot is the shape of the distribution in 2018 and the dotted line in the 2018 plot represents the distribution in 2000. Bar heights represent the total number of infants under 6 months who were not exclusively breastfed by district-level units, with corresponding non-EBF prevalence. Bins are a width of one non-EBF infant per 100 infants. The colour of each bar represents the global region as defined by the subset legend map. As such, the sum of heights of all bars represents the total number of non-EBF infants across the 94 countries.
Four countries have more than an estimated million infants each that were not exclusively breastfed in 2018 (Fig. 5), accounting for 39.9% of the total: India (5,351,900 (4,825,700–5,904,700); 19.1% of the total non-EBF infants), Nigeria (2,899,100 (2,850,500–2,945,200); 10.4%), Pakistan (1,770,300 (1,653,400–1,889,400); 6.3%) and Brazil (1,157,000 (1,116,400–1,200,800); 4.1%). Eight additional countries each had an estimated half-million children or more that were not exclusively breastfed in 2018, accounting for 17.4% of the total non-EBF infants: Indonesia (982,200 (897,700–1,063,100); 3.5% of the total), the Philippines (782,100 (658,100–893,800); 2.8%), Mexico (760,500 (699,500–813,800); 2.7%), DRC (741,200 (623,700–848,700); 2.7%), Ethiopia (626,800 (427,600–851,000); 2.2%), Bangladesh (609,900 (402,100–832,000); 2.1%), Egypt (574,100 (480,700–670,500); 2.0%) and Vietnam (515,500 (455,800–564,800); 1.8%). Although some of these countries were close to achieving the original WHO GNT of 50% prevalence by 2018, with >45% mean national prevalence, Mexico has had low EBF prevalence scattered throughout its units and the Philippines has consistently had some of the largest subnational inequalities. Nigeria, Brazil and Vietnam have the dual complications of high geographic inequalities and relatively low national EBF prevalence (<30%).
Fig. 5. Number of infants under 6 months who are not being exclusively breastfed at the district level, 2000 and 2018.
a,b, Number of infants under 6 months who are not being exclusively breastfed, aggregated to district-level units in 2000 (a) and 2018 (b). c, Difference in number of infants under 6 months who are not being exclusively breastfed between 2018 and 2000, aggregated to district-level units. Maps reflect administrative boundaries, land cover, lakes and population; grey-coloured grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’ or were not included in this analysis50–55.
Projected EBF prevalence in 2025 and 2030
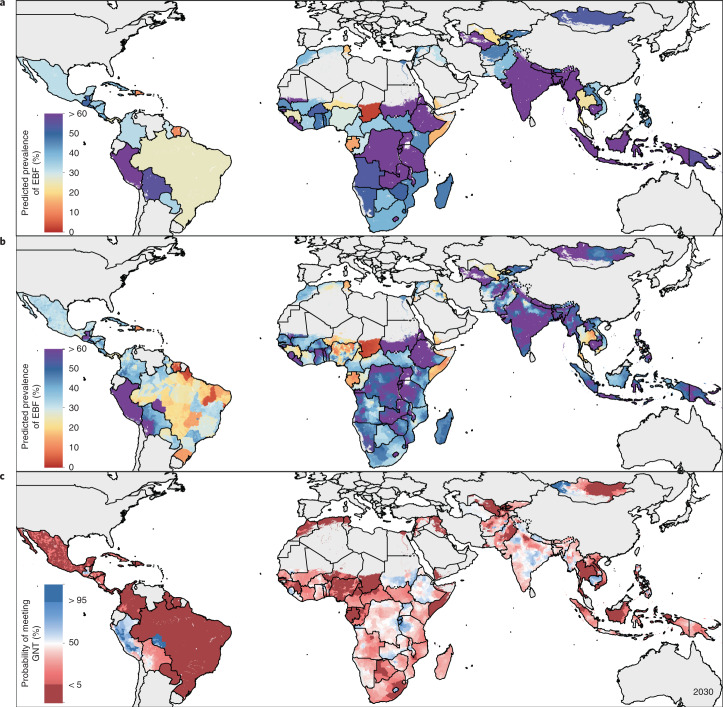
On the basis of previous spatiotemporal historical trends and the assumption that recent trends will continue, we projected EBF estimates for the year 2025 (Supplementary Fig. 17a,b) and 2030 (Fig. 6a,b). Overall, EBF prevalence across LMICs is expected to increase from 38.7% (28.3–49.9%) in 2018 to 42.6% (25.6–60.5%) in 2025 and to reach 45.2% (23.9–67.2%) by 2030. National EBF prevalence is expected to vary by as much as 56.6 times across all LMICs (1.6% (0.5–3.8%) in Chad; 87.9% (67.4–97.0%) in Rwanda) in 2025, while within-country differences are expected to range from 1.1 to 62.9 times, with the most variation in the Philippines, Brazil and Nigeria (ninefold or more difference). By 2030, national-level prevalence is projected to vary by 71.3 times across LMICs (1.2 (0.3–3.7%) in Chad; 87.7% (59.9–98.1%) in Rwanda), with subnational variation ranging from 1.1 to 80.4 times; Brazil, the Philippines and Nigeria are expected to maintain a ninefold or greater difference between districts.
Fig. 6. Projected prevalence for EBF for 2030 and probability of meeting the ≥70% WHO GNT by 2030.
a,b, Projected EBF prevalence for 2030 at the national (a) and district (b) levels. c, Probability of meeting the WHO GNT of at least 70% EBF prevalence by 2030 at the district level. Dark blue indicates a high probability (>95% posterior probability) and dark red indicates a low probability (<5% posterior probability) of meeting the WHO GNT by 2030. Maps reflect administrative boundaries, land cover, lakes and population; grey-coloured grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’ or were not included in this analysis50–55.
Our predictions for 2025 and 2030 show similar levels of EBF and patterns of subnational inequalities throughout LMICs as in 2018, with a few notable exceptions. On the basis of current trajectories, some of the largest projected gains are expected throughout sub-Saharan Africa. In Guinea-Bissau, Mauritania, Sierra Leone, Namibia, Zimbabwe and Gambia, most districts had <50% mean prevalence in 2018, but these countries are estimated to meet or exceed the original 50% EBF target in most districts in 2025. Outside of sub-Saharan Africa, Turkmenistan, Myanmar, Indonesia and Kyrgyzstan are also expected to exceed the 50% EBF mean prevalence target in most of their districts by 2025. Projected declines are expected to lead to districts in 15 LMICs that had mean estimates of EBF of ≥50% in 2018 to drop below this threshold by 2025; for example, Argo (Badakshan) in northeastern Afghanistan is expected to decrease from 52.4% (32.6–71.9%) in 2018 to 48.9% (17.8–60.45%) in 2025. By 2025, 33 LMICs are projected to have national mean EBF prevalence that meet the original WHO GNT of ≥50%, while 16 LMICs are predicted to have mean EBF prevalence meeting this target in all of their province-level units; 11 LMICs are expected to meet this target in all of their district-level units by 2025.
By 2030, six LMICs (Burundi, Cambodia, Lesotho, Peru, Rwanda and Sierra Leone) are projected to have mean national EBF prevalence that meet the updated WHO GNT of ≥70%, while three LMICs (Burundi, Lesotho and Rwanda) are predicted to meet this target in all their province-level and district-level units. Five LMICs (the Philippines, India, Peru, Ghana and Bolivia) had districts that met the ≥70% WHO GNT in 2018 which are expected to fall below this threshold in 2030, such as in Sandia (Puno), Peru (70.6% (51.2–88.4%) in 2018; 64.0% (33.8–87.1%) in 2030) and Mallig (Isabella), the Philippines (70.5% (54.5–83.2%) in 2018; 69.1% (38.2–90.0%) in 2030).
Progress towards the 2030 WHO GNT of ≥70% EBF
We mapped the probabilities of meeting the updated WHO GNT of ≥70% EBF by 2018 and 2030 at various scales (Supplementary Fig. 18 and Fig. 6c). Across LMICs, 86.2% (81 of 94), 63.8% (60 of 94) and 52.1% (49 of 94) had a low probability (<5%) of having achieved the updated WHO GNT of ≥70% EBF at the national level, in all provinces, or in all districts, respectively, by 2018 (Supplementary Table 10a). Rwanda was the only LMIC that had a high probability (>95%) of having already achieved the 70% target in 2018 at the national level, as well as the only LMIC to have had a high probability of meeting the target in all province-level units. No LMIC, however, had a high probability of meeting WHO GNT of 70% in all their district-level units in 2018. Across LMICs, 84.4% (20,717 of 24,556) of districts located in 88 LMICs had a low probability, while only 1.0% (256 of 24,556) of districts in five LMICs had a high probability of having achieved the updated target of 70% by 2018. Three LMICs had districts with both high and low probability of having met the new 70% target by 2018: Brazil, Peru and the Philippines.
In analysing probabilities of meeting the updated WHO GNT of ≥70% EBF by the year 2030, most LMICs (56.4% (53 of 94)) are expected to have a low probability (<5%) of nationally achieving this goal; 23.4% (22 of 94) and 13.8% (13 of 94) of LMICs have a low probability of meeting this goal in all of their province- and district-level units, respectively (Supplementary Table 10b). No LMIC has a high probability (>95%) of meeting the ≥70% target by 2030 at the national level or in all their province- or district-level units. Across LMICs, only 0.7% (177 of 24,556) of districts located in seven LMICs have a high probability, while 59.1% (14,518 of 24,556) of districts in 56 LMICs have a low probability of meeting the ≥70% target by 2030. Extreme subnational inequalities in probabilities (both <5% and >95% probability) of achieving the 70% EBF target by 2030 are expected to occur in 3.2% (3 of 94) of LMICs: Brazil, the Philippines and Mongolia. See Supplementary Table 9 and Supplementary Fig. 17 for probabilities of meeting the original WHO GNT of ≥50% by 2025.
Discussion
EBF practice has been known to vary by region, country and population25–27 but an understanding of the subnational distribution of this heterogeneity is hampered by several limitations in the previously available estimates. Previous studies have estimated EBF prevalence and interest groups such as UNICEF26 and Countdown to 203027 have compiled EBF datasets and country profiles; some of these results have been stratified by urban–rural status or wealth quintiles or mapped at the first-administrative level (for example, states, provinces). These maps and datasets, however, are limited to select countries or years and do not allow for comparisons across countries for each year or within countries at more detailed geographic scales. Understanding subnational variation in EBF is critical to determining where increased breastfeeding support efforts are needed to lead to the most improvement. This study maps comparable subnational estimates of EBF prevalence across most LMICs over an almost 20-yr period, projects these estimates to WHO GNT target years and quantifies within-country inequalities. Not only can these estimates aid tracking progress toward WHO GNTs but also toward the United Nation’s Sustainable Development Goal (SDG)28 to reduce national inequalities in health opportunities and outcomes, both between and within countries, by 2030.
Although EBF is considered a cost-effective intervention, it is not free; it requires investment of time and energy from mothers and support from wider networks, including their families, communities, workplaces, health systems and government leadership. Manipulative marketing of breast-milk substitutes29,30, inadequate workplace support31, late or lack of attendance at antenatal care32, lack of skilled lactation support or breastfeeding counselling in health facilities11,32 and societal beliefs favouring mixed feeding11,32–34 all contribute to low rates of EBF25,26,35. The WHO-UNICEF Global Breastfeeding Collective (GBC) initiative stresses the need for advocacy at global, national and subnational levels to improve breastfeeding rates for the betterment of maternal and child health and wellbeing36. The GBC’s Breastfeeding Advocacy Toolkit outlines seven key policy actions to increase breastfeeding practices, which are: increasing funding to support EBF and continued breastfeeding to 2 years; fully adopting and monitoring the International Code of Marketing of Breast-Milk Substitutes (‘the Code’); enacting workplace breastfeeding policies and paid family leave; implementing the ‘baby-friendly’ hospital’s ‘ten steps to successful breastfeeding’; improving access to skilled breastfeeding counselling in health facilities; strengthening links between health facilities and communities to support breastfeeding; and strengthening monitoring systems to track progress36. Inconsistent implementation of these policies could contribute to the between- and within-country variation we see in EBF practice across LMICs. Combined with information on breastfeeding interventions, our mapped estimates can aid policy-makers in monitoring the success of breastfeeding policy and programme investments.
The World Bank estimates that an investment of US$4.70 per live newborn is needed to meet the WHO GNT for EBF by 202537,38. According to the Global Breastfeeding Scorecard, however, only five LMICs in this analysis meet or exceed estimated funding needs (Guinea-Bissau, Haiti, Nepal, Somalia and Timor-Leste), while 50 spend <US$1 per live birth on breastfeeding support programmes, as of 201839. Aggressive marketing of breast-milk substitutes (BMS) disrupts mothers’ informed choices by providing misleading information. In response to controversial marketing strategies, the World Health Assembly established the Code in 1981 to regulate the promotion and safety of BMS and ensure the adequate nutrition of infants40. The Code bans point-of-sale promotion of BMS or bottles, distribution of free samples and misleading promotional materials suggesting a product’s superiority over mother’s natural milk40. In 2018, however, only 24 of the 94 LMICs in this study had comprehensive Code legislation in place and 25 had no legal measures protecting consumers from aggressive BMS marketing tactics39. A study on global infant formulas sales showed that the steepest market increases were in Asia Pacific (18% increase) and Middle East and Africa (14% increase) regions within just 1 year (2012–2013)30; by 2025, the infant formula industry is expected to surpass US$98 billion in sales41, and increase in marketing and sales will likely negatively affect breastfeeding42,43. Additionally, few LMICs have national policies that satisfy the International Labour Organization’s Convention minimum recommendations for 14 weeks of paid maternity leave and appropriate workplace nursing areas; Colombia, Cuba, India, Paraguay, Tajikistan and Vietnam are the only six LMICs in our analysis that fully met these recommendations in 201839. Individual breastfeeding counselling was reported to be implemented in all primary healthcare facilities in just 28 LMICs39. Of the LMICs with available data, at least half of births were in baby-friendly hospitals and maternities in only six countries (Costa Rica, Cuba, Eswatini, Tajikistan, Thailand and Turkmenistan)39. By subnational reporting, 29 LMICs in the analysis had implemented community programmes in all districts in 201839. Our estimates, combined with the WHO’s Breastfeeding Scorecard Data, can be used to decide where additional resources to support breastfeeding are most needed (Supplementary Information section 5.4).
Positive exemplars in EBF uptake due to policy implementation and financial investments could provide lessons learned for policy-makers to apply towards their countries. The 2018 Global Nutrition Report spotlighted Burkina Faso’s strong commitment to supporting breastfeeding through the rapid roll-out of a national infant and young feeding programme that led to all primary healthcare facilities providing counselling and 70% of districts with community programmes for breastfeeding support35. Furthermore, Burkina Faso passed legislation providing 14 weeks of state-funded maternity leave and laws prohibiting advertising breast-milk substitutes35; by our estimates, most districts experienced >5% annualized increase in EBF over the modelled study period. In Nepal, USAID’s integrated nutrition programme combined water and sanitation, family planning and agricultural activities along with essential nutrition and breastfeeding counselling to children and caregivers in 42 of 77 districts and the recommended minimum US$4.70 per live-birth investment was met in 201835; by our estimates, all districts in Nepal had annualized EBF increases between 2000 and 2018. The USAID’s programming in Malawi worked with the Ministry of Health to achieve ‘baby-friendly’ status in hospitals, develop a nutrition training for nurses and midwives and provide deworming and vitamin A supplementation35; these combined efforts may have contributed to many of Malawi’s districts being >50% of mean EBF prevalence in 2018. Turkmenistan’s success in achieving >5% annualized increase in all of its districts by 2018 may be attributed to the high proportion of births in baby-friendly hospitals (86.9%) and community breastfeeding programmes implemented in all its districts39. Gambia and Côte d’Ivoire, which had reduced absolute inequalities by at least a third, fared well on the Breastfeeding Scorecard; basic maternity provisions, as well as community programmes in all districts and counselling in all facilities were reported for Côte d’Ivoire and Gambia had full legal status of the Code, met recommended maternity leave length and all facilities offered counselling. Although we identified Cambodia as having among the highest EBF prevalence levels in 2018, and Myanmar as having among the highest annualized increases, and both countries experienced large reductions in relative inequalities, they did not have widespread supportive breastfeeding policies implemented, according to their 2018 national scorecard39. Additional local investigations are needed to document subnational policy implementation and determine associations between breastfeeding policies and interventions and EBF progress.
This study provides a comprehensive picture of the unmet need for EBF by mapping both prevalence of EBF and the absolute number of children not exclusively breastfed for their first 6 months of life. Our mapped estimates provide a tool to visualize subnational inequalities otherwise masked by national-level estimates and areas left behind in EBF uptake. These subnational EBF estimates can aid policy- and decision-makers in tracking progress towards the international target and in identifying where additional breastfeeding support efforts are needed to improve child health and survival. Comparisons against additional health indicators could inform the development of more comprehensive approaches to improve health in populations most in need. Future research could compare these estimates with breastfeeding policies and interventions, or lack thereof, to determine which were most successful in achieving increased practice of EBF and what barriers still need to be addressed.
Methods
Overview
For this study, we used a similar methodology to that of our previous work on mapping EBF prevalence in Africa14 and extended our scope to include all LMICs with available relevant data. LMIC status was determined by sociodemographic index (SDI), which indicates a country’s level of development on the basis of poverty, education and fertility as defined in the GBD study. Here we map estimates of countries that have low, low-middle or middle SDI status (Supplementary Table 4). We excluded several countries from our analysis despite low, low-middle or middle status due to lack of relevant input data (Cape Verde, Dominica, Djibouti, Ecuador, Grenada, Iran, Libya, Malaysia, Seychelles, Sri Lanka and Venezuela). This study complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER; http://gather-statement.org; Supplementary Information section 1.0).
Data
Surveys and EBF indicator data
When searching the Global Health Data Exchange (GHDx; http://ghdx.healthdata.org) for the keyword ‘breastfeeding’, we compiled an extensive geo-located dataset that includes 345 household surveys (including the Demographic and Health Surveys (DHS), Multiple Indicator Cluster Surveys (MICS) and other country-specific or multinational surveys) conducted in years 1998 to 2018 in LMICs. Of these, we assigned data from 21 surveys conducted in years 1998 or 1999 to the year 2000 to address data scarcity. This dataset represents 302,435 infants aged 0–5 months (infants up to the age of 6 months) across 94 LMICs and was geocoded to 69,179 coordinates corresponding to cluster-level boundaries and 67,750 subnational polygon boundaries. Across the 94 countries in the analysis, there were 1,727 first-administrative-level boundaries (for example, provinces) and 24,556 second-administrative-level boundaries (for example, districts). Overall inclusion criteria for surveys included: conducted in an LMIC between 1998 and 2018; responses available at the individual level; contains subnational geographic identifiers (that is, GPS coordinates, cluster or administrative units) with survey weights for each observation; and contains questions and responses about child’s age and breastfeeding status. A survey was included if it contained questions and responses regarding whether the child had consumed other food or liquids besides breast-milk. We only included observations of children who were under 6 months at the time of the survey (0–5 months). See the Supplementary Information sections 2.1 and 2.2 for further details on exclusion and inclusion criteria.
Spatial covariates
In these analyses, we included 11 socioeconomic and health-related covariates identified as conceivably associated with breastfeeding practices: (1) travel time to the nearest settlement >50,000 inhabitants, (2) nighttime lightsTV, (3) populationTV, (4) number of children under 5 yr per woman of childbearing ageTV, (5) urban proportion of the locationTV, (6) number of people whose daily vitamin A needs could be met, (7) educational attainment in women of reproductive age (15–49-years-old)TV, (8) human development index (HDI)TV, (9) human immunodeficiency virus (HIV) prevalenceTV, (10) healthcare access and quality indexTV and (11) proportion of pregnant women who received four or more antenatal care visitsTV (where superscript TV indicates time-varying covariates). Of these, the covariates for the Healthcare Access and Quality Index44 and the proportion of pregnant women who received four or more antenatal care visits45 were indexed at the national level, while all others were indexed at the subnational level. The spatial covariates were selected because they are factors or proxies for factors that previous literature has identified to be associated (not necessarily causally) with EBF prevalence.
Variance inflation factor46 (VIF) analysis was used to filter covariates for multicollinearity. We performed temporal processing for covariates that did not have information for every year of the modelled study period and filled in intervening years with the value from the nearest neighbouring year or used an exponential growth rate model. Detailed information on covariates can be found in Supplementary Table 5 and Supplementary Fig. 8.
Analysis
The technical descriptions of methods for the underlying geostatistical model, model validation and postestimation are consistent with those previously used in the geospatial modelling of EBF across Africa14.
Geostatistical model
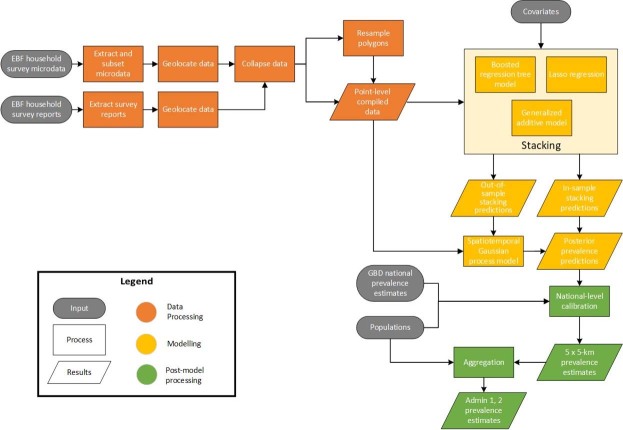
EBF was modelled using a Bayesian geostatistical approach. This approach uses a hierarchical logistic regression model that is spatially and temporally explicit, and assumes points close in space and time and with similar covariate patterns will have similar levels of EBF. Using a stacked generalization technique, we also incorporated potential nonlinear relationships between covariates and EBF input data. For all model parameters and hyper-parameters, we used the R-INLA statistical package to approximate posterior distributions47. We used 1,000 draws from these approximate joint posterior distributions to calculate uncertainty intervals (UI), determining and reporting the 2.5th and 97.5th percentiles of those 1,000 draws. Further details on methodology can be found in Supplementary Information section 4.0. Extended Data Fig. 1 provides an overview of analytical processing steps involved in the analysis.
Extended Data Fig. 1. Analytic process overview.
The process used to produce EBF prevalence estimates across LMICs involved three main parts. In the data-processing steps (orange), data were identified, extracted, and prepared for use in the models. In the modelling phase (yellow), we used these data and covariates in stacked generalization ensemble models and spatiotemporal Gaussian process models for each EBF indicator. In post-processing (green), we calibrated the prevalence estimates to match the GBD 201912 study estimates and aggregated the estimates to the first- and second-administrative levels in each country.
Model validation
We used fivefold cross validation to validate models, as summarized below. Complete methods used for validation and related results are available in the Supplementary Information. First, we combined randomized sets of cluster-level data points at the first-administrative level to create holdout sets. Afterwards, we fit the geostatistical model five times, sequentially excluding each of the five groups of data, and provided out-of-sample predictions that correspond to all included surveys in the analysis. We summarized the performance of the model using 95% data coverage within prediction intervals, correlation between predictions and observed data and the mean error (a measure of bias) and root-mean-square error (a measure of total variance). Model estimates were also compared with other existing estimates, as possible.
Postestimation
To estimate EBF prevalence at various levels (province, district and country), we aggregated each of the 1,000 draws of coverage at the 5 × 5-km grid-cell level, weighted by population. We preformed posthoc calibration of our estimates to the GBD 2019 estimates12. This allowed us to include data sources outside of the scope of our geospatial modelling framework. On the basis of the estimates, we calculated absolute differences between lowest and highest administrative units and relative differences between a country’s average and each administrative unit in that country to quantify geographic inequality. We performed a simple projection calculation by comparing the estimated rates of EBF improvement between 2000 and 2018 with the improvements needed between 2018 and 2030 to meet the WHO GNT (Supplementary Information section 4.4.4). The national time series and aggregated input data in our estimates can be found in Extended Data Fig. 2.
Extended Data Fig. 2. National time series plots and aggregated input data.
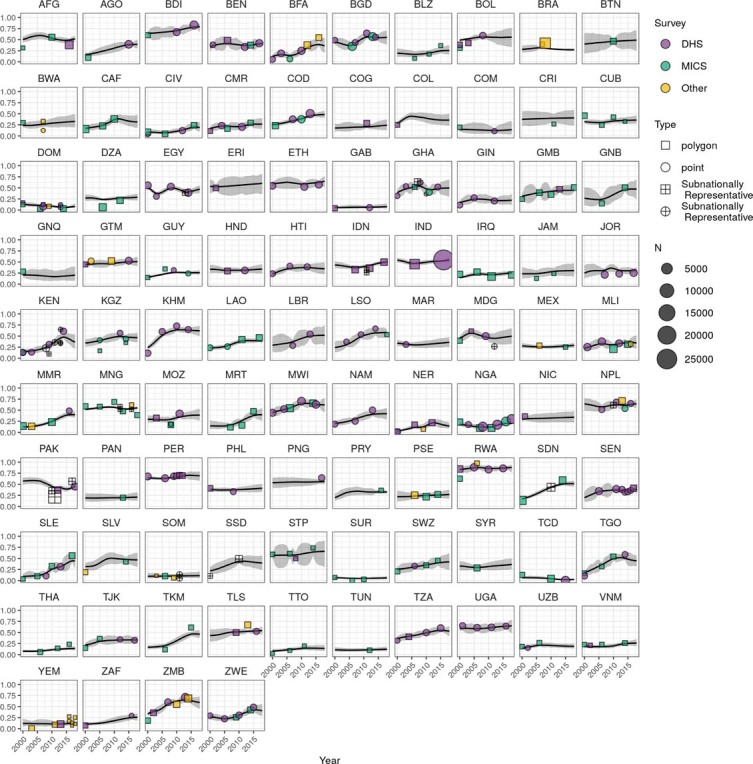
National time series plots of the post-GBD calibration final estimates by country during 2000–2018. Uncertainty ranges are presented in grey, and aggregated input data are classified by survey series (purple for country-specific, green for DHS, and yellow for MICS surveys), data type (square for polygon, circle for point data), and whether the survey is nationally or subnationally representative).
Modelling limitations
The modelling limitations in this work are consistent with those previously described in the geospatial modelling of EBF across Africa14.
While we have attempted to propagate uncertainty from various sources through the different modelling stages, there are some sources of uncertainty that have not been propagated. In particular, it was not computationally feasible to propagate uncertainty from the submodels in stacking through the geostatistical model. Similarly, although the WorldPop population raster is also composed of estimates associated with some uncertainty, this uncertainty is difficult to quantify and not currently reported and we were unable to propagate this uncertainty into our estimates of EBF prevalence for administrative units that were created using population-weighted averages of grid-cell estimates. Model fitting was carried out using an integrated nested Laplace approximation to the posterior distribution, as implemented in the R-INLA package47. Prediction from fitted models was subsequently carried out using the inla.posterior.sample() function, which generates samples from the approximated posterior of the fitted model. Both model fitting and prediction thus require approximations, and these approximations may introduce error.
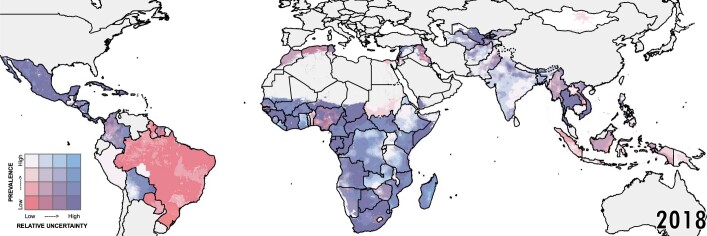
To estimate projections of EBF prevalence levels in 2025 and 2030, we used previous historical trends and the assumption that recent trends will continue. These assumptions in turn lend to modelling limitations, as we were not able to project underlying drivers of changes in EBF, such as increasing urbanization or changes in population35,48,49, and the certainty of our estimates and projections were critically dependent on data quality and availability. Availability of relevant data varies both spatially and temporally across LMICs (Supplementary Figs. 1–5) and lack of relevant data is one of the main sources of uncertainty around our estimates (as seen in Extended Data Fig. 3). We have mapped EBF prevalence levels against the relative uncertainty of our estimates in Extended Data Fig. 3.
Extended Data Fig. 3. Relative uncertainty in EBF estimates for 2018.
Relative uncertainty in second-administrative-level estimates compared with mean estimated EBF prevalence in each second-administrative-level unit for 2018. Mean prevalence and relative uncertainty are split into population-weighted quartiles. These cut-off points for relative uncertainty (calculated as the absolute range of the uncertainty intervals divided by the estimate) are 0.684 (25th percentile), 0.916 (50th percentile), and 1.271 (75th percentile), respectively. The cut-off points for EBF prevalence are 25.8% (25th percentile), 35.4% (50th percentile), and 49.4% (75th percentile), respectively. Units in which our estimates are more uncertain are coloured with a scale of increasing blue hue, whereas areas in which the mean estimates of EBF are low are coloured with a scale of increasing red hue. Purple areas have low, but uncertain, estimates of EBF. White areas have high EBF estimates that are fairly certain. Relative uncertainty is defined as the ratio of the width of the 95% uncertainty interval to mean estimate. Maps reflect administrative boundaries, land cover, lakes, and population; grey-coloured grid cells had fewer than ten people per 1 × 1-km grid cell and were classified as ‘barren or sparsely vegetated’, or were not included in this analysis50–55.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Supplementary Figs. 1–23, Supplementary Tables 1–12, Supplementary Methods, Supplementary Results, Supplementary Limitations, Supplementary Discussion, Supplementary References; Author Contributions.
Acknowledgements
This work was primarily supported by grant no. OPP1132415 from the Bill & Melinda Gates Foundation. Co-authors used by the Bill & Melinda Gates Foundation (E.G.P. and R.R.3) provided feedback on initial maps and drafts of this manuscript. L.G.A. has received support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Código de Financiamento 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant nos. 404710/2018-2 and 310797/2019-5). O.O.Adetokunboh acknowledges the National Research Foundation, Department of Science and Innovation and South African Centre for Epidemiological Modelling and Analysis. M.Ausloos, A.Pana and C.H. are partially supported by a grant from the Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI, project no. PN-III-P4-ID-PCCF-2016-0084. P.C.B. would like to acknowledge the support of F. Alam and A. Hussain. T.W.B. was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professor award, funded by the German Federal Ministry of Education and Research. K.Deribe is supported by the Wellcome Trust (grant no. 201900/Z/16/Z) as part of his international intermediate fellowship. C.H. and A.Pana are partially supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNDS-UEFISCDI, project no. PN-III-P2-2.1-SOL-2020-2-0351. B.Hwang is partially supported by China Medical University (CMU109-MF-63), Taichung, Taiwan. M.Khan acknowledges Jatiya Kabi Kazi Nazrul Islam University for their support. A.M.K. acknowledges the other collaborators and the corresponding author. Y.K. was supported by the Research Management Centre, Xiamen University Malaysia (grant no. XMUMRF/2020-C6/ITM/0004). K.Krishan is supported by a DST PURSE grant and UGC Centre of Advanced Study (CAS II) awarded to the Department of Anthropology, Panjab University, Chandigarh, India. M.Kumar would like to acknowledge FIC/NIH K43 TW010716-03. I.L. is a member of the Sistema Nacional de Investigación (SNI), which is supported by the Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT), Panamá. M.L. was supported by China Medical University, Taiwan (CMU109-N-22 and CMU109-MF-118). W.M. is currently a programme analyst in Population and Development at the United Nations Population Fund (UNFPA) Country Office in Peru, which does not necessarily endorses this study. D.E.N. acknowledges Cochrane South Africa, South African Medical Research Council. G.C.P. is supported by an NHMRC research fellowship. P.Rathi acknowledges support from Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India. Ramu Rawat acknowledges the support of the GBD Secretariat for supporting the reviewing and collaboration of this paper. B.R. acknowledges support from Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal. A.Ribeiro was supported by National Funds through FCT, under the programme of ‘Stimulus of Scientific Employment—Individual Support’ within the contract no. CEECIND/02386/2018. S.Sajadi acknowledges colleagues at Global Burden of Diseases and Local Burden of Disease. A.M.S. acknowledges the support from the Egyptian Fulbright Mission Program. F.S. was supported by the Shenzhen Science and Technology Program (grant no. KQTD20190929172835662). A.Sheikh is supported by Health Data Research UK. B.K.S. acknowledges Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal for all the academic support. B.U. acknowledges support from Manipal Academy of Higher Education, Manipal. C.S.W. is supported by the South African Medical Research Council. Y.Z. was supported by Science and Technology Research Project of Hubei Provincial Department of Education (grant no. Q20201104) and Outstanding Young and Middle-aged Technology Innovation Team Project of Hubei Provincial Department of Education (grant no. T2020003). The funders of the study had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All maps presented in this study are generated by the authors and no permissions are required to publish them.
Extended data
Author contributions
S.I.H. and L.D.-L. conceived and planned the study. D.L., S.J.S., J.Albright, W.M.G., B.V.P., C.L., K.M.D., E.G.P., Rahul Rawat and B.Sartorius identified and obtained data for analysis. D.L., S.J.S., J.Albright, W.M.G., N.J.H., C.L., A.L.-A., B.V.P. and K.M.D. extracted, processed and geopositioned the data. N.V.B. carried out the statistical analyses to produce estimates. A.E.O.-Z., N.J.H., M.L.C., M.A.C., D.K.K., J.F.M., A.Deshpande, L.W., J.D.V., K.E.W., R.C.R. and L.D.-L. provided input on the methods. N.V.B., D.L., K.B.J., I.D.L. and K.M.D. prepared the figures and tables. L.E.S., N.Davis Weaver and L.B.M. managed the publications processes and N.V.B., M.F.S., L.D.-L., N.J.K. and S.I.H. managed the estimation processes. M.F.S. served as project manager for the study. N.V.B. and L.E.S. wrote the first draft of the manuscript and all authors contributed to subsequent revisions. All authors provided intellectual input into aspects of this study; additional author contributions can be found in the Supplementary Information.
Data availability
The information given here is mostly consistent with our previous study modelling EBF across Africa14. The findings of this study are supported by data that are available in public online repositories, data that are publicly available on request from the data provider and data that are not publicly available due to restrictions by the data provider and which were used under license for the current study. Details on data sources can be found on the GHDx website (http://ghdx.healthdata.org/lbd-publication-data-input-sources?field_rec_ihme_publication_tid=29093), including information about the data provider and links to where the data can be accessed or requested (where available). We have also provided maps of the data included in our models in Supplementary Figs. 1–5. Outputs of these EBF analyses can be explored at various spatial levels (national, administrative and 5 × 5-km levels) through our customized visualization tool (https://vizhub.healthdata.org/lbd/ebf) and are publicly available at the GHDx (http://ghdx.healthdata.org/record/ihme-data/global-exclusive-breastfeeding-prevalence-geospatial-estimates-2000-2019). Administrative boundaries were retrieved from the Database of Global Administrative Areas (GADM)50. Land cover was retrieved from the online Data Pool, courtesy of the NASA EOSDIS Land Processes Distributed Active Archive Center, USGS/Earth Resources Observation and Science Center, Sioux Falls, South Dakota51. Lakes were retrieved from the Global Lakes and Wetlands Database, courtesy of the World Wildlife Fund and the Center for Environmental Systems Research, University of Kassel52. Populations were retrieved from WorldPop53. All maps in this study were produced using ArcGIS Desktop 10.6.
Code availability
All codes used for these analyses are publicly available online at https://github.com/ihmeuw/lbd/tree/ebf-lmic-2021.
Competing interests
R.A. reports he received consultancy or speakers fees from UCB, Sandoz, Abbvie, Zentiva, Teva, Laropharm, CEGEDIM, Angelini, Biessen Pharma, Hofigal, AstraZeneca and Stada. A.Deshpande reports grants from Bill & Mellinda Gates Foundation, during the conduct of the study. J.J.J. reports personal fees from Boehringer Ingelheim, Zentiva, Amgen and Teva, all outside the submitted work. K.Krishan reports grants from DST PURSE and UGC Centre of Advanced Study, CAS II, awarded to the Department of Anthropology, Panjab University, Chandigarh, India, outside the submitted work. J.F.M. reports grants from Bill & Melinda Gates Foundation during the conduct of the study. S.R.P. reports non-financial support from Somnogen Canada Inc. and personal fees from editorial services, during the conduct of the study. E.U. reports having a Patent A system and method of reusable filters for anti-pollution mask pending and a Patent A system and method for electricity generation through crop stubble by using microbial fuel cells pending. All other authors declare no competing interests.
Footnotes
Peer review informationNature Human Behaviour thanks Ranadip Chowdhury, Paulo Augusto Neves and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Simon I. Hay, Email: sihay@uw.edu
Local Burden of Disease Exclusive Breastfeeding Collaborators:
Dan Lu, Megan F. Schipp, Alice Lazzar-Atwood, Katie M. Donkers, Gdiom Gebreheat Abady, Foad Abd-Allah, Ahmed Abdelalim, Zeleke Hailemariam Abebo, Ayenew Negesse Abejie, Akine Eshete Abosetugn, Lucas Guimarães Abreu, Michael R. M. Abrigo, Eman Abu-Gharbieh, Abdelrahman I. Abushouk, Aishatu L. Adamu, Isaac Akinkunmi Adedeji, Adeyinka Emmanuel Adegbosin, Victor Adekanmbi, Olatunji O. Adetokunboh, Marcela Agudelo-Botero, Budi Aji, Oluwaseun Oladapo Akinyemi, Alehegn Aderaw Alamneh, Fahad Mashhour Alanezi, Turki M. Alanzi, James Albright, Jacqueline Elizabeth Alcalde-Rabanal, Biresaw Wassihun Alemu, Robert Kaba Alhassan, Beriwan Abdulqadir Ali, Saqib Ali, Cyrus Alinia, Vahid Alipour, Arianna Maever L. Amit, Dickson A. Amugsi, Etsay Woldu Anbesu, Robert Ancuceanu, Mina Anjomshoa, Fereshteh Ansari, Carl Abelardo T. Antonio, Davood Anvari, Jalal Arabloo, Amit Arora, Kurnia Dwi Artanti, Mulusew A. Asemahagn, Wondwossen Niguse Asmare, Maha Moh’d Wahbi Atout, Marcel Ausloos, Nefsu Awoke, Beatriz Paulina Ayala Quintanilla, Martin Amogre Ayanore, Yared Asmare Aynalem, Muluken Altaye Ayza, Zelalem Nigussie Azene, B. B. Darshan, Ashish D. Badiye, Atif Amin Baig, Shankar M. Bakkannavar, Maciej Banach, Palash Chandra Banik, Till Winfried Bärnighausen, Huda Basaleem, Mohsen Bayati, Bayisa Abdissa Baye, Neeraj Bedi, Sefealem Assefa Belay, Akshaya Srikanth Bhagavathula, Dinesh Bhandari, Nikha Bhardwaj, Pankaj Bhardwaj, Zulfiqar A. Bhutta, Ali Bijani, Tsegaye Adane Birhan, Binyam Minuye Birihane, Zebenay Workneh Bitew, Somayeh Bohlouli, Mahdi Bohluli, Hunduma Amensisa Bojia, Archith Boloor, Oliver J. Brady, Nicola Luigi Bragazzi, Andre R. Brunoni, Shyam S. Budhathoki, Sharath Burugina Nagaraja, Zahid A. Butt, Rosario Cárdenas, Joao Mauricio Castaldelli-Maia, Franz Castro, Achille Cernigliaro, Jaykaran Charan, Pranab Chatterjee, Souranshu Chatterjee, Vijay Kumar Chattu, Sarika Chaturvedi, Mohiuddin Ahsanul Kabir Chowdhury, Dinh-Toi Chu, Michael L. Collison, Aubrey J. Cook, Michael A. Cork, Rosa A. S. Couto, Baye Dagnew, Haijiang Dai, Lalit Dandona, Rakhi Dandona, Parnaz Daneshpajouhnejad, Aso Mohammad Darwesh, Amira Hamed Darwish, Ahmad Daryani, Jai K. Das, Rajat Das Gupta, Claudio Alberto Dávila-Cervantes, Adrian Charles Davis, Nicole Davis Weaver, Edgar Denova-Gutiérrez, Kebede Deribe, Assefa Desalew, Aniruddha Deshpande, Awrajaw Dessie, Keshab Deuba, Samath Dhamminda Dharmaratne, Meghnath Dhimal, Govinda Prasad Dhungana, Daniel Diaz, Alireza Didarloo, Isaac Oluwafemi Dipeolu, Linh Phuong Doan, Bereket Duko, Andre Rodrigues Duraes, Laura Dwyer-Lindgren, Lucas Earl, Maysaa El Sayed Zaki, Maha El Tantawi, Teshome Bekele Elema, Hala Rashad Elhabashy, Shaimaa I. El-Jaafary, Pawan Sirwan Faris, Andre Faro, Farshad Farzadfar, Valery L. Feigin, Berhanu Elfu Feleke, Tomas Y. Ferede, Florian Fischer, Nataliya A. Foigt, Morenike Oluwatoyin Folayan, Richard Charles Franklin, Mohamed M. Gad, Shilpa Gaidhane, William M. Gardner, Biniyam Sahiledengle Geberemariyam, Birhan Gebresillassie Gebregiorgis, Ketema Bizuwork Gebremedhin, Berhe Gebremichael, Fariborz Ghaffarpasand, Syed Amir Gilani, Themba G. Ginindza, Mustefa Glagn, Mahaveer Golechha, Kebebe Bekele Gonfa, Bárbara Niegia Garcia Goulart, Nachiket Gudi, Davide Guido, Rashid Abdi Guled, Yuming Guo, Samer Hamidi, Demelash Woldeyohannes Handiso, Ahmed I. Hasaballah, Amr Hassan, Khezar Hayat, Mohamed I. Hegazy, Behnam Heidari, Nathaniel J. Henry, Claudiu Herteliu, Hagos Degefa de Hidru, Hung Chak Ho, Chi Linh Hoang, Ramesh Holla, Julia Hon, Mostafa Hosseini, Mehdi Hosseinzadeh, Mowafa Househ, Mohamed Hsairi, Guoqing Hu, Tanvir M. Huda, Bing-Fang Hwang, Segun Emmanuel Ibitoye, Olayinka Stephen Ilesanmi, Irena M. Ilic, Milena D. Ilic, Leeberk Raja Inbaraj, Usman Iqbal, Seyed Sina Naghibi Irvani, M. Mofizul Islam, Chidozie C. D. Iwu, Chinwe Juliana Iwu, Animesh Jain, Manthan Dilipkumar Janodia, Tahereh Javaheri, Yetunde O. John-Akinola, Kimberly B. Johnson, Farahnaz Joukar, Jacek Jerzy Jozwiak, Ali Kabir, Leila R. Kalankesh, Rohollah Kalhor, Ashwin Kamath, Naser Kamyari, Other Tanuj Kanchan, Neeti Kapoor, Behzad Karami Matin, Salah Eddin Karimi, Habtamu Kebebe Kasaye, Getinet Kassahun, Nicholas J. Kassebaum, Gbenga A. Kayode, Ali Kazemi Karyani, Peter Njenga Keiyoro, Bayew Kelkay, Nauman Khalid, Md. Nuruzzaman Khan, Khaled Khatab, Amir M. Khater, Mona M. Khater, Mahalaqua Nazli Khatib, Yun Jin Kim, Ruth W. Kimokoti, Damaris K. Kinyoki, Adnan Kisa, Sezer Kisa, Soewarta Kosen, Kewal Krishan, Vaman Kulkarni, G. Anil Kumar, Manasi Kumar, Nithin Kumar, Pushpendra Kumar, Om P. Kurmi, Dian Kusuma, Carlo La Vecchia, Sheetal D. Lad, Faris Hasan Lami, Iván Landires, Van Charles Lansingh, Savita Lasrado, Paul H. Lee, Kate E. LeGrand, Ian D. Letourneau, Sonia Lewycka, Bingyu Li, Ming-Chieh Li, Shanshan Li, Xuefeng Liu, Rakesh Lodha, Jaifred Christian F. Lopez, Celia Louie, Daiane Borges Machado, Venkatesh Maled, Shokofeh Maleki, Deborah Carvalho Malta, Abdullah A. Mamun, Navid Manafi, Mohammad Ali Mansournia, Chabila Christopher Mapoma, Laurie B. Marczak, Francisco Rogerlândio Martins-Melo, Man Mohan Mehndiratta, Fabiola Mejia-Rodriguez, Tefera Chane Mekonnen, Walter Mendoza, Ritesh G. Menezes, Endalkachew Worku Mengesha, Abera M. Mersha, Ted R. Miller, G. K. Mini, Erkin M. Mirrakhimov, Sanjeev Misra, Masoud Moghadaszadeh, Dara K. Mohammad, Abdollah Mohammadian-Hafshejani, Jemal Abdu Mohammed, Shafiu Mohammed, Ali H. Mokdad, Pablo A. Montero-Zamora, Masoud Moradi, Rahmatollah Moradzadeh, Paula Moraga, Jonathan F. Mosser, Seyyed Meysam Mousavi, Amin Mousavi Khaneghah, Sandra B. Munro, Moses K. Muriithi, Ghulam Mustafa, Saravanan Muthupandian, Ahamarshan Jayaraman Nagarajan, Gurudatta Naik, Mukhammad David Naimzada, Vinay Nangia, Bruno Ramos Nascimento, Vinod C. Nayak, Rawlance Ndejjo, Duduzile Edith Ndwandwe, Ionut Negoi, Georges Nguefack-Tsague, Josephine W. Ngunjiri, Cuong Tat Nguyen, Diep Ngoc Nguyen, Huong Lan Thi Nguyen, Samuel Negash Nigussie, Tadesse T. N. Nigussie, Rajan Nikbakhsh, Chukwudi A. Nnaji, Virginia Nunez-Samudio, Bogdan Oancea, Onome Bright Oghenetega, Andrew T. Olagunju, Bolajoko Olubukunola Olusanya, Jacob Olusegun Olusanya, Muktar Omer Omer, Obinna E. Onwujekwe, Doris V. Ortega-Altamirano, Aaron E. Osgood-Zimmerman, Nikita Otstavnov, Stanislav S. Otstavnov, Mayowa O. Owolabi, P. A. Mahesh, Jagadish Rao Padubidri, Adrian Pana, Anamika Pandey, Seithikurippu R. Pandi-Perumal, Helena Ullyartha Pangaribuan, Shradha S. Parsekar, Deepak Kumar Pasupula, Urvish K. Patel, Ashish Pathak, Mona Pathak, Sanjay M. Pattanshetty, George C. Patton, Kebreab Paulos, Veincent Christian Filipino Pepito, Brandon V. Pickering, Marina Pinheiro, Ellen G. Piwoz, Khem Narayan Pokhrel, Hadi Pourjafar, Sergio I. Prada, Dimas Ria Angga Pribadi, Zahiruddin Quazi Syed, Mohammad Rabiee, Navid Rabiee, Fakher Rahim, Shadi Rahimzadeh, Azizur Rahman, Mohammad Hifz Ur Rahman, Amir Masoud Rahmani, Rajesh Kumar Rai, Chhabi Lal Ranabhat, Sowmya J. Rao, Prateek Rastogi, Priya Rathi, David Laith Rawaf, Salman Rawaf, Reza Rawassizadeh, Rahul Rawat, Ramu Rawat, Lemma Demissie Regassa, Maria Albertina Santiago Rego, Robert C. Reiner, Jr, Bhageerathy Reshmi, Aziz Rezapour, Ana Isabel Ribeiro, Jennifer Rickard, Leonardo Roever, Susan Fred Rumisha, Godfrey M. Rwegerera, Rajesh Sagar, S. Mohammad Sajadi, Marwa Rashad Salem, Abdallah M. Samy, Milena M. Santric-Milicevic, Sivan Yegnanarayana Iyer Saraswathy, Abdur Razzaque Sarker, Benn Sartorius, Brijesh Sathian, Deepak Saxena, Alyssa N. Sbarra, Debarka Sengupta, Subramanian Senthilkumaran, Feng Sha, Omid Shafaat, Amira A. Shaheen, Masood Ali Shaikh, Ali S. Shalash, Mohammed Shannawaz, Aziz Sheikh, B. Suresh Kumar Shetty, Ranjitha S. Shetty, Kenji Shibuya, Wondimeneh Shibabaw Shiferaw, Jae Il Shin, Diego Augusto Santos Silva, Narinder Pal Singh, Pushpendra Singh, Surya Singh, Yitagesu Sintayehu, Valentin Yurievich Skryabin, Anna Aleksandrovna Skryabina, Amin Soheili, Shahin Soltani, Muluken Bekele Sorrie, Emma Elizabeth Spurlock, Krista M. Steuben, Agus Sudaryanto, Mu’awiyyah Babale Sufiyan, Scott J. Swartz, Eyayou Girma Tadesse, Animut Tagele Tamiru, Leili Tapak, Md. Ismail Tareque, Ingan Ukur Tarigan, Getayeneh Antehunegn Tesema, Fisaha Haile Tesfay, Abinet Teshome, Zemenu Tadesse Tessema, Kavumpurathu Raman Thankappan, Rekha Thapar, Nihal Thomas, Roman Topor-Madry, Marcos Roberto Tovani-Palone, Eugenio Traini, Bach Xuan Tran, Phuong N. Truong, Berhan Tsegaye B. T. Tsegaye, Irfan Ullah, Chukwuma David Umeokonkwo, Bhaskaran Unnikrishnan, Era Upadhyay, Benjamin S. Chudi Uzochukwu, John David VanderHeide, Francesco S. Violante, Bay Vo, Yohannes Dibaba Wado, Yasir Waheed, Richard G. Wamai, Fang Wang, Yafeng Wang, Yuan-Pang Wang, Nuwan Darshana Wickramasinghe, Kirsten E. Wiens, Charles Shey Wiysonge, Lauren Woyczynski, Ai-Min Wu, Chenkai Wu, Tomohide Yamada, Sanni Yaya, Alex Yeshaneh, Yigizie Yeshaw, Yordanos Gizachew Yeshitila, Mekdes Tigistu Yilma, Paul Yip, Naohiro Yonemoto, Tewodros Yosef, Mustafa Z. Younis, Abdilahi Yousuf Yousuf, Chuanhua Yu, Yong Yu, Deniz Yuce, Shamsa Zafar, Syed Saoud Zaidi, Leila Zaki, Josefina Zakzuk, Maryam Zamanian, Heather J. Zar, Mikhail Sergeevich Zastrozhin, Anasthasia Zastrozhina, Desalege Amare Zelellw, Yunquan Zhang, Zhi-Jiang Zhang, Xiu-Ju George Zhao, Sanjay Zodpey, Yves Miel H. Zuniga, and Simon I. Hay
Extended data
is available for this paper at 10.1038/s41562-021-01108-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s41562-021-01108-6.
References
- 1.Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025: The Integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD) (WHO & UNICEF, 2013); https://www.who.int/maternal_child_adolescent/documents/global_action_plan_pneumonia_diarrhoea/en/ [DOI] [PubMed]
- 2.Victora CG, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 3.Bhutta ZA, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381:1417–1429. doi: 10.1016/S0140-6736(13)60648-0. [DOI] [PubMed] [Google Scholar]
- 4.Darmstadt G, et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;11:977–988. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan A, Farver M, Smilowitz JT. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr. Metab. Insights. 2015;8:1–9. doi: 10.4137/NMI.S29530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012;554:63–77. doi: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.From the First Hour of Life: Making The Case for Improved Infant and Young Child Feeding Everywhere (UNICEF, 2016); https://www.unicef.org/publications/index_93027.html
- 9.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:30–37. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 10.Horta, B. L. et al. Evidence on the Long-term Effects of Breastfeeding (WHO, 2007).
- 11.Global Nutrition Targets 2025—Breastfeeding Policy Brief (WHO & UNICEF, 2014); http://www.who.int/nutrition/publications/globaltargets2025_policybrief_breastfeeding/en/
- 12.Murray CJL, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Extension of the 2025 Maternal, Infant and Young Child Nutrition Targets to 2030 (WHO & UNICEF, 2018); https://www.who.int/nutrition/global-target-2025/discussion-paper-extension-targets-2030.pdf?ua=1
- 14.Bhattacharjee NV, et al. Mapping exclusive breastfeeding in Africa between 2000 and 2017. Nat. Med. 2019;25:1205–1212. doi: 10.1038/s41591-019-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osgood-Zimmerman A, et al. Mapping child growth failure in Africa between 2000 and 2015. Nature. 2018;555:41–47. doi: 10.1038/nature25760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinyoki DK, et al. Mapping child growth failure across low- and middle-income countries. Nature. 2020;577:231–234. doi: 10.1038/s41586-019-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinyoki DK, et al. Mapping local patterns of childhood overweight and wasting in low- and middle-income countries between 2000 and 2017. Nat. Med. 2020;26:750–759. doi: 10.1038/s41591-020-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray CJ, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 19.Reiner RC, Jr., et al. Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000–17: analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:1779–1801. doi: 10.1016/S0140-6736(20)30114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burstein R, et al. Mapping 123 million neonatal, infant and child deaths between 2000 and 2017. Nature. 2019;574:353–358. doi: 10.1038/s41586-019-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiens KE, et al. Mapping geographical inequalities in oral rehydration therapy coverage in low-income and middle-income countries, 2000–17. Lancet Glob. Health. 2020;8:e1038–e1060. doi: 10.1016/S2214-109X(20)30230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshpande A, et al. Mapping geographical inequalities in access to drinking water and sanitation facilities in low-income and middle-income countries, 2000–17. Lancet Glob. Health. 2020;8:e1162–e1185. doi: 10.1016/S2214-109X(20)30278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Maio FG. Income inequality measures. J. Epidemiol. Community Health. 2007;61:849–852. doi: 10.1136/jech.2006.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, H. et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet396, 1160–1203 (2020). [DOI] [PMC free article] [PubMed]
- 25.Roberts TJ, Carnahan E, Gakidou E. Can breastfeeding promote child health equity? A comprehensive analysis of breastfeeding patterns across the developing world and what we can learn from them. BMC Med. 2013;11:254. doi: 10.1186/1741-7015-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Infant and Young Child Feeding (UNICEF, 2019); https://data.unicef.org/topic/nutrition/infant-and-young-child-feeding/
- 27.The Countdown Country Profile: A Tool for Action (Countdown to 2030, accessed 10 February 2020); http://countdown2030.org/country-and-regional-networks/country-profiles
- 28.Sustainable Development Goals (UN, accessed 10 February 2020); https://www.un.org/sustainabledevelopment/sustainable-development-goals/
- 29.Marketing of Breast-milk Substitutes: National Implementation of the International Code, Status Report 2018 (WHO, UNICEF & IBFAN, 2018).
- 30.Piwoz EG, Huffman SL. The impact of marketing of breast-milk substitutes on WHO-recommended breastfeeding practices. Food Nutr. Bull. 2015;36:373–386. doi: 10.1177/0379572115602174. [DOI] [PubMed] [Google Scholar]
- 31.Chai Y, Nandi A, Heymann J. Does extending the duration of legislated paid maternity leave improve breastfeeding practices? Evidence from 38 low-income and middle-income countries. BMJ. Glob. Health. 2018;3:e001032. doi: 10.1136/bmjgh-2018-001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kavle J, LaCroix E, Dau H, Engmann C. Addressing barriers to exclusive breast-feeding in low- and middle-income countries: a systematic review and programmatic implications. Public Health Nutr. 2017;20:3120–3134. doi: 10.1017/S1368980017002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinn VJ, et al. Improving breastfeeding practices on a broad scale at the community level: success stories from Africa and Latin America. J. Hum. Lact. 2005;21:345–354. doi: 10.1177/0890334405278383. [DOI] [PubMed] [Google Scholar]
- 34.A Successful Start in Life: Improving Breastfeeding in West and Central Africa (UNICEF, 2010); https://www.unicef.org/health/files/wcaro_improving_breastfeeding_en.pdf
- 35.2018 Global Nutrition Report (Global Nutrition Report, 2018); https://globalnutritionreport.org/reports/global-nutrition-report-2018/
- 36.Global Breastfeeding Collective. Breastfeeding Advocacy Toolkit. Knowledge for Health (K4Health) (Johns Hopkins Center for Communication Programs, 2 August 2019); https://www.k4health.org/toolkits/breastfeeding-advocacy-toolkit
- 37.Walters, D., Eberwein, J. D., Sullivan, L., D’Alimonte, M. & Shekar, M. An Investment Framework for Meeting the Global Nutrition Target for Breastfeeding (World Bank Group, 2017).
- 38.Increasing Commitment to Breastfeeding Through Funding and Improved Policies and Programmes: Global Breastfeeding Scorecard 2019 (WHO & UNICEF, 2019); http://www.who.int/nutrition/publications/infantfeeding/global-bf-scorecard-2019/en/
- 39.Global Breastfeeding Collective. Scorecard Data (Tableau Software, 2018); https://public.tableau.com/profile/gbc2364#!/vizhome/Tables2/Dashboard1?publish=yes
- 40.International Code of Marketing of Breast-milk Substitutes (WHO, 1981); https://www.who.int/nutrition/publications/infantfeeding/9241541601/en/ [PubMed]
- 41.Infant Formula Market Value to Hit $98 billion by 2025: Global Market Insights, Inc. (Global Market Insights, 2019); http://www.globenewswire.com/news-release/2019/04/17/1805213/0/en/Infant-formula-Market-value-to-hit-98-billion-by-2025-Global-Market-Insights-Inc.html
- 42.Rollins NC, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet. 2016;387:491–504. doi: 10.1016/S0140-6736(15)01044-2. [DOI] [PubMed] [Google Scholar]
- 43.Sinha B, et al. Interventions to improve breastfeeding outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:114–134. doi: 10.1111/apa.13127. [DOI] [PubMed] [Google Scholar]
- 44.Fullman N, et al. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236–2271. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lozano R, et al. Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:2091–2138. doi: 10.1016/S0140-6736(18)32281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faraway, J. J. Linear Models with R Ch. 4 (CRC Press, 2004).
- 47.Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations (with discussion) J. R. Stat. Soc. B. 2009;71:319–392. doi: 10.1111/j.1467-9868.2008.00700.x. [DOI] [Google Scholar]
- 48.Lugina, H. I. Breastfeeding commitments and challenges in Africa. Afr. J. Midwifery Women’s Health10.12968/ajmw.2011.5.1.4 (2011).
- 49.Perez-Escamilla R. Breastfeeding in Africa and the Latin American and Caribbean Region: the potential role of urbanization. J. Trop. Pediatr. 1994;40:137–143. doi: 10.1093/tropej/40.3.137. [DOI] [PubMed] [Google Scholar]
- 50.GADM Database of Global Adminstrative Areas (GADM, 2018); http://www.gadm.org
- 51.Terra and Aqua Combined MODIS Version 5.1 (Land Processes Distributed Active Archive Center, accessed 24 July 2019).
- 52.Global Lakes and Wetlands Database, Level 3 (WWF, 2004); https://www.worldwildlife.org/pages/global-lakes-and-wetlands-database
- 53.WorldPop Datasets (WorldPop, accessed 22 January 2019); http://www.worldpop.org.uk/data/get_data/
- 54.Tatem AJ. WorldPop, open data for spatial demography. Sci. Data. 2017;4:170004. doi: 10.1038/sdata.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehner B, Döll P. Development and validation of a global database of lakes, reservoirs and wetlands. J. Hydrol. 2004;296:1–22. doi: 10.1016/j.jhydrol.2004.03.028. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–23, Supplementary Tables 1–12, Supplementary Methods, Supplementary Results, Supplementary Limitations, Supplementary Discussion, Supplementary References; Author Contributions.
Data Availability Statement
The information given here is mostly consistent with our previous study modelling EBF across Africa14. The findings of this study are supported by data that are available in public online repositories, data that are publicly available on request from the data provider and data that are not publicly available due to restrictions by the data provider and which were used under license for the current study. Details on data sources can be found on the GHDx website (http://ghdx.healthdata.org/lbd-publication-data-input-sources?field_rec_ihme_publication_tid=29093), including information about the data provider and links to where the data can be accessed or requested (where available). We have also provided maps of the data included in our models in Supplementary Figs. 1–5. Outputs of these EBF analyses can be explored at various spatial levels (national, administrative and 5 × 5-km levels) through our customized visualization tool (https://vizhub.healthdata.org/lbd/ebf) and are publicly available at the GHDx (http://ghdx.healthdata.org/record/ihme-data/global-exclusive-breastfeeding-prevalence-geospatial-estimates-2000-2019). Administrative boundaries were retrieved from the Database of Global Administrative Areas (GADM)50. Land cover was retrieved from the online Data Pool, courtesy of the NASA EOSDIS Land Processes Distributed Active Archive Center, USGS/Earth Resources Observation and Science Center, Sioux Falls, South Dakota51. Lakes were retrieved from the Global Lakes and Wetlands Database, courtesy of the World Wildlife Fund and the Center for Environmental Systems Research, University of Kassel52. Populations were retrieved from WorldPop53. All maps in this study were produced using ArcGIS Desktop 10.6.
All codes used for these analyses are publicly available online at https://github.com/ihmeuw/lbd/tree/ebf-lmic-2021.