Abstract
The entire globe is affected by the novel disease of coronavirus 2019 (COVID-19 or 2019-nCoV), which is formally recognised as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organisation (WHO) announced this disease as a global pandemic. The presence of SARS-CoV-2 RNA in unprocessed wastewater has become a cause of worry due to these emerging pathogens in the process of wastewater treatment, as reported in the present study. This analysis intends to interpret the fate, environmental factors and route of transmission of SARS-CoV-2, along with its eradication by treating the wastewater for controlling and preventing its further spread. Different recovery estimations of the virus have been depicted by the detection of SARS-CoV-2 RNA in wastewater through the viral concentration techniques. Most frequently used viral concentration techniques include polyethylene glycol (PEG) precipitation, ultrafiltration, electronegative membrane, and ultracentrifugation, after which the detection and quantification of SARS-CoV-2 RNA are done in wastewater samples through quantitative reverse transcription-polymerase chain reaction (RT-qPCR). The wastewater treatment plant (WWTP) holds the key responsibility of eliminating pathogens prior to the discharge of wastewater into surface water bodies. The removal of SARS-CoV-2 RNA at the treatment stage is dependent on the operations of wastewater treatment systems during the outbreak of the virus; particularly, in the urban and extensively populated regions. Efficient primary, secondary and tertiary methods of wastewater treatment and disinfection can reduce or inactivate SARS-CoV-2 RNA before being drained out. Nonetheless, further studies regarding COVID-19-related disinfectants, environment conditions and viral concentrations in each treatment procedure, implications on the environment and regular monitoring of transmission need to be done urgently. Hence, monitoring the SARS-CoV-2 RNA in samples of wastewater under the procedure of wastewater-based epidemiology (WBE) supplement the real-time data pertaining to the investigation of the COVID-19 pandemic in the community, regional and national levels.
Keywords: Transmission routes, Untreated wastewater, Wastewater treatment process, Virus concentration method, Disinfection, Wastewater–based epidemiology (WBE), Surveillance
Graphical abstract
1. Introduction
The concurrent pandemic due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is commonly known as COVID-19 and was initially encountered at Wuhan City, China, in December 2019. This is induced by a novel human coronavirus and has emerged as a crucial disease and public health issue (Usman et al., 2020); thus, affecting millions of people across the globe (Dhama et al., 2021). The World Health Organisation (WHO) had informed about the attack of COVID-19 pandemic on March 11, 2020, owing to its global spread (Agrawal et al., 2021; WHO, 2020). The commonly recognised mode of transmission of SARS-CoV-2 is by inhaling the droplets and establishing person-to-person contact (Mandal et al., 2020; Morawska and Cao, 2020). Current scholars are investing their attempts in studying the transmission routes of SARS-CoV-2 prevalent on treatment plants for wastewater.
SARS-CoV-2 was recently found in stool samples, and its continued dropping into environmental sections like sewage and wastewater indicates the potential menace to the route of transference of the virus (Dhama et al., 2021). Recently, the passage of SARS-CoV into the sewage by way of patients' stool and urine has been highlighted (Wang et al., 2005a). The sewage is contaminated by the presence of SARS–CoV–2 RNA, which has been excreted by the human body through saliva, sputum and faeces (Lahrich et al., 2021). SARS-CoV-2 is incessant in human bodies and may prevail in body fluids, including urine (Kujawski et al., 2020; Lo et al., 2020; Sun et al., 2020) and faecal samples (Amirian, 2020; Chen et al., 2020; Kujawski et al., 2020; Ling et al., 2020; Park et al., 2020; Tang et al., 2020; Wu et al., 2020a) that are passed out of the body through their excretion into the wastewater (Cervantes-Avilés et al., 2021). Previously conducted research reported the presence of SARS-CoV-2 in the faeces, along with gastrointestinal symptoms in 17.1% of patients infected by COVID-19 (95% CI, 6.9–36.7) (Cheung et al., 2020). SARS-CoV-2 RNA was constantly excreted in the faeces of almost 50% of symptomatic patients with a concentration of 108 RNA per sample of stool (Chen et al., 2020; Wang et al., 2020a; Wölfel et al., 2020; Wu et al., 2020a; Xiao et al., 2020; Xu et al., 2020). Foladori et al. (2020) examined the viral load in the COVID-19 positive patients' faeces and revealed the presence of SARS-CoV-2 RNA at the concentration level of 5 × 103–5 × 107.6 copies/mL, wherein its sequential form are determined and isolated in human faeces (HFs). It means that a single person can discard billions of copies of SARS-CoV-2 RNA, thereby contaminating the wastewater (Trottier et al., 2020). A recent study held at Bangkok Metropolitan and suburbs attempted to track the occurrence of COVID-19 in wastewater for understanding the asymptomatic transmission and exposed the increasing count of SARS-CoV-2 RNA in the wastewater in line with the growing number of COVID-19 positive patients (Wannigama et al., 2021). Additionally, the viral RNA has been found in the faeces of almost 15–83% SARS-CoV-2 infected patients (Foladori et al., 2020). A recent study established the high prevalence of SARS-CoV-2 RNA in faeces rather than urine (Cervantes-Avilés et al., 2021; Collivignarelli et al., 2020). Subsequent to the excretion of the virus from the faeces, it is initially diluted in toilet water, post which it mixes with wastewater and then enters WWTP, along with the greywater released from showers and washing machines (Foladori et al., 2020; Larsen and Wigginton, 2020). The excreted virus leaves a possibility for the faecal-oral transmission of the virus (Pandey et al., 2021), whereas the stool acts as a major cause of viral genomic units prevailing in wastewater. SARS-CoV-2 RNA's survival in wastewater is possible for multiple days (Ahmed et al., 2020a), thus probing the uncontrolled impact of SARS-CoV-2 on the environment (Abu Ali et al., 2021).
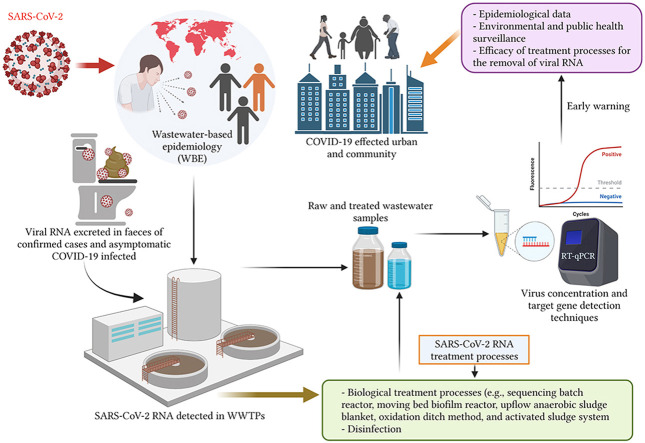
It is virtually non-practical to continue with the clinical testing method of swabbing every individual of a country for a long time due to the restricted availability of resources and funds as well as due to the requirement of multiple resources and personnel (Hong et al., 2021). Contrarily, directly monitoring the wastewater for the SARS-CoV-2 RNA over a long period will help in consummating the current process of clinical surveillance. The virus is ejected out by way of faeces by almost 39–65% of infected patients as well as asymptomatic hosts, whereas merely 6% of infected carriers pass it out through urine (Chan et al., 2020; Cheung et al., 2020; Hong et al., 2021). Hence, the studies done for detection of SARS-CoV-2 RNA in untreated wastewater explored a molecular test, namely wastewater-based epidemiology (WBE), that can be done to determine the invasive prevalence of the virus (Ahmed et al., 2020a; Kitajima et al., 2020). Considering the fact that it would not be possible to examine many individuals clinically, proactive detection of SARS-CoV-2 in the wastewater can furnish a technique for non-invasive warning to warn the communities against a new COVID-19 infection (Orive et al., 2020).
The surveillance of wastewater streams to identify the COVID-19 offers an opportunity to observe the pandemic in closed areas by way of sewage monitoring to prevent the further dispersal of the pathogen. The present study relates to the dispersal of SARS-CoV-2 in wastewater and the environment, whereas further study needs to be pursued to clarify its link with COVID-19 infection and faecal-oral transmission channels of the virus. Hence, the initial detection of the virus through rapid testing, faeces testing and examining its presence in municipal wastewater can prove to be efficient techniques of controlling the spread of COVID-19 infection (Orive et al., 2020; Thakur et al., 2021). In the same context, reduction of possibly identified risks of transmission of SARS-CoV-2 through faecal-oral channels need to be addressed in subsequent research studies along with filling the knowledge gaps pertaining to the effect of population density on quantification of SARS-CoV-2 detected in wastewater and the classical method of detecting virus. SARS-CoV-2 is spread through aerosols and droplets, due to which the viral detection studies involve in-situ wastewater and other segments of the environment owing to the intensity of the disease. The mechanism through which this virus enters the environment can be well understood by interpreting the significance of the SARS-CoV-2 virus's diffusion and survival in wastewater and its eradication through treatment procedures. This study has been compiled to critically review the fate and transmission route of SARS-CoV-2 RNA along with the methods for detecting SARS-CoV-2 RNA in wastewater. This review also enables the surveillance of public health monitoring by way of critical analysis of wastewater-based epidemiology (WBE) and the current information on wastewater treatment procedures for eradicating SARS-CoV-2. This knowledge will assist in strategic decision making for drafting policies, initiating measures, planning for prevention and control of SARS-CoV-2 in addition to scrutinising the wastewater-based epidemiology process prior to and post-COVID-19 epidemic.
2. Methodology
The review has been facilitated by performing a literature examination on the databases that are available online. The search has been done by reviewing the articles published on or before June 2021; the data has been extracted majorly from the PubMed, Web of Science, ScienceDirect and Scopus databases. The researcher encompasses the keyword search by focusing on SARS-CoV-2, coronavirus 2019, wastewater, wastewater treatment, and the Boolean operators of ‘OR’ and ‘AND’. The article search was conducted by focusing on the English language, and it was administered by identifying, screening, classifying and selected the best articles on wastewater treatment plants and their disinfection. The research has been delved by even considering the pre-print and the pre-reviewed articles as well. The articles had been predominantly reported on the SARS-CoV-2 detection in wastewater and its concerning process such as the process to remove RNA of SARS-CoV-2 from the wastewater. The articles gave data on the fate, route transmission, method of discerning SARS-CoV-2. The other concepts narrate the use of wastewater treatment plants (WWTPs) and wastewater-based epidemiology (WBE) in order to remove SARS-CoV-2 RNA in wastewater.
3. Fate and environmental factors of SARS-CoV-2 in wastewater
SARS-CoV-2 is known to be a single-stranded enveloped RNA genesis that develops due to the Coronaviridae, and its size range falls between 60 and 140 nm (Bogler et al., 2020; Lesimple et al., 2020). It was explored in the past that SARS-CoV-2 RNA in faeces of viral load accounted to 1.7 × 109–4.1 × 1010 copies/L existed in between the patients was approximately 27 days old neonate (Han et al., 2020). This viral RNA exists at approximately 3.2 × 105 copies/L concentration level (Peng et al., 2020). Therefore, the amount of pathogen time such as SARS-CoV-2 can get through outside the human body is supplied by the environmental persistence. The more likely the virus persists outside the human body, the more likely chances of spread are there (Lahrich et al., 2021). In the studies conducted earlier, it was evidenced that the ability of the SARS-CoV to live under varied atmosphere is higher such as humidity and high temperature as well as equipment provided patients in hospitals such as sterile sponges, aluminium, latex gloves and biological fluids (Geller et al., 2012). The fate of coronavirus in wastewater is bifurcated into two processes, namely the ability to survive in wastewater and the removal of various stages during the treatment of wastewater (Amoah et al., 2020). It has also been identified that pH, organic matter and antagonistic bacteria are present during the removal of the wastewater (Arslan et al., 2020). The sunlight's presence, including the effects of humidity and the organic substance matter, oppose the outcomes (Kataki et al., 2021). Contrastingly, it has been stated by Nemudryi et al. (2020) that SARS-CoV-2 RNA levels in the wastewater contaminate the onset by several days. Moreover, the study conducted in the primary sewage sludge from NE division of US reported a time of SARS-CoV-2 RNA concentrations in the spring season amidst COVID-19 outbreak followed by wastewater contamination. It was found out that virus RNA concentration in the COVID-19 is associated with epidemiological curve and admission in the local hospital (R2 = 0.99). SARS-CoV-2 RNA concentrations used to remain on a seven-day leading indicator ahead of the virus testing data and headed towards the local hospital admission (Coil, 2020). In the viewpoints of Venugopal et al. (2020), it has been reported that the virus has a prolonged survival even at a low temperature. Therefore, this factor excreted coronavirus possibility for the residual treatment plants. As per the previous study conducted by Wang et al. (2005b), demonstrated SARS-CoV is disabled after 2 days at 20 °C.
In addition to the studies above, a recent study proclaims that coronaviruses can stay in hospital or household water for up to a period of 14 days, even at 4 °C in dechlorinated treatment. The virus can sustain at a temperature of 20 °C, even though it survives for only 2 or 3 days (García-Ávila et al., 2020; Kitajima et al., 2020; La Rosa et al., 2020a; Ren et al., 2020). It has also been explored that the average T 90 (time required for 1 log10) of coronavirus RNA ranged from 8.0 days to 27.8 days in untreated wastewater. García-Ávila et al. (2020) opined that SARS-CoV-2 virus could last in untreated sewage for more than 25 days span and it has a high potential of transmission by the faecal-oral transmission. As per the report conducted by Shutler et al. (2021), it has been revealed that the SARS-CoV-2 virus can stay longer in the aquatic ecosystems depending upon the characteristics of wastewater. The risk of developing the risk in the wastewater increases in the cool period as the virus becomes viable in low temperature and sustain longer (Carraturo et al., 2020). Amoah et al. (2020) and Arslan et al. (2020) suggested that various factors influence the coronavirus in wastewater and in varied environmental conditions (for instance, sewage characteristics, pH, weather, the structure of the virus and the wastewater attributes). In line with the same direction; the persistence of SARS-CoV-2 in wastewater depends on the aura and the attributes of the wastewater (Bilal et al., 2020a; Carducci et al., 2020; Foladori et al., 2020; Kumar et al., 2020a). The factors have depicted that SARS-CoV-2 infectivity may have a larger impact on the organic compound found in the sewage water and the pH (Chin et al., 2020; Geller et al., 2012; Wang et al., 2005b). Additionally, the pH of faeces, for instance, used to have a major effect on the sustenance of SARS-CoV-1 that can range from 3 h in newborn faeces and approximately to four days in an adult's diarrheal faeces along with having a pH up till 9 (Lai et al., 2005). While SARS-CoV-1 is deferred, there has been no traceable reduction in the infection titers even post 1 h over a wide range of pH range of 3–10 (Chin et al., 2020). In contrast to it, greywater has received water discharge, namely shower, sink and drain within the periphery of health care units. It is unexpected to be a transmitter to the SARS-CoV-2 spread (Wang et al., 2020a; Wölfel et al., 2020). The low virus of concentrates in greywater can be affected by disinfectants, soaps and detergents to SARS-CoV-2 sensitivity (Chin et al., 2020; Kampf et al., 2020).
The study undertaken by Arora et al. (2020) suggests that there is either very low or no risk in the instance of SARS-CoV-2 regarding the effluent that has been treated for non-potable applications. As per another assertion, raw untreated wastewater can potentially be transmitted by the transmission of the virus to the workers. SARS-CoV-2 is like SARS-CoV that could develop into wastewater by the surface water, and another mode of transmission could be possibly due to bioaerosols emissions sources.
4. The probability of transmission of SARS-CoV-2 in wastewater
Since the year 2003, WHO had been reporting on the vast spread of the severe acute respiratory syndrome inside the housing in the province of Hong Kong (WHO, 2003). In the year 2003, confirmed cases of COVID-19 were approximately 342 in number, causing a high morbidity rate such as occurrence of 42 fatalities on the 50-storey building (Gormley et al., 2020). Another study proclaimed that at the time; bathroom exhaust fans were running, there used to be a high chance of the concentration of viral aerosols in wastewater plumbing systems (Gormley et al., 2011, 2013). The phenomenon had led to transmit the virus-laden droplet nuclei's via the empty U-bends into the bathroom as a source of airborne route transmission inside of a building (Gormley et al., 2020; McKinney et al., 2006; Nghiem et al., 2020). The scientific study suggests that a possible risk of coronavirus transmission through the water indicates the existence of SARS-CoV-2 in WWTPs (Kataki et al., 2021). The main route of transmitting SARS-CoV-2 is via respiratory droplets that get manifold by coming in direct contact with the infected person (Mandal et al., 2020). It has been evidenced by the plumbing system of wastewater as the key source of pathogens (Farkas et al., 2020; Gormley et al., 2011, 2013). It has been observed that droplet fallout results in surface contamination, such as in free space and devices (Gormley et al., 2020). It had been found out that adenovirus was more resistant to eradication in comparison with the polyomavirus and torque teno virus (Sidhu et al., 2018). The studies depict that biological characteristics of wastewater would manifold if the sources of the faecal-oral route get contaminated if not managed properly. It has been indicated that the virus gets spread with the effluent stream, most particularly through untreated water that is usually seen in developing nations (Elsaid et al., 2021).
Wastewater workers occupationally get exposed to the bioaerosols in environment operation during the influent treatment process carried by numerous microorganisms. Most of the bioaerosols in wastewater or sewage contains bacteria, fungus and other metabolites products such as endotoxin plus viruses (Thakur et al., 2021). Carducci et al. (2018) postulated that a higher average risk in the wastewater gets affected by biological oxidation calculated through quantitative microbial risk assessment (QMRA). The risk in QMRA ranges between 15.6% and 12.7% for exposure within 3 min. As explored, the concentration of human adenovirus (HAdv) acts as a predominant factor for estimating the risk and sensitivity based on biological risk evaluation. The high exposure concentration is parameters have a strong effect on health (Chen et al., 2021). There are potential biological health hazards associated with the emitted bioaerosols from WWTPs with the externalities such as bubble aeration, dewatering and mechanical aeration. The spread in WTTPs boundaries has shown that document of the plumbing system in wastewater reservoir for the pathogen agents (Gormley et al., 2020). Pathogenic viruses are one of the major threats to human beings since SARS-CoV-2 becomes inherent in wastewater and cannot be extracted out easily. Inhalation directly to the aerosols is reported to be the main route of transmission (Ge et al., 2020).
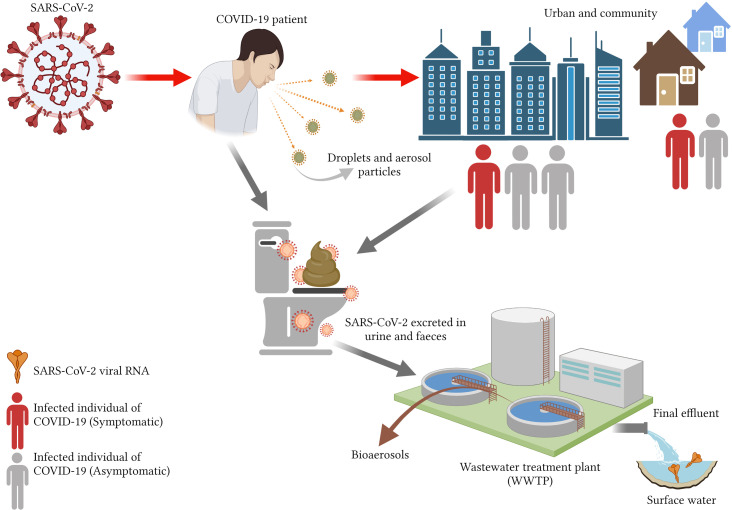
The survival of the coronaviruses with the exposure of aerosols, for instance, concentration of viral inhaled particle, high host emission rate, viral inactivation and meteorological factors manifold the possible threat of exposure within WWTPs is made through bioaerosol inhalation or direct contact with infectious viral fragments (Amoah et al., 2020; Panchal et al., 2021). It has also been identified that the extent of infection in SARS-CoV-2 in wastewater is limited (Kitajima et al., 2020). The discovery of SARS-CoV-2 RNA during the entire process emphasises the need for infectivity investigations and showcases that the danger to WWTP operators and in the surrounding community is low (Abu Ali et al., 2021). In addition, in plentiful environmental settings, the persistence of SARS-CoV-2 is measured in different environments such as dust particles, tap water, airborne particulates and domestic sewage that calls for urgent systemic investigations (Bilal et al., 2020b). There becomes a high risk of the pathogenic viruses present in human health as it has a high potentiality of secondary transmission, particularly in wastewater during the COVID-19 pandemic (Liu et al., 2020; Thakur et al., 2021). The viruses are the major causes of transmitting respiratory diseases such as gastrointestinal and hepatitis (Bhatt et al.). There has been a doubted debate regarding the infectivity of SARS-CoV-2 via the material in the aquatic environment owing to the presence of the detected gene in the wastewater system (Kumar et al., 2020b). In addition, SARS-CoV-2 RNA can be both waterborne and non-waterborne viruses (Bhatt et al., 2020). Another technique of transmittance of SARS-CoV-2 coronavirus was explored by Bogler et al. (2020), Bhatt et al. (2020), Dhama et al. (2021) and Tran et al. (2021). They concluded possible routes in wastewater and environment as depicted in Fig. 1 .
Fig. 1.
The possible transmission routes for SARS-CoV-2 RNA through contaminated water and the environment (Created with BioRender.com).
5. SARS-CoV-2 RNA detection method in wastewater and wastewater-based epidemiology (WBE)
5.1. Method for SARS-CoV-2 RNA detection and virus concentration in wastewater
Detecting SARS-CoV-2 in wastewater is an impressive technique of supporting the surveillance of dissemination of COVID-19 through the biomarker analysis of wastewater contaminated through human sewage. SARS-CoV-2 detection in wastewater can also indicate symptomatic and asymptomatic instances of COVID-19 in the populations of each area that differs from clinical laboratory tests wherein individualised results have been exhibited by patients. Presently, the quantitative reverse transcription PCR (RT-qPCR) and real-time reverse transcription-polymerase chain reaction (rRT-PCR) techniques (Kumar et al., 2020b; Mathuria et al., 2020) are most commonly used for molecular analysis of SARS-CoV-2 RNA along with nested RT-PCR technique. The RNA target genes of SARS-CoV-2 are made up of nucleocapsid (N), RNA-dependent RNA polymerase (RdRP), spike (S) protein, regions of the open reading frame (ORF) 1a and 1b and envelope (E) genes (Carter et al., 2020; Mathuria et al., 2020). Mostly, the discovery and quantification of SARS-CoV-2 RNA in wastewater done through RT-qPCR entails detection methods that are based on molecular techniques. In the current analytical study, the virus was detected in 61% wastewater units out of 126 samples, wherein the level of viral gene copies per ml was determined by RT-qPCR (Weidhaas et al., 2021). Additionally, the identification methods of aqueous as well as solid-phase SARS-CoV-2 should be taken into consideration during quantification of SARS-CoV-2 gene concentrations and loads shed in wastewater and accordingly, meticulous periodic protocols should be developed for measuring clean-up and RT-qPCR (Weidhaas et al., 2021). Currently, multiple studies have recorded the detection of SARS-CoV-2 RNA in wastewater (Ahmed et al., 2020b; Kocamemi et al., 2020; Arora et al., 2020; Balboa et al., 2021, Bar Or et al., 2020; Chavarria-Miró et al., 2020; Collivignarelli et al., 2020; Green et al., 2020; Haramoto et al., 2020; Hata et al., 2021; Kumar et al., 2020b; La Rosa et al., 2020b; Medema et al., 2020; Nemudryi et al., 2020; Peccia et al., 2020; Randazzo et al., 2020a, 2020b; Rimoldi et al., 2020; Sharif et al., 2020; Wu et al., 2020b; Wurtzer et al., 2020). The summary of the prevalence of SARS-CoV-2 RNA in the category of wastewater samples, the viral concentration and the detection technique engaged in each continent and country are represented in Table 1 .
Table 1.
Occurrence of SARS-CoV-2 RNA in wastewater and various viral detection techniques.
| Continent | Country | Type of wastewater sample | Virus concentration method | Detection method | Positive result rate (%) | Range of concentration (copies/L) | RNA target genes | References |
|---|---|---|---|---|---|---|---|---|
| North America | USA | Untreated wastewater | Ultrafiltration and adsorption-eluting using electronegative membrane | Nested PCR and RT-qPCR | 2/7 = 28.6% | 3.1 × 103–7.5 × 103 | ORF 1a, S | Sherchan et al. (2020) |
| Secondary treated | 0/4 = 0% | N | ||||||
| Final effluent | 0/4 = 0% | |||||||
| USA | Untreated wastewater | Polyethylene glycol-8000 (PEG 8000) | RT-qPCR | 2/2 = 100% | 57–303 copies/mL | N1, N2, N3 | Wu et al. (2020b) | |
| 10/10 = 100% | ||||||||
| USA | Untreated wastewater | Corning Spin X-ultrafiltration | RT-qPCR | 7/7 = 100% | >3 × 104 | N | Nemudryi et al. (2020) | |
| Europe | Italy | Untreated wastewater | Polyethylene glycol (PEG) and dextran (DEX) or PEG-dextran | Nested PCR and RT-qPCR | 6/12 = 50% | Not detected | ORF 1 ab | La Rosa et al. (2020b) |
| Italy | Untreated wastewater | Ultrafiltration | RT-qPCR | 4/8 = 50.0% | – | 2019-nCoV, ORF 1 ab, E_Sarbeco | Rimoldi et al. (2020) | |
| Treated wastewater | Not detected | – | ||||||
| Italy | Untreated wastewater | Ultrafiltration over ultracentrifugation or PEG precipitation | RT-qPCR | 4/9 = 44.4% | – | ORF ab, N | Baldovin et al. (2021) | |
| Tertiary treated | 2/2 = 100% | |||||||
| Germany | Untreated wastewater | Electronegative membrane filter | RT-qPCR | 2/2 = 100% | 4 × 1011–1 × 1015 copies/day | N, S, ORF 1 ab | Agrawal et al. (2021) | |
| Spain | Untreated wastewater | Aluminium flocculation-beef exact precipitation | RT-qPCR | 12/15 = 80.0% | 104–105 | N | Randazzo et al. (2020a) | |
| Treated wastewater | 0/9 = 0% | 0 | ||||||
| Spain | Untreated wastewater | Aluminum hydroxide adsorption-precipitation | RT-qPCR | 35/42 = 83.3% | 1 × 105–3.4 × 105 | N | Randazzo et al. (2020b) | |
| Treated secondary | 2/18 = 11.1% | <2.5 × 104 | ||||||
| Treated tertiary | 0/12 = 0% | Not available | ||||||
| Spain | Untreated wastewater | Amicon ultrafiltration of centrifugated supernatant | RT-qPCR | 5/5 = 100% | – | N, E, RdRP | Balboa et al. (2021) | |
| Treated wastewater | 1/4 = 25.0% | |||||||
| France | Untreated wastewater | Ultracentrifugation | RT-qPCR | 23/23 = 100% | 5 × 104 GU/L | RdRP, E | Wurtzer et al. (2020) | |
| Treated wastewater | 6/8 = 75.0% | 3 × 106 GU/L | ||||||
| Netherlands | Untreated wastewater | Ultrafiltration | RT-qPCR | 14/24 = 58.3% | 2 × 103–2.2 × 106 | N, E | Medema et al. (2020) | |
| Asia | Japan | Untreated wastewater | Electronegative membrane-direction RNA exaction; ultrafiltration | RT-qPCR | 0/5 = 0% | Not available | N | Haramoto et al. (2020) |
| Secondary wastewater | 1/5 = 20.0% | 2 .4 × 103 | ||||||
| Japan | Untreated wastewater | PEG precipitation | RT-qPCR | 7/17 = 41.2% | 4.4 × 104 | N2, N3, NIID_2019-nCoV_N | Hata et al. (2021) | |
| China | Untreated wastewater | PEG precipitation of centrifugation supernatant | RT-qPCR | 0/4 = 0% | Not available | ORF 1, N | Zhang et al. (2020) | |
| Treated wastewater | 7/9 = 77.8% | 0.5 × 103–18.7 × 103 | ||||||
| India | Untreated wastewater | PEG and NaCl centrifugation | RT-qPCR | 5/12 = 41.7% | – | RdRP, ORF 1 ab, E, S, N | Arora et al. (2020) | |
| Treated wastewater | 0/6 = 0% | |||||||
| India | Untreated wastewater | PEG precipitation | RT-qPCR | 2/2 = 100% | 0.78 × 102–8.05 × 102 | ORF 1 ab, N, S | Kumar et al. (2020b) | |
| Untreated wastewater | Adsorption | rRT-PCR | 6/17 = 35.3% | RdRP, ORF 1 ab, E, S, N | ||||
| India | Upflow anaerobic sludge blanket (UASB) | PEG precipitation of centrifugated supernatant | RT-qPCR | – | 3.5 × 103 | ORF 1 ab, N, S | Kumar et al. (2021) | |
| Aeration pond | 1.5 × 102 | ORF 1 ab | ||||||
| Iran | Treated wastewater (Final effluent) | Adsorption-elution technique using electronegative filter | RT-qPCR | 8/10 = 80.0% | 101–103 | ORF 1 ab, N | Tanhaei et al. (2021) | |
| Iran | Untreated wastewater | PEG 6000 | RT-qPCR | 12/12 = 100% | – | ORF 1 ab, N | Nasseri et al. (2021) | |
| Treated wastewater | 2/12 = 16.7% | |||||||
| Israel | Untreated wastewater | Primary: PEG or Alum; precipitation; secondary: Amicon ultrafiltration | RT-qPCR | 10/26 = 38.5% | – | E | Bar Or et al. (2020) | |
| Turkey | Untreated wastewater | Ultracentrifugation, PEG8000-adsorption electronegative membrane and ultrafiltration | RT-qPCR | 7/9 = 77.8% | 9.33 × 104 | RdRP | Kocamemi et al. (2020) | |
| The United Arab Emirates (UAE) | Untreated wastewater-WWTP | Ultrafiltration columns, and PEG/TRIzol | RT-qPCR | 28/36 = 77.8% | 7.50E + 02 – 3.40E + 04 | RdRP | Hasan et al. (2021) | |
| Untreated wastewater (38 other locations) | 30/38 = 78.9% | 2.86E + 02 – 2.90E + 04 | ||||||
| 11 WWTP treated effluent | Not detected | – | ||||||
| Qatar | Influent wastewater (Untreated wastewater) | PEG | RT-qPCR | 100% | 7,889 ± 1421 – 542,056 ± 25,775 copy/L | N | Saththasivam et al. (2021) | |
| Saudi Arabia | Untreated wastewater | Electronegative membrane | RT-qPCR | 43/57 = 75.4% | – | N1, N2, N3 | Hong et al. (2021) | |
| Pakistan | Untreated wastewater | PEG/dextran precipitation of centrifuged supernatant | RT-qPCR | 21/78 = 26.9% | – | ORF 1a | Sharif et al. (2020) | |
| Oceania | Australia | Untreated wastewater | Electronegative membrane-direct RNA exaction; ultrafiltration | RT-qPCR | 2/9 = 22.2% | 1.9 × 101–1.2 × 102 | N | Ahmed et al. (2020b) |
The main constraint in the detection of SARS-CoV-2 RNA in wastewater is associated with the lack of a reliable virus concentration method owing to the low plethora of SARS-CoV-2 in the sample of wastewater. Hence, virus concentration technique would likely be crucial to increase the chances of analytical determination of SARS-CoV-2 RNA in wastewater; a virus concentration test needs to be performed prior to the RT-qPCR analyses of SARS-CoV-2 RNA for accomplishing virus detection in wastewater, including untreated water samples considering the differing concentrations of the virus at each WWTP site. Furthermore, as revealed by Ibrahim et al. (2021), precipitation of polyethylene glycol (PEG) is appropriate for analysing middle and large-sized sample aggregates due to the reliability and high efficiency of this technique's outcomes. It is a broadly adapted technique that is also characterised by bulky input volumes and low-speed centrifugation (<12,000 xg). Multiple limitations of this technique include the requirement of precipitation for long before undergoing centrifugation. This method can induce the precipitation of other proteins and non-viruses linked with extracellular nucleic acids (ENAs) and is used in dangerous chemical solutions (e.g., TRIzol reagent) for efficient extraction of total RNA and concurrent isolation of DNA, RNA and protein (Ahmed et al., 2020c; Ibrahim et al., 2021; McNamara and Dittmer, 2020; Prata et al., 2012). This technique can also be used for long precipitation hours prior to the actual centrifuging stage; it is a spend time-protracted procedure (Ibrahim et al., 2021). Though the virus concentration methodology employed in small-scale samples such as the ultracentrifugation technique gets benefited due to the quick processing of the samples used as well as the reliability and effectiveness of the isolation procedure, there are certain limitations linked with techniques like pre-filtration, such as low volume availability between 20 and 50 mL, need for removal of deterring substances from the sample, high centrifugation speed of ≥100,000 xg (Ibrahim et al., 2021; McNamara and Dittmer, 2020; Prata et al., 2012) Similarly, the adsorption techniques engaging electronegative filters cost lesser and can enhance the recovery rate in the sample due to the optimisation of the enormous sample volumes for enteric viruses; however, inhibitions like the need for adjusting the pH of the samples containing beef extract, acids and salt and requirement of sample filtration prior to adsorption for preventing clogging of filtration make this technique disadvantageous (Cashdollar and Wymer, 2013; Hata et al., 2015; Ibrahim et al., 2021). The major goal of virus concentration techniques is to accomplish better recovery of viruses so that viral contagion in wastewater can be well-monitored and assessed for mitigation and prevention against virus outbreaks anticipated in future times. Both previous and latest studies have highlighted multiple methods of virus concentration such as polyethylene glycol (PEG) precipitation (Hasan et al., 2021; La Rosa et al., 2020b; Nasseri et al., 2021; Saththasivam et al., 2021), ultrafiltration and adsorption-eluting using an electronegative membrane (Sherchan et al., 2020) and ultrafiltration columns, ultrafiltration over ultracentrifugation or PEG precipitation (Baldovin et al., 2021) as depicted in Table 1.
5.2. Wastewater-based epidemiology (WBE) surveillance of the SARS–CoV–2
The detection of SARS-CoV-2 in faecal samples has made the presence of coronavirus in human wastewater quite explicit and obvious (Usman et al., 2020). The detection of SARS-CoV-2 virus in the faeces or stool samples of asymptomatic patients who are tested negative in nasopharyngeal samples paves the way for wastewater surveillance by wastewater-based epidemiology (WBE) (Haramoto et al., 2020; Jiang et al., 2020; Lu et al., 2020; Pandey et al., 2021), especially in regions with low funding resources so as to assist in the fulfilment of clinical research requirements (Barceló, 2020; Gonzalez et al., 2020; Hart and Halden, 2020). Therefore, wastewater-based epidemiology (WBE) plays the role of a crucial epidemiological instrument engaged to determine the movement of virus in communities and understand the status of viral outbreaks through viral load investigation in designated containment regions (Choi et al., 2018; Kitajima et al., 2020; Saawarn and Hait, 2021). WBE raises the alarm to control the spread of the virus by symptomatic as well as asymptomatic patients (Chavarria-Miró et al., 2020; Thompson et al., 2020). The COVID-19 pandemic can be effectively managed by employing the technique of examination of wastewater (Street et al., 2020) that further addresses the urgent concern regarding ineffective diagnostic assessment that can also introduce a proactive low-cost detection technique in the current epidemic of COVID-19 (Barcelo, 2020). This exploration will carve the way for innovative approaches to supervising population with the aid of wastewater-based epidemiology considering the limited and cost-intensive testing capacity for the entire pool of citizens in low and middle-income nations (Pandey et al., 2021). Though for executing wastewater-based epidemiology precisely, population normalisation, representative sampling of viral concentration in wastewater and ethical recommendations are critical deliberations (Polo et al., 2020). Maximum studies based on the usefulness of wastewater analysis can effectively assist in monitoring the spread of coronavirus and predicting the novel pandemic, thereby limiting its spread across the borders (Lapolla et al., 2020). This confirms the vitality of wastewater in monitoring the prevalence of the virus in the communities (Nemudryi et al., 2020).
Nonetheless, the implementation of wastewater-based epidemiology will be indispensable for monitoring and managing public health surveillance during and after the COVID-19 pandemic (Cervantes-Avilés et al., 2021). Additionally, this technique will use computational scrutiny and modelling to test the feasibility, cost-effectiveness and challenges linked with the quantification of the active cases of coronavirus infection in communities as well as global regions (Hart and Halden, 2020). Thus, the enumeration of SARS-CoV-2 in wastewater enables monitoring and surveillance techniques for detecting the existence of viral infection in the population via WBE amid the global outbreak of pandemic (Ahmed et al., 2020b; Barceló, 2020; Gonzalez et al., 2020; Michael-Kordatou et al., 2020). However, proactive detection of the virus in sewage can be a non-invasive alarm to warn people regarding new COVID-19 infections, as many people would not be screened through standard clinical laboratory assessments (Orive et al., 2020).
Since SARS-CoV-2 are excreted in the faeces of COVID-19 infected people, an increased emphasis should be laid on surveillance through wastewater-based epidemiology (Nghiem et al., 2020) in order to predict upcoming waves or outbreaks of the virus (Wannigama et al., 2021). Hence, developed as well as developing nations should use WBE tools for public health surveillance during and after the COVID-19 pandemic to gather epidemiological data and support suitable disinfection measures of the wastewater recycling process to make water useable. WBE is an effective tool for developing countries having a limited scope of clinical diagnosis for SARS-CoV-2 (Carrillo-Reyes et al., 2021). Moreover, as per the existing data, an exhaustive understanding of wastewater's status as a potential source of epidemiological information and a risk factor for environmental and public health is of utmost requirement. Thus, WBE is used as a proactive warning system regarding the health of the public as well as the environment. Indication or prediction of recurrence of SARS-CoV-2 will assist in initiating measures of public health surveillance, including planning and adapting to inhibit the spread of COVID-19 at the local, community, city, state and country-level via lockdown measures, including social distancing and quarantine and prohibition of travel across borders or high-risk regions.
6. Approaches to processes for the removal of SARS-CoV-2 in wastewater processing plants
The sewage and untreated wastewater released from residential areas, offices, institutions and other commercial buildings, specifically urban areas with highly dense populations, reaches the municipal wastewater treatment sites (Liu et al., 2020). Usually, multiple physicochemical treatment procedures like adsorption, precipitation, ultrafiltration and biodegradation are used for removing pollutants from the wastewater (Barebita et al., 2020), along with filtration and other disinfection methods like ozonation, chlorination and ultraviolet (UV) disinfection employed in tertiary stages of wastewater processing, especially when wastewater is to be treated for reuse or recycling (Zhang et al., 2016).
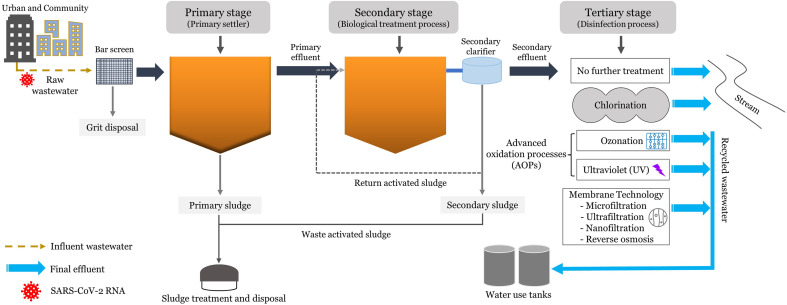
Wastewater treatment plants (WWTPs) are not just specially designed for wastewater treatment but also for effective sludge treatment. The deficiency of wastewater and faecal sludge treatment facilities, particularly in developing countries, may cause the mixing of wastewater with the surface water without disinfection processing (Pandey et al., 2021). Unfortunately, there is no legislation defining the requirement of any protocol for discharging wastewater containing SARS-CoV-2 RNA back into surface water after being treated. The prevalence of effective wastewater treatment systems is crucially important (Bhatt et al., 2020), thereby establishing the need for wastewater treatment plants (WWTPs) to process the wastewater discharge before being released into the environmental water bodies. Additionally, disinfection should also be adopted as a primary method of inactivation and eradication of pathogens to avoid the downward transference of waterborne diseases. The wastewater treatment via primary, secondary, and tertiary processing stages should be an integral part of municipal wastewater management during COVID-19, as portrayed in Fig. 2 . The current analysis marks the prevalence of SARS-CoV-2 RNA in wastewater and a comparative assessment of the efficiency of secondary and tertiary wastewater treatment methods for removing the virus during COVID-19, as briefed in Table 2 . The wastewater treatment steps implemented for the removal of SARS-CoV-2 are elaborated below.
Fig. 2.
Schematics of wastewater treatment stages in WWTPs.
Table 2.
Various efficient wastewater treatment processes employed by selected countries for the eradication of SARS-CoV-2 RNA.
| Country | Treatment stage | Wastewater treatment Processes | Results | References |
|---|---|---|---|---|
| India | Secondary + tertiary treatment | – Sequencing batch reactor (SBR) + Cl2 (site: 3 and 4) | All negative | Arora et al. (2020) |
| – Moving bed biofilm reactor (MBBR) + UV (site: 5) | All negative | |||
| – Sequencing batch reactor (SBR) + without tertiary (site: 6) | All negative | |||
| Iran – Tehran | Secondary + tertiary treatment | – Extended aeration activated sludge (EAAS) system + Cl2 disinfection (Modules: 1–4) | Positive samples: 2/4 = 50.0% | Nasseri et al. (2021) |
| – Oxidation ditch method + UV disinfection (Modules: 5–6) | All negative | |||
| – Anzali | – Extended aeration activated sludge (EAAS) system + Cl2 disinfection | All negative | ||
| – Qom | – Conventional activated sludge (CAS) system with diffuser aeration + Cl2 disinfection | All negative | ||
| Japan | Secondary treatment | – Activated sludge (AS) system | Positive sample: 1/5 = 20.0% | Haramoto et al. (2020) |
| Italy (Padua, Veneto, NE Italy) | Secondary + tertiary treatment | – Activated sludge (AS) system + peracetic acid and terminal UV lamps | Positive sample: 2/2 = 100% | Baldovin et al. (2021) |
| Spain | Secondary treatment | – Activated sludge (AS) system | Positive sample: 2/18 = 11.1% | Randazzo et al. (2020b) |
| Tertiary treatment | – Coagulation, flocculation, and filtration, UV and NaClO disinfection | Positive sample: 0/13 = 0% | ||
| Spain (North–Western) | Primary treatment | – Primary settler | Positive sample: 1/2 = 50% | Balboa et al. (2021) |
| Secondary treatment | – SBR and microfiltration | Positive sample: 0/5 = 0% | ||
| USA | Secondary treatment | – Activated sludge (AS) system | Positive sample: 0/4 = 0% | Sherchan et al. (2020) |
| Tertiary treatment (Final effluent) | – Cl2 disinfection | Positive sample: 0/4 = 0% | ||
| UAE | Secondary treatment | – Activated sludge process (ASP)/clarification | Positive sample | Hasan et al. (2021) |
| Tertiary treatment | – Sand filtration, disinfection, and chlorination | None of the 11 WWTPs' treated effluents tested positive | ||
| Israel | Secondary treatment | – Activated sludge process | – ~1 log10 RNA removal by the primary and secondary treatment | Abu Ali et al. (2021) |
| Tertiary treatment | – Sand filtration and disinfection through chlorination | – > 100 copies/L of SARS-CoV-2 RNA remained the secondary effluent | ||
| Saudi Arabia | Secondary treatment | – Activated sludge (AS) system | N1: 0.3 log10 reduction (50%) | Hong et al. (2021) |
| Tertiary treatment | – Chlorination | N3: 0.5 log10 reduction (70%) | ||
| (Hospital WWTP) |
6.1. Primary stage of wastewater treatment
The first stage of a WWTP's physical operations involves the eradication of fixed and volatile suspended solids (VSS) from the wastewater via the usage of physical barriers (Saawarn and Hait, 2021). The research exposed that initial processes of wastewater treatment involved physical procedures like flocculent precipitation, adsorption, and gravity precipitation (Ferraa et al., 2021). Previous studies have indicated that 0–50% viruses could be removed by the primary stage of the treatment process depending on the settling time (Gerba, 1981). According to a recent study, SARS-CoV-2 RNA is present in 50% of discharging samples after being allowed to settle down in the primary stage of wastewater treatment (Balboa et al., 2021). This analytical study proclaimed the insufficiency of gravitational settling for the absolute removal of viruses from the wastewater under the primary stage of the wastewater treatment process at a WWTP (Saawarn and Hait, 2021). The current information can be inferred from the existing scientific literature that SARS-CoV-2 RNA can be removed in the first stage of the wastewater treatment process through physical procedures like gravitational precipitation of suspended colloids or settling of organic matter with larger diameters and attached viruses. A recent study conducted by Abu Ali et al. (2021) reported that the wastewater treatment process had reduced total suspended solids (TSS) to the extent of 50%, thereby removing the viral particles affixed with suspended solids. However, complete removal of SARS-CoV-2 RNA from wastewater is not possible through this stage.
6.2. Secondary stage of wastewater treatment
Under the COVID-19 pandemic, SARS-CoV-2 are rapidly growing pathogens in drainage and collection systems. In the secondary treatment stage, biological methods are engaged to eliminate biodegradable organic compounds and suspended solids from wastewater by relying on the cellular activity of microorganisms under aerobic and anaerobic conditions to enable the oxidation of the organic materials present in wastewater (Thakur et al., 2021). Additional secondary treatment (biological process) tends to remove almost 90–99% range of pathogens. However, secondary treatment of sewage water does not remove rotaviruses as effectively as enteroviruses (Gerba, 1981). Multiple varieties of wastewater treatment processes at WWTPs include membrane bioreactor (MBR), sequencing batch reactor (SBR), pond system, moving bed biofilm reactor (MBBR), upflow anaerobic sludge blanket (UASB), activated sludge process (ASP), and membrane treatment.
The WWTP is a ventilated tank treatment in which residual organic materials of wastewater, including sludge, are dissolved through the microorganism activity. The prevalence of the SARS-CoV-2 RNA in the volumes of 20% and 10%, respectively, has been exhibited in the discharged wastewater samples after being treated by secondary treatment methods in a study performed by Haramoto et al. (2020) and Randazzo et al. (2020b). After employing the secondary treatment stage of ASP, the viral load of SARS-CoV-2 RNA. SARS-CoV-2 RNA had reduced remarkably to the mark of <2.5 × 104 copies/L (Randazzo et al., 2020b). Coincidentally, in a study performed in the US, 100% of wastewater samples that had been treated through the secondary stage treatment of ASP reflected the negative presence of SARS-CoV-2 RNA. On the contrary, a study by Arora et al. (2020) revealed the presence of SARS-CoV-2 RNA in the samples that were treated through SBR, AS and MBBR wastewater treatment processes. Notably, considering the COVID-19 pandemic, SBR and MBBR have been proved to be efficient secondary treatment techniques for removing SARS-CoV-2 RNA from the wastewater, according to the latest research of Balboa et al. (2021). Kumar et al. (2021) recently reported a reduction in the existence of viruses after upflow anaerobic sludge blanket (UASB) for municipal wastewater treatment exceeded the level of 1.5 log10, which was also seen in the case of other genomes linked with SARS-CoV-2 RNA. Furthermore, an additional study marked the importance of hydraulic retention time (HRT) in the wastewater treatment done by the pond system to achieve the target of virus removal. According to the study, the wastewater retention for a duration of 14.5–20.9 days results in an average reduction of pathogens by 1 log10 (Feachem et al., 1983). As per the findings of a recent study, the wastewater kept at room temperature for 1.5 days can result in the 90% removal of SARS-CoV-2 RNA from the wastewater sample (Bivins et al., 2020). Hence, the retention time under the secondary treatment stage is longer in WWTPs as higher temperatures tend to impact the inactivation of SARS-CoV-2 in wastewater (Saawarn and Hait, 2021). Similarly, thermophilic digestion also confirms the finding of the sludge treatment process and establishes it on the grounds of the consensus of high sensitivity or inactivity of coronaviruses to the increased temperatures (Foladori et al., 2020). According to a recent study Hong et al. (2021), the removal rate of SARS-CoV-2 target genes in a biological sludge tank within 9 h of treatment of hospital wastewater was N1 (0.3 log10), whereas the reduction rate was N3 (0.5 log10). Additionally, many factors in each process may affect the efficacy of the treatment stage, such as pH, HRT, biological solids retention time (BSRT), temperature (Bogler et al., 2020; Saawarn and Hait, 2021; Wigginton and Ellenberg, 2015) in addition to wastewater characteristics, the flow rate of wastewater and weather conditions and other related criteria. The impermeability of virus on filters can lead to abundant growth of microbial communities, after which it needs to be subjected to the disinfection process. The extra focus needs to be laid upon additional research on the elimination of SARS-CoV-2 in membrane technology with special reference to the MBR system of treatment. Adherence to the disinfection process in WWTPs is the key to the successful inactivation of SARS-CoV-2 in wastewater (Cervantes-Avilés et al., 2021).
Resultantly, there is a risk of infection from non-potable exposures, which can affect the dispersed greywater and domestic wastewater. These wastewater treatments were facilitated by membrane bioreactors (MBRs) in association with treatment through the chlorination process. The rotavirus, norovirus, cryptosporidium and Campylobacter jejuni have been referred to as pathogens in microbial risk investigation. MBR technique tends to decrease the health risks from the reuse of non-potable water (Schoen et al., 2018). MBR technology has many advantages like the creation of high-quality treated water available for reuse and removal of hazardous bacteria and viruses; however, the MBR technique has certain drawbacks also such as limited aeration, fouling smell of sludge in extrinsic MBRs and high operational costs (Kweinor et al., 2020). The simplest technique to manage wastewater is through channelising sewage storage pipelines from the urban areas to the treatment plants and refurbishing municipal wastewater treatment sites into pond systems (Lesimple et al., 2020). Nonetheless, this option proves to be advantageous as the interaction with sunlight reduces the number of SARS-CoV-2 in the wastewater, which lies in alignment with the guidelines of WHO stating that in the presence of 6–7 log10 units, treatment of wastewater can make it reusable for irrigation purposes (Lesimple et al., 2020; WHO, 2006). Thus, ponds systems should be effectively used as a secondary treatment mechanism for wastewater. The pond or lagoon system's efficacy in this treatment method remains ambiguous, owing to its dependency on chemical features, sediment characteristics and environmental conditions in the pond (Verbyla and Mihelcic, 2015). In a generic sense, enveloped viruses can be inactivated far more easily as compared to non-enveloped viruses. Unfortunately, there is scarce information available regarding the efficiency of wastewater treatment methods like MBR, ASP, sedimentation-coagulation, and disinfection methods that are used for removing SARS-CoV-2 (Kitajima et al., 2020). Considering the data scarcity for analysing the removal of SARS-CoV-2 RNA, additional research is required to understand the secondary treatment methods of SARS-CoV-2 or another pathogen removal from the wastewater. A review of the existing research, however, revealed the likelihood of removal of SARS-CoV-2 RNA from wastewater through secondary wastewater treatment systems in WWTPs. Nonetheless, the evidence is insufficient to draw firm conclusions about the removal of SARS-CoV-2 from wastewater at the current stage. Considerably, future studies should incorporate computational modelling and optimisation techniques for managing environmental factors such as humidity, pH, temperature, solar radiation, regional climate and other related criteria against growth and dissemination of viruses as a directive for determining viral load as well as inactivation of the virus through wastewater treatment methodologies.
6.3. Tertiary stage of wastewater treatment
The final stage of treating wastewater before releasing the treated water of improved quality into the environment is known as the tertiary stage of wastewater treatment in WWTPs. The wastewater treatment could be processed through many ways like chlorination, UV disinfection, ozonation, membrane technology and so on (Gerba and Pepper, 2019; Saawarn and Hait, 2021). The treatment frees the wastewater from turbidity, multiple inorganic compounds and components like phosphorous, nitrogen and metals. The involvement of chemical coagulation in the tertiary treatment method is likely to eliminate a huge number of pathogens from the wastewater (Gerba, 1981), thereby pushing out the viruses, bacteria and other parasitic organisms that harm public health. This section is focused on the distinct wastewater treatment processes engaged in this stage; for instance, membrane, ozonation and disinfection that are especially relevant in the reference of COVID-19. Furthermore, WWTPs should consider practising an additional cleansing process during the tertiary stage to prevent the likely risk posed by microorganisms, particularly SARS-CoV-2 RNA, before discharging effluent into surface water. Abu Ali et al. (2021) recent study explored the prevalence of SARS-CoV-2 RNA in the secondary effluent, which was greater than 100 copies/L, implying the need for tertiary processing methods like sand distillation and disinfection to ensure virus-free effluent.
A critical study highlighting the efficacy of disinfectants on the inactivation of SARS-CoV-2 is significant for ensuring the adequate protection and disinfection of surface waters based on the river loading and transmission of pathogens via WWTPs. This can also ensure the complete elimination of SARS-CoV-2 for making the wastewater reusable. Wang et al. (2020b) study proposes the dependency of determination of a specific type of disinfection technology on multiple economic and feasible considerations like safety requirements, the quantity of wastewater, availability of disinfectants, cost of investment and disinfection services, the distance between the WWTP and populated areas and the extent of operational monitoring. Furthermore, evident knowledge and relevant data about the impact of SARS-CoV-2 on the quality of treated wastewater, drinking water, and surface water must be continuously monitored by wastewater treatment plants and experts should consistently monitor the water quality (García-Ávila et al., 2020).
The investigation of activated sludge in WWTP revealed weak removal of SARS-CoV-2, whereas particles of SARS-CoV-2 can be decreased in the effluent by using full-scale ozonation (Westhaus et al., 2021). These approaches can be employed alternatively in place of wastewater disinfection treatment procedures amid the COVID-19 pandemic. Contrastingly, the spreading of COVID-19 can also be restricted by the famous method of disinfection practised in wastewater treatment plants. However, excessive use of disinfectants in public facilities to combat the epidemic, especially based on chlorine disinfectants, may impact the surface water and the environment negatively. WWTPs undergoing large-scale treatment processes like ultrafiltration, chlorination and inactivation by ultraviolet radiation can employ promising tactics to upgrade the treatment, particularly in pandemic hotspots (Venugopal et al., 2020). Contrastingly, haloacetamides (HAcAms), haloacetonitriles (HANs) and trichloronitromethane (TCNM) enhanced dramatically after being exposed to UV/chlorine treatment (Hua et al., 2021), and are now used as chemical indicators for all potentially harmful substances produced by the addition of chlorine to water (García-Ávila et al., 2020). The presence of these substances caused cytotoxicity and considerably increased the disinfection by product (DBPs) genotoxicity after processing with UV/chlorine (Hua et al., 2021). Additionally, it has been established that it is not possible to treat the wastewater released from hospitals in just one step of conventional treating methods (Top et al., 2020). To reduce the threats for WWTPs, it is required to conduct studies on the performance of commonly used disinfection technologies for inactivating SARS-CoV-2 in municipal wastewater.
6.3.1. Chlorine disinfection
The usage of chlorine as a disinfectant is most common due to its oxidising characteristics, and while it was effective at low concentrations, it was expensive and left a residue if used in sufficient amounts (Lahrich et al., 2021). Chlorine is utilised in multiple forms like gas, hypochlorite and hypochlorous acid (HOCl) and can also be used largely as a combination of hypochlorite and gas (chlorine dioxide). Chlorine reacts with water readily and forms HCl and hypochlorous acid (HOCl), which then separates to release another derivative called hypochlorite ion. Hence, HOCl is the main component required for the treatment of wastewater. Higher volumes of HOCl are produced at neural or lower pH, leading to better disinfection ability. HOCl and OCl are freely formed forms of chlorine. The presence of ammonia and nitrogen compounds cause a reaction between effluent wastewater and HOCl, leading to the production of chloramines. As a result of high volumes of dissolved NH3 and organic matter (OM) in the wastewater, toxic residues called chloramines are produced, along with substitute derivatives for the disinfection treatment process; thus, causing ecotoxicological effects (Luan et al., 2020; Verbyla and Mihelcic, 2015). The studies of Dunkin et al. (2018) reported the reasons for the resistance of variable human noroviruses (hNoV) to free chlorine and examined the endurance of GI and GII fragments of hNoV at the time of disinfection of public wastewater effluent. As observed, prior to the cultivation of viruses in wastewater, the selection of the hNoV purification method assists in gaining significant disinfection outcomes. The tertiary wastewater treatment stage should employ free chlorine disinfection, which is beneficial for protecting public health from virus pollution (Dunkin et al., 2018).
Recent research found that 6.5 mg/L free chlorine and a contact time of 1.5 h could sufficiently reduce SARS-CoV-RNA levels from 0.5 × 103 to 18.7 × 103 copies/L to the levels that could not be detected (Zhang et al., 2020). Contrastingly, a recent study Hong et al. (2021) discovered that after 2 h of contact time, the final disinfection stage in hospital WWTPs used a chlorine concentration of 100 mg/L, implying the likely removal or complete inactivation of any residual SARS-CoV-2 RNA before being discharged. Furthermore, SARS–CoV has been shown to be limited, particularly at temperatures above 20 °C, and the virus has been easily worn out with the use of chlorine solution at 0.5 mg/L for 30 min (Collivignarelli et al., 2020; Zhang et al., 2020), whereas as recommended by the Chinese Centers for Disease Control and Prevention, it should be treated at 6.5 mg/L after 1.5 h contact with NaCl. The efficiency of free chlorine for inactivating SARS–CoV has been proved to be greater than chlorine dioxide (Kitajima et al., 2020). The study conducted by Lahrich et al. (2021) and Wang et al. (2005b) has recommended the application of free chlorine residual for disinfecting a low concentration of wastewater and inactivate SARS-CoV-1 effectively. However, free chlorine in the range of 0.2 and 0.5 mg/L is sufficient to disinfect municipal wastewater. As a result, the effectiveness and safety of the wastewater method for virus removal are dependent on the optimal selection of disinfectant dose and the duration of contact in the treatment process. The aqueous solutions of sodium hypochlorite in the range of 5.25%–6.15% are commonly used for disinfection, whereas the complete inactivation of SARS-CoV can be effectuated in less than 1 min by using 0.05% of hypochlorite solution (Singh et al., 2021).
6.3.2. Ozonation disinfection
Ozone (O3) is a very powerful and virucidal oxidant that reduces the effect of bacteria, viruses and protozoans through oxidising agent processing reaction (Hudson et al., 2009) and is extensively used in the treatment of wastewater as well as tap water (Chiang et al., 2003; Wang et al., 2020b). The ozone disinfection process (EPA, 1999a) involves the following steps: (i) direct demolition and oxidation of the cellular wall leading to outburst of cellular components from the cell, (ii) reacting with radical derivatives of ozone decomposition, (iii) destruction of elements of nucleic acid (pyrimidines and purines), and (iv) detachment of bonds between carbon-nitrogen causing depolymerisation. Therefore, the ability of ozone to effectively destroy the composition of SARS-CoV-2 and inactivate the viruses suggests its usefulness as an efficient oxidant against COVID-19 (Tizaoui, 2020). The reaction between ozone and wastewater leads to the production of radicals (Zhang et al., 2016), which destroys proteins, viral nucleic acids and lipids of spikes and envelopes (Kataki et al., 2021; Zhang et al., 2016). Additional advantages include high efficiency of viral inactivation and removal of colour and odour from the wastewater (Kataki et al., 2021).
In a past study, Wang et al. (2018) conducted the traditional ozone treatment of human pathogenic viruses found in wastewater and determined that the concentration of prominent viruses like parvovirus, astrovirus, picobirnairus, norovirus, adenovirus, hepatitis E virus (HEV), sapovirus, pecovirus, parechovirus and gokushovirus decreased to a remarkable level by 1–4 log10 due to standard treatment; however, adenovirus and parvovirus were eliminated only to a little extent. Further reduction of these viruses was possible through ozone treatment by a level of 1–2 log10, although adenovirus was yet not eliminated completely Wang et al. (2018). Presently, there is limited evidence of the effectiveness of ozonation in disinfecting the wastewater comprising SARS-CoV-2; nonetheless, this technique is presumed to be equally successful as it was in the disinfection of wastewater having SARS-CoV-1 (Kataki et al., 2021). It can be suggested to use ozonation for the probable removal of viruses from wastewater. Ozone disinfection can reduce the concentrations of some viruses to undetectable levels, implying that during the COVID-19 pandemic, promising strategies for preventing the spread of certain pathogenic humans need to be preferred for the final disinfection of treated wastewater, particularly in wastewater, recycled water, and drinking water treatment plants. However, ozonation treatment is appropriate for small-scale WWTPs, especially wastewater treatment systems with high effluent quality (Wang et al., 2020b). Furthermore, Kataki et al. (2021) reported that the combined treatment process, including UV disinfection, ozonation and chlorination, effectively resulted in 99.99% inactivation of faecal coliform based on the quality of influent water and the treated water was found to be negative of SARS-CoV-2 after being disinfected by ozonation which improved UV transmission by 20–30%; thus indicating the removal of coronavirus. While strategising upon the installation of modern water treatment systems to eliminate bacterial pathogens and microcontaminants, it is justifiable to consider the capability of ozone treatment in reducing the risk of transmission of human pathogens (Wang et al., 2018). Ozone is a strong disinfectant that improves the quality of water containing microbiological organisms to a greater concentration in a shorter period of time with higher efficacy.
6.3.3. Ultraviolet (UV) disinfection
An ultraviolet (UV) irradiation disinfection system transmits electromagnetic waves with wavelengths ranging from 200 nm to 400 nm. UV radiation is emitted by a mercury arc lamp at either low or medium pressure, and it is absorbed by an organism's genetic material comprising of DNA and RNA (EPA, 1999b). The ultraviolet spectrum is divided into four wavelengths, incorporating ultraviolet A (315–400 nm), ultraviolet B (280–315 nm), and ultraviolet C (200–280 nm). The wavelength bands between 200 nm and 300 nm have the potential to damage and break down the structure of RNA and DNA in bacteria, viruses, and single-cell microorganisms, thereby inhibiting and damaging protein synthesis (Wang et al., 2020b); thus, causing viruses to lose their ability of replication due to genome and protein destruction (Wigginton and Kohn, 2012). Furthermore, the study that found human norovirus (NoV) in untreated sewage through UV disinfection is more effective than the other studies wherein average removal rates of GI was recorded as 99.5% (2.3 log10) and 99.7% (2.6 log10) for GII (Campos et al., 2016). On the contrary, a study performed by Darnell et al. (2004) proclaimed the inactivation of SARS-CoV by the ultraviolet radiation of 254 nm. Furthermore, Qiu et al. (2018) investigated pre–ultraviolet and post–UV samples obtained from wastewater plants, and the results revealed that both pre and post–UV samples contained relatively high concentrations of viruses like noroviruses, rotaviruses, reoviruses, sapoviruses, astroviruses, enteroviruses, and adenoviruses. According to the findings, quantifying reovirus infection could provide a useful index of enteric virus inactivation during full-scale treatment of wastewater (Qiu et al., 2018). Furthermore, a previous study by Zyara et al. (2017) reported that the efficacy of UV–LED inactivation of DNA and RNA coliphages separated from wastewater exhibited that a wavelength of 270 nm for 2 min induced a decrease of 0.93–2.73 log10 in the coliphage test in a 5.2 L reactor, while a decrease of 4.30–5.16 log10 improved with a 10 min irradiation time. The conventional mercury (Hg–UV) lamp, on the contrary, led to a reduction of 0.67–4.08 log10 in 2 min and a reduction of 4.56–7.21 log10 in 10 min at a wavelength of 254 nm in 10 mL of water. Hence, UV–light-emitting diode (LED) is a successful approach to disinfection of UV and Cl− resistant viruses (Zyara et al., 2017). As a result, the optimal wavelength for UVB and UVC inactivation of microorganisms is 250 nm and 270 nm, respectively (EPA, 1999b). Kataki et al. (2021) latest study recommends using a secondary disinfectant followed by UV to ensure latency of microbial defence. A former study discovered the efficiency of prevention of virus invasion routed by air or by touching infected objects through the production of deep ultraviolet light-emitting diode (DUV–LED) fitted devices with a wavelength of 280 ± 5 nm (Inagaki et al., 2020). Recently, the microorganism inactivation by newly emerging microplasma UV lamps has been studied to see if it can actually impact the nucleic acid repair–deficiency caused by microplasma UV. These revelations resulted in the eradication of the ssRNA virus, thus demonstrating far–UVC resistance to SARS-CoV-2 elimination from wastewater and water treatment, making way for sustainable disinfection systems to undergo high-quality treatment of wastewater effluent (Raeiszadeh and Taghipour, 2020). However, some countries had adopted centralised water treatment and safe piped water supplies, whereas, in developing countries, wastewater is not available because of the expensive and intensive method; thus, sun light-driven UV has been used at a relatively low cost. Furthermore, the benefits of UV include non-corrosiveness, ease of installation and operation, but one concern is that it is hard to monitor the performance of the equipment (Kataki et al., 2021).
6.3.4. Membrane technology
Micro-and nano-sieves can be used for a variety of applications; however, they can also be used as an aid for analysing the particles, microorganisms, and bilayer lipids attached to the sieve's surface (Baker, 2012). Furthermore, low energy consumption is advanced in membrane filtration, as separation can be carried out continuously, and upscaling is simple (Saleh and Gupta, 2016). Thus, during the COVID-19 pandemic, the type of membranes to be used for removing viruses in the tertiary wastewater treatment process is selected by way of effective sequence starting with reverse osmosis (RO), succeeded by nanofiltration (NF), ultrafiltration (UF), and microfiltration (MF), respectively due to the multiple sizes of SARS-CoV-2 ranging between 60 and 140 nm or 0.06–0.16 μm (Bogler et al., 2020). Considering the virus of the size of 0.01–0.1 μm (Lesimple et al., 2020), the membrane required for RO should be capable of removing this virus. The usage of these membranes is potential for this virus before considering microfiltration and membrane bioreactor (MBR) technology (Lesimple et al., 2020), as the membrane technology of virus removal from water has been employed for a long time (Cervantes-Avilés et al., 2021). In the nanofiltration and reverse osmosis process, the characteristics of the membranes were efficacious in separating viruses targeted retentates (Baker, 2012; Saleh and Gupta, 2016). As a result, reusing treated wastewater should be done in a mixed or combined system; for example, disinfection or ultrafiltration followed by nanofiltration and reverse osmosis for wastewater treatment should be exercised during the outbreak of COVID-19.
7. Conclusion
This review focuses on the transmission route, detection of the virus and wastewater treatment plants to eradicate SARS-CoV-2 RNA in the ongoing pandemic of COVID-19. The occurrence of SARS-CoV-2 RNA in the wastewater is due to its shedding as a part of the faeces of COVID-19 patients. Post excretion through the faeces, the virus gets diluted in water and spreads across the WWTPs through the municipal wastewater. Under favourable parameters of pH, temperature, wastewater characteristics, humidity level, suspended solids, the structure of the virus and environmental factors, it is possible for SARS-CoV-2 to survive in the wastewater for multiple days. Hence, the surveillance technique of wastewater-based epidemiology (WBE) on the grounds of quantitative data linked with the virus concentration method like PEG precipitation, ultrafiltration, electronegative membrane, and ultracentrifugation, after which the RT-qPCR assay. Moreover, the difference in viral concentration of recovery rate in wastewater samples also depends on each virus concentration technique. Therefore, viral RNA levels in wastewater samples by detecting SARS-CoV-2 from the RT-qPCR technique proves to be a warning tool to prevent the spread of COVID-19 at the community level. It aids in detecting viruses proactively and conducting WBE for the assessment of COVID-19 infection and surveillance of public health. It also adds to the contemporary knowledge linked with the efficacy of processes of wastewater treatment to remove the SARS-CoV-2 in WWTPs subsequent to primary process including settler and sedimentation and secondary treatment processes like sequencing batch reactor (SBR), activated sludge process (ASP), oxidation ditch method (ODM), moving bed biofilm reactor (MBBR), conventional activated sludge (CAS), extended aeration activated sludge (EAAS), and upflow anaerobic sludge blanket (UASB). This technique effectively undergoes biological processing of wastewater and additional higher temperature, and hydraulic retention time could assist in effective degradation and deactivation of SARS-CoV-2 RNA to inhibit the dissemination of SARS-CoV-2 in the wastewater. This process is succeeded by the tertiary stage of wastewater treatment, including disinfection via ozonation, chlorination, ultraviolet radiation, membrane technology and few others like coagulation, filtration and flocculation to remove and inactivate SARS-CoV-2 RNA in WWTPs. The biological, physical and physical-chemical treatment procedures combined together can assist in accomplishing total deactivation of SARS-CoV- RNA. Promising results have been derived from the initial studies based on the removal of SARS-CoV-2 from wastewater through secondary and tertiary treatment processes. Future studies should focus on collecting evidence to confirm the detection of SARS-CoV-2 RNA in different conditions of wastewater treatment at every stage such as pH, viral RNA load, temperature, the flow of wastewater, the daily volume of wastewater, population density, disinfectants, type of WWTPs, environmental conditions and operational parameters in line with the number of COVID-19 infected patients. Thus, an efficient model of detection, estimation and analysis of SARS-CoV-2 RNA in wastewater, and the efficacy of wastewater treatment process, will guide the strategists in implementing suitable interventions and adopting mandatory sanitation measures to ensure the clean surrounding which is virus-free and restrict the spread of SARS-CoV-2 pandemic in the environment.
Funding
No funding.
Declaration of competing interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author wishes to express thanks to the editor along with the seven anonymous reviewers for their excellent contributions to enhance the quality of this paper. The images and graphical summaries are created with the aid of resources from BioRender.com.
References
- Abu Ali H., Yaniv K., Bar-Zeev E., Chaudhury S., Shagan M., Lakkakula S., Ronen Z., Kushmaro A., Nir O. Tracking SARS-CoV-2 RNA through the wastewater treatment process. ACS ES&T Water. 2021;1:1161–1167. doi: 10.1021/acsestwater.0c00216. http://doi:10.1021/acsestwater.0c00216 [DOI] [PubMed] [Google Scholar]
- Agrawal S., Orschler L., Lackner S. Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Sci. Rep. 2021;11:5372. doi: 10.1038/s41598-021-84914-2. http://doi:10.1038/s41598-021-84914-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS- CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirian E.S. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Int. J. Infect. Dis. 2020;95:363–370. doi: 10.1016/j.ijid.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoah I.D., Kumari S., Bux F. Coronaviruses in wastewater processes: source, fate and potential risks. Environ. Int. 2020;143 doi: 10.1016/j.envint.2020.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., Gupta A.B. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Sci. Technol. 2020;82:2823–2836. doi: 10.2166/wst.2020.540. http://doi:10.2166/wst.2020.540 [DOI] [PubMed] [Google Scholar]
- Arslan M., Xu B., Gamal El-Din M.G. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.W. In: Membrane Technology and Applications. Baker R.W., editor. 2012. Overview of membrane science and technology.http://doi:10.1002/9781118359686.ch1 [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodriguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., Lema J.M. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Sci. Total Environ. 2021;772 doi: 10.1016/j.scitotenv.2021.145268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldovin T., Amoruso I., Fonzo M., Buja A., Baldo V., Cocchio S., Bertoncello C. SARS-CoV-2 RNA detection and persistence in wastewater samples: an experimental network for COVID-19 environmental surveillance in Padua, Veneto Region (NE Italy) Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar Or I., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E., Mannasse B., Shirazi R., Kramarsky-Winter E., Nir O., Abu-Ali H., Ronen Z., Rinott E., Lewis Y.E., Friedler E., Bitkover E., Paitan Y., Berchenko Y., Kushmaro A. Regressing SARS-CoV- 2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. medRxiv. 2020 doi: 10.3389/fpubh.2021.561710. http://doi:10.1101/2020.04.26.20073569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló D. Wastewater-Based Epidemiology to monitor COVID-19 outbreak: present and future diagnostic methods to be in your radar. Case Stud. Chem. Environ. Eng. 2020;2 doi: 10.1016/j.cscee.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barebita H., Naciri Y., Ferraa S., Nimour A., Guedira T. Investigation of structural and photocatalytic behavior of Bi13B1-2xVxPxO20.95+2x (0 ≤ x ≤ 0.5) Solid State Sci. 2020;108 doi: 10.1016/j.solidstatesciences.2020.106389. [DOI] [Google Scholar]
- Bhatt A., Arora P., Prajapati S.K. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: a review with emphasis on SARS-CoV-2. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal M., Nazir M.S., Rasheed T., Parra-Saldivar R., Iqbal H.M.N. Water matrices as potential source of SARS-CoV-2 transmission – an overview from environmental perspective. Case Stud. Chem. Environ. Eng. 2020;2:100023. doi: 10.1016/j.cscee.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal M., Munir H., Nazir M.S., Iqbal H.M.N. Persistence, transmission, and infectivity of SARS-CoV-2 in inanimate environments. Case Stud. Chem. Environ. Eng. 2020;2:100047. doi: 10.1016/j.cscee.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:937–942. doi: 10.1021/acs.estlett.0c00730. http://doi:10.1021/acs.estlett.0c00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler A., Packman A., Furman A., Gross A., Kushmaro A., Ronen A., Dagot C., Hill C., Vaizel-Ohayon D., Morgenroth E., Bertuzzo E., Wells G., Kiperwas H.R., Horn H., Negev I., Zucker I., Bar-Or I., Moran-Gilad J., Balcazar J.L., Bibby K., Elimelech M., Weisbrod N., Nir O., Sued O., Gillor O., Alvarez P.J., Crameri S., Arnon S., Walker S., Yaron S., Nguyen T.H., Berchenko Y., Hu Y., Ronen Z., Bar-Zeev E. Rethinking wastewater risks and monitoring in light of the COVID-19 pandemic. Nat. Sustain. 2020 http://doi:10.1038/s41893-020-00605-2 [Google Scholar]
- Campos C.J.A., Avant J., Lowther J., Till D., Lees D.N. Human norovirus in untreated sewage and effluents from primary, secondary and tertiary treatment processes. Water Res. 2016;103:224–232. doi: 10.1016/j.watres.2016.07.045. [DOI] [PubMed] [Google Scholar]
- Carducci A., Donzelli G., Cioni L., Federigi I., Lombardi R., Verani M. Quantitative microbial risk assessment for workers exposed to bioaerosol in wastewater treatment plants aimed at the choice and setup of safety measures. Int. J. Environ. Res. Publ. Health. 2018;15:1490. doi: 10.3390/ijerph15071490. http://doi:10.3390/ijerph15071490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making Waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179:115907. doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraturo F., Del Giudice C., Morelli M., Cerullo V., Libralato G., Galdiero E., Guida M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020;265:115010. doi: 10.1016/j.envpol.2020.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reyes J., Barragán-Trinidad M., Buitrón G. Surveillance of SARS-CoV-2 in sewage and wastewater treatment plants in Mexico. J. Water Process. Eng. 2021;40:101815. doi: 10.1016/j.jwpe.2020.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., Sasso J.M., Gregg A.C., Soares D.J., Beskid T.R., Jervey S.R., Liu C. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020;6:591–605. doi: 10.1021/acscentsci.0c00501. http://doi:10.1021/acscentsci.0c00501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar J.L., Wymer L. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J. Appl. Microbiol. 2013;115:1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- Cervantes-Avilés P., Moreno-Andrade I., Carrillo-Reyes J. Approaches applied to detect SARS-CoV-2 in wastewater and perspectives post-COVID-19. J. Water Process. Eng. 2021;40:101947. doi: 10.1016/j.jwpe.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V.W.-S., Chiu P.K.-F., Yee C.-H., Yuan Y., Ng C.-F., Teoh J.Y.-C. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J. Urol. 2020 doi: 10.1007/s00345-020-03246-4. http://doi:10.1007/s00345- 020- 03246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Guix S., Paraira M., Galofré B., Sáanchez G., Pintó R.M., Bosch A. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. medRxiv. 2020 http://doi:10.1101/2020.06.13.20129627 [Google Scholar]
- Chen Y.-H., Yan C., Yang Y.-F., Ma J.-X. Quantitative microbial risk assessment and sensitivity analysis for workers exposed to pathogenic bacterial bioaerosols under various aeration modes in two wastewater treatment plants. Sci. Total Environ. 2021;755:142615. doi: 10.1016/j.scitotenv.2020.142615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., Yang J., Ye G., Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. http://doi:10.1002/jmv.25825 [DOI] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R., Yip C.C.Y., Leung K.-H., Fung A.Y.-F., Zhang R.R., Lin Y., Cheng H.M., Zhang A.J.X., To K.K.W., Leung W.K. Gastrointestinal manifestations of SARS-CoV-2 infection and virus Load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.-F., Tsai C.-T., Lin S.-T., Huo C.-P., Victor Lo K. Disinfection of hospital wastewater by continuous ozonization. J. Environ. Sci. Health A. 2003;38:2895–2908. doi: 10.1081/ese-120025839. http://doi:10.1081/ESE-120025839 [DOI] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P.M., Tscharke B.J., Donner E., O'Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O'Malley E., Crosbie N.D., Thomas K.V., Mueller J.F. Wastewater-based epidemiology biomarkers: past, present and future. Trends Anal. Chem. 2018;105:453–469. doi: 10.1016/j.trac.2018.06.004. [DOI] [Google Scholar]
- Coil D. 2020. COVID19 Journal Club: “SARS-CoV-2 RNA Concentrations in Primary Municipal Sewage Sludge as a Leading Indicator of COVID-19 Outbreak Dynamics”.https://microbe.net/2020/05/26/covid19-journal-club-sars-cov-2-rna-concentrations-in- primary-municipal-sewage-sludge-as-a-leading-indicator-of-covid-19-outbreak- dynamics/ accessed. [Google Scholar]
- Collivignarelli M.C., Collivignarelli C., Carnevale Miino M., Abbà A., Pedrazzani R., Bertanza G. SARS-CoV-2 in sewer systems and connected facilities. Process Saf. Environ. Protect. 2020;143:196–203. doi: 10.1016/j.psep.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome. SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. http://doi:10.1016/j.jviromet.2004.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Patel S.K., Yatoo M.I., Tiwari R., Sharun K., Dhama J., Natesan S., Malik Y.S., Singh K.P., Harapan H. SARS-CoV-2 existence in sewage and wastewater: a global public health concern? J. Environ. Manag. 2021;280:111825. doi: 10.1016/j.jenvman.2020.111825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkin N., Weng S., Coulter C.G., Jacangelo J.G., Schwab K.J. Impacts of virus processing on human norovirus GI and GII persistence during disinfection of municipal secondary wastewater effluent. Water Res. 2018;134:1–12. doi: 10.1016/j.watres.2018.01.053. [DOI] [PubMed] [Google Scholar]
- Elsaid K., Olabi V., Sayed E.T., Wilberforce T., Abdelkareem M.A. Effects of COVID- 19 on the environment: an overview on air, water, wastewater, and solid waste. J. Environ. Manag. 2021;292:112694. doi: 10.1016/j.jenvman.2021.112694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA . 1999. Wastewater Technology Fact Sheet Ozone Disinfection.https://www3.epa.gov/npdes/pubs/ozon.pdf accessed. [Google Scholar]
- EPA . 1999. Wastewater Technology Fact Sheet Ultraviolet Disinfection.https://www.stlawco.org/sites/default/files/PublicHealth/UV%20Disinfection.pdf accessed. [Google Scholar]
- Farkas K., Hillary L.S., Malham S.K., McDonald J.E., Jones D.L. Wastewater and public health: the potential of wastewater surveillance for monitoring COVID-19. Curr Opin Environ Sci Health. 2020;17:14–20. doi: 10.1016/j.coesh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem R.G., Bradley D.J., Garelick H., Mara D.D. John Wiley and Sons; 1983. Sanitation and Disease: Health Aspects of Excreta and Wastewater Management. [Google Scholar]
- Ferraa S., Naciri Y., Hsini A., Barebita H., Bouziani A., Albourine A., Nimour A., Guedira T. Evolution of the physicochemical and photocatalytic properties of BaO embedded in bismuth phosphovanadates glasses. Chem. Phys. Lett. 2021;763:138173. doi: 10.1016/j.cplett.2020.138173. [DOI] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ávila F., Valdiviezo-Gonzales L., Cadme-Galabay M., Gutiérrez-Ortega H., Altamirano-Cárdenas L., Arévalo C.Z., Flores del Pino L. Considerations on water quality and the use of chlorine in times of SARS-CoV-2 (COVID-19) pandemic in the community. Case Stud. Chem. Environ. Eng. 2020;2:100049. doi: 10.1016/j.cscee.2020.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z.-Y., Yang L.-M., Xia J.-J., Fu X.-H., Zhang Y.-Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. - Sci. B. 2020;21:361–368. doi: 10.1631/jzus.B2010010. http://doi:10.1631/jzus.B2010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller C., Varbanov M., Duval R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. http://doi:10.3390/v4113044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P. In: Viruses and Wastewater Treatment. Goddard M., Butler M., editors. Pergamon; 1981. Virus survival in wastewater treatment; pp. 39–48. [Google Scholar]
- Gerba C.P., Pepper I.L. In: Environmental and Pollution Science. third ed. Brusseau M.L., Pepper I.L., Gerba C.P., editors. Academic Press; 2019. Chapter 22 - municipal wastewater treatment; pp. 393–418. [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., Thompson H., Keeling D., Mitchell J., Gonzalez D. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186:116296. doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8:e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Swaffield J.A., Sleigh P.A., Noakes C.J. An assessment of, and response to, potential cross-contamination routes due to defective appliance water trap seals in building drainage systems. Build. Serv. Eng. Technol. 2011;33:203–222. http://doi:10.1177/0143624411410619 [Google Scholar]
- Gormley M., Templeton K.E., Kelly D.A., Hardie A. Environmental conditions and the prevalence of norovirus in hospital building drainage system wastewater and airflows. Building Build Serv Eng Res Technol. 2013;35:244–253. http://doi:10.1177/0143624413485080 [Google Scholar]
- Green H., Wilder M., Middleton F.A., Collins M., Fenty A., Gentile K., Kmush B.L., Zeng T., Middleton F.A., Larsen D.A. Quantification of SARS-CoV-2 and cross- assembly phage (crAssphage) from wastewater to monitor coronavirus transmission within communities. medRxiv. 2020 http://doi:10.1101/2020.05.21.20109181 [Google Scholar]
- Han M.S., Seong M.-W., Heo E.Y., Park J.H., Kim N., Shin S., Cho S.I., Park S.P., Choi E.H. Sequential analysis of viral load in a neonate and her mother infected with severe acute respiratory syndrome coronavirus 2. Clin. Infect. Dis. 2020;71:2236–2239. doi: 10.1093/cid/ciaa447. http://doi:10.1093/cid/ciaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.W., Ibrahim Y., Daou M., Kannout H., Jan N., Lopes A., Alsafar H., Yousef A.F. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: surveillance of COVID-19 epidemic in the United Arab Emirates. Sci. Total Environ. 2021;764:142929. doi: 10.1016/j.scitotenv.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;758:143578. doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Matsumori K., Kitajima M., Katayama H. Concentration of enteric viruses in large volumes of water using a cartridge-type mixed cellulose ester membrane. Food Environ Virol. 2015;7:7–13. doi: 10.1007/s12560-014-9169-x. http://doi:10.1007/s12560-014-9169-x [DOI] [PubMed] [Google Scholar]
- Hong P.-Y., Rachmadi A.T., Mantilla-Calderon D., Alkahtani M., Bashawri Y.M., Al Qarni H., O'Reilly K.M., Zhou J. Estimating the minimum number of SARS-CoV-2 infected cases needed to detect viral RNA in wastewater: to what extent of the outbreak can surveillance of wastewater tell us? Environ. Res. 2021;195:110748. doi: 10.1016/j.envres.2021.110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z., Li D., Wu Z., Wang D., Cui Y., Huang X., Fang J., An T. DBP formation and toxicity alteration during UV/chlorine treatment of wastewater and the effects of ammonia and bromide. Water Res. 2021;188:116549. doi: 10.1016/j.watres.2020.116549. [DOI] [PubMed] [Google Scholar]
- Hudson J.B., Sharma M., Vimalanathan S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009;31:216–223. http://doi:10.1080/01919510902747969 [Google Scholar]
- Ibrahim Y., Ouda M., Kadadou D., Banat F., Naddeo V., Alsafar H., Yousef A.F., Barceló D., Hasan S.W. Detection and removal of waterborne enteric viruses from wastewater: a comprehensive review. J. Environ. Chem. Eng. 2021;9:105613. doi: 10.1016/j.jece.2021.105613. [DOI] [Google Scholar]
- Inagaki H., Saito A., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerg. Microb. Infect. 2020;9:1744–1747. doi: 10.1080/22221751.2020.1796529. http://doi:10.1080/22221751.2020.1796529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Luo M., Zou Z., Wang X., Chen C., Qiu J. Asymptomatic SARS-CoV-2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. J. Med. Virol. 2020 doi: 10.1002/jmv.25941. http://doi:10.1002/jmv.25941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataki S., Chatterjee S., Vairale M.G., Sharma S., Dwivedi S.K. Concerns and strategies for wastewater treatment during COVID-19 pandemic to stop plausible transmission. Resour. Conserv. Recycl. 2021;164:105156. doi: 10.1016/j.resconrec.2020.105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Hacıoglu S., Yaralı C., Saatci A.M., Pakdemirli B. medRxiv; 2020. First Data-Set on SARS-CoV-2 Detection for Istanbul Wastewaters in Turkey.http://doi:10.1101/2020.05.03.20089417 [Google Scholar]
- Kujawski S.A., Wong K.K., Collins J.P., Epstein L., Killerby M.E., Midgley C.M., et al. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat. Med. 2020;26:861–868. doi: 10.1038/s41591-020-0877-5. http://doi:10.1038/s41591- 020-0877-5 [DOI] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Patel A.K., Patel N., Bhattacharya P., Joshi M., Joshi C.G. Decay of SARS-CoV-2 RNA along the wastewater treatment outfitted with Upflow Anaerobic Sludge Blanket (UASB) system evaluated through two sample concentration techniques. Sci. Total Environ. 2021;754:142329. doi: 10.1016/j.scitotenv.2020.142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Thakur A.K., Mazumder P., Kuroda K., Mohapatra S., Rinklebe J., Ramanathan A.L., Cetecioglu Z., Jain S., Tyagi V.K., Gikas P., Chakraborty S., Islam M.T., Ahmad A., Shah A.V., Patel A.K., Watanabe T., Vithanage M., Bhattacharya P. Frontier review on the propensity and repercussion of SARS- CoV-2 migration to aquatic environment. J. Hazard. Mater. Lett. 2020;1:100001. doi: 10.1016/j.hazl.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweinor Tetteh E., Opoku Amankwa M., Armah E.K., Rathilal S. Fate of COVID-19 occurrences in wastewater systems: emerging detection and treatment technologies—a review. Water. 2020;12 http://doi:10.3390/w12102680 [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahrich S., Laghrib F., Farahi A., Bakasse M., Saqrane S., El Mhammedi M.A. Review on the contamination of wastewater by COVID-19 virus: impact and treatment. Sci. Total Environ. 2021;751:142325. doi: 10.1016/j.scitotenv.2020.142325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y., Cheng P.K., Lim W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41:e67–71. doi: 10.1086/433186. http://doi:10.1086/433186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapolla P., Lee R., Mingoli A. Wastewater as a red flag in COVID-19 spread. Publ. Health. 2020;185:26. doi: 10.1016/j.puhe.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen D.A., Wigginton K.R. Tracking COVID-19 with wastewater. Nat. Biotechnol. 2020;38:1151–1153. doi: 10.1038/s41587-020-0690-1. http://doi:10.1038/s41587-020-0690-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. J. Water Process. Eng. 2020;38:101544. doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.-B., Lin Y.-X., Tian D., Zhu Z.-Q., Dai F.-H., Wu F., Song Z.-G., Huang W., Chen J., Hu B.-J., Wang S., Mao E.-Q., Zhu L., Zhang W.-H., Lu H.-Z. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. http://doi:10.1097/cm9.0000000000000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Thompson J.R., Carducci A., Bi X. Potential secondary transmission of SARS- CoV-2 via wastewater. Sci. Total Environ. 2020;749:142358. doi: 10.1016/j.scitotenv.2020.142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T.H., Zhong X., Tian Y., Sin N.N. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16:1698–1707. doi: 10.7150/ijbs.45357. http://doi:10.7150/ijbs.45357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Huang Z., Luo J., Zhang X., Sha S. Primary concentration – the critical step in implementing the wastewater based epidemiology for the COVID-19 pandemic: a mini- review. Sci. Total Environ. 2020;747:141245. doi: 10.1016/j.scitotenv.2020.141245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X., Liu X., Fang C., Chu W., Xu Z. Ecotoxicological effects of disinfected wastewater effluents: a short review of in vivo toxicity bioassays on aquatic organisms. Environ. Sci. Water Res. Technol. 2020;6:2275–2286. http://doi:10.1039/D0EW00290A [Google Scholar]
- Mandal P., Gupta A.K., Dubey B.K. A review on presence, survival, disinfection/removal methods of coronavirus in wastewater and progress of wastewater- based epidemiology. J. Environ. Chem. Eng. 2020;8:104317. doi: 10.1016/j.jece.2020.104317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathuria J.P., Yadav R., Rajkumar Laboratory diagnosis of SARS-CoV-2 - a review of current methods. J. Infect. Public Health. 2020;13:901–905. doi: 10.1016/j.jiph.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at amoy gardens. J. Environ. Health. 2006;68(9):26–30. quiz 51-22. [PubMed] [Google Scholar]
- McNamara R.P., Dittmer D.P. Modern techniques for the isolation of extracellular vesicles and viruses. J. Neuroimmune Pharmacol. 2020;15:459–472. doi: 10.1007/s11481-019-09874-x. http://doi:10.1007/s11481- 019-09874-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS- coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. http://doi:10.1021/acs.estlett.0c00357 [DOI] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020;8:104306. doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri S., Yavarian J., Baghani A.N., Azad T.M., Nejati A., Nabizadeh R., Hadi M., Jandaghi N.Z.S., Vakili B., Vaghefi S.K.A., Baghban M., Yousefi S., Nazmara S., Alimohammadi M. The presence of SARS-CoV-2 in raw and treated wastewater in 3 cities of Iran: tehran, Qom and Anzali during coronavirus disease 2019 (COVID-19) outbreak. J. Environ. Health Sci. Eng. 2021;19:573–584. doi: 10.1007/s40201-021-00629-6. http://doi:10.1007/s40201-021-00629- 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud. Chem. Environ. Eng. 2020;1:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage- based epidemiology. Sci. Total Environ. 2020;732:139298. doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal D., Prakash O., Bobde P., Pal S. SARS-CoV-2: sewage surveillance as an early warning system and challenges in developing countries. Environ. Sci. Pollut. Res. 2021;28:22221–22240. doi: 10.1007/s11356-021-13170-8. http://doi:10.1007/s11356-021-13170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey D., Verma S., Verma P., Mahanty B., Dutta K., Daverey A., Arunachalam K. SARS-CoV-2 in wastewater: challenges for developing countries. Int. J. Hyg Environ. Health. 2021;231:113634. doi: 10.1016/j.ijheh.2020.113634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-K., Lee C.-W., Park D.-I., Woo H.-Y., Cheong H.S., Shin H.C., Ahn K., Kwon M.-J., Joo E.-J. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2020;19:1387–1394. doi: 10.1016/j.cgh.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Omer S.B. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. medRxiv. 2020 http://doi:10.1101/2020.05.19.20105999 [Google Scholar]
- Peng L., Liu J., Xu W., Luo Q., Chen D., Lei Z., Huang Z., Li X., Deng K., Lin B., Gao Z. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020;92:1676–1680. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo D., Quintela-Baluja M., Corbishley A., Jones D.L., Singer A.C., Graham D.W., Romalde J.L. Making waves: wastewater-based epidemiology for COVID-19 – approaches and challenges for surveillance and prediction. Water Res. 2020;186:116404. doi: 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata C., Ribeiro A., Cunha Â., Gomes N.C.M., Almeida A. Ultracentrifugation as a direct method to concentrate viruses in environmental waters: virus-like particle enumeration as a new approach to determine the efficiency of recovery. J. Environ. Monit. 2012;14:64–70. doi: 10.1039/c1em10603a. http://doi:10.1039/C1EM10603A [DOI] [PubMed] [Google Scholar]
- Qiu Y., Li Q., Lee B.E., Ruecker N.J., Neumann N.F., Ashbolt N.J., Pang X. UV inactivation of human infectious viruses at two full-scale wastewater treatment plants in Canada. Water Res. 2018;147:73–81. doi: 10.1016/j.watres.2018.09.057. [DOI] [PubMed] [Google Scholar]
- Raeiszadeh M., Taghipour F. Inactivation of microorganisms by newly emerged microplasma UV lamps. Chem. Eng. J. 2020;127490 doi: 10.1016/j.cej.2020.127490. [DOI] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuán R., Domingo-Calap P., Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg Environ. Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S.Y., Wang W.B., Hao Y.G., Zhang H.R., Wang Z.C., Chen Y.L., Gao R.D. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. 2020;8:1391–1399. doi: 10.12998/wjcc.v8.i8.1391. http://doi:10.12998/wjcc.v8.i8.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saawarn B., Hait S. Occurrence, fate and removal of SARS-CoV-2 in wastewater: current knowledge and future perspectives. J. Environ. Chem. Eng. 2021;9:104870. doi: 10.1016/j.jece.2020.104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh T.A., Gupta V.K. In: Nanomaterial and Polymer Membranes. Saleh T.A., Gupta V.K., editors. Elsevier; 2016. Chapter 3 - membrane classification and membrane operations; pp. 55–82. [Google Scholar]
- Saththasivam J., El-Malah S.S., Gomez T.A., Jabbar K.A., Remanan R., Krishnankutty A.K., Ogunbiyi O., Rasool K., Ashhab S., Rashkeev S., Bensaad M., Ahmed A.A., Mohamoud Y.A., Malek J.A., Abu Raddad L.J., Jeremijenko A., Abu Halaweh H.A., Lawler J., Mahmoud K.A. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021;774:145608. doi: 10.1016/j.scitotenv.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen M.E., Jahne M.A., Garland J. Human health impact of non-potable reuse of distributed wastewater and greywater treated by membrane bioreactors. Microb. Risk Anal. 2018;9:72–81. doi: 10.1016/j.mran.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif S., Ikram A., Khurshid A., Salman M., Mehmood N., Arshad Y., Ahmad J., Safdar R.M., Angez M., Alam M.M., Rehman L., Mujtaba G., Hussain J., Ali J., Akthar R., Malik M.M., Baig Z.I., Rana M.S., Usman M., Ali M.Q., Ahad A., Badar N., Umair M., Tamim S., Ashraf A., Tahir F., Ali N. Detection of SARS-Coronavirus-2 in wastewater, using the existing environmental surveillance network: an epidemiological gateway to an early warning for COVID-19 in communities. medRxiv. 2020 http://doi:10.1101/2020.06.03.20121426 [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutler J.D., Zaraska K., Holding T., Machnik M., Uppuluri K., Ashton I.G.C., Migdał Ł., Dahiya R.S. Rapid assessment of SARS-CoV-2 transmission risk for fecally contaminated river water. ACS EST Water. 2021;1:949–957. doi: 10.1021/acsestwater.0c00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu J.P.S., Sena K., Hodgers L., Palmer A., Toze S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2018;616–617:669–677. doi: 10.1016/j.scitotenv.2017.10.265. [DOI] [PubMed] [Google Scholar]
- Singh S., Kumar V., Kapoor D., Dhanjal D.S., Bhatia D., Jan S., Singh N., Romero R., Ramamurthy P.C., Singh J. Detection and disinfection of COVID-19 virus in wastewater. Environ. Chem. Lett. 2021;19:1917–1933. doi: 10.1007/s10311-021-01202-1. http://doi:10.1007/s10311-021-01202-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street R., Malema S., Mahlangeni N., Mathee A. Wastewater surveillance for covid-19: an african perspective. Sci. Total Environ. 2020;743:140719. doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S.-B., Liu X., Dai J., Li X., Huang S., Huang X., Luo L., Wen L., Zhuo J., Li Y., Wang Y., Zhang L., Zhang Y., Li F., Feng L., Chen X., Zhong N., Yang Z., Huang J., Zhao J., Li Y.-M. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microb. Infect. 2020;9:991–993. doi: 10.1080/22221751.2020.1760144. http://doi:10.1080/22221751.2020.1760144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tong Z.-D., Wang H.-L., Dai Y.-X., Li K.-F., Liu J.-N., Wu W.-J., Yuan C., Yu M.-L., Li P., Yan J.-B. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26:1337. doi: 10.3201/eid2606.200301. http://doi:10.3201/eid2606.200301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhaei M., Mohebbi S.R., Hosseini S.M., Rafieepoor M., Kazemian S., Ghaemi A., Shamloei S., Mirjalali H., Aghdaei H.A., Zali M.R. The first detection of SARS- CoV-2 RNA in the wastewater of Tehran, Iran. Environ. Sci. Pollut. Res. 2021 doi: 10.1007/s11356-021-13393-9. http://doi:10.1007/s11356-021-13393-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A.K., Sathyamurthy R., Velraj R., Lynch I., Saidur R., Pandey A.K., Sharshir S.W., Kabeel A.E., Hwang J.-Y., GaneshKumar P. Secondary transmission of SARS- CoV-2 through wastewater: concerns and tactics for treatment to effectively control the pandemic. J. Environ. Manag. 2021;290:112668. doi: 10.1016/j.jenvman.2021.112668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal V.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184:116181. doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizaoui C. Ozone: a potential oxidant for COVID-19 virus (SARS-CoV-2) Ozone: Sci. Eng. 2020;42:378–385. http://doi:10.1080/01919512.2020.1795614 [Google Scholar]
- Top S., Akgün M., Kıpçak E., Bilgili M.S. Treatment of hospital wastewater by supercritical water oxidation process. Water Res. 2020;185:116279. doi: 10.1016/j.watres.2020.116279. [DOI] [PubMed] [Google Scholar]
- Tran H.N., Le G.T., Nguyen D.T., Juang R.-S., Rinklebe J., Bhatnagar A., Lima E.C., Iqbal H.M.N., Sarmah A.K., Chao H.-P. SARS-CoV-2 coronavirus in water and wastewater: a critical review about presence and concern. Environ. Res. 2021;193:110265. doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J., Darques R., Ait Mouheb N., Partiot E., Bakhache W., Deffieu M.S., Gaudin R. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10:100157. doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman M., Farooq M., Hanna K. Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. Environ. Sci. Technol. 2020;54:7758–7759. doi: 10.1021/acs.est.0c02777. http://doi:10.1021/acs.est.0c02777 [DOI] [PubMed] [Google Scholar]
- Venugopal A., Ganesan H., Sudalaimuthu Raja S.S., Govindasamy V., Arunachalam M., Narayanasamy A., Sivaprakash P., Rahman P.K.S.M., Gopalakrishnan A.V., Siama Z., Vellingiri B. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Curr Opin Environ Sci Health. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Wang H., Sikora P., Rutgersson C., Lindh M., Brodin T., Björlenius B., Larsson D.G.J., Norder H. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int. J. Hyg Environ. Health. 2018;221:479–488. doi: 10.1016/j.ijheh.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. http://doi:10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262:114665. doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Guo T.-K., Zhen B., Kong Q.-X., Yi B., Li Z., Song N., Jin M., Xiao W.-J., Zhu X.-M., Gu C.-Q., Yin J., Wei W., Yao W., Liu C., Li J.-F., Ou G.-R., Li J.-W. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th hospital. J. Virol. Methods. 2005;128:156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J., Si B.-Y., Guo B.-Z., Liu C., Ou C.-R., Wang M.-N., Fang T.-Y., Chao F.-H., Li J.-W. Study on the resistance of severe acute respiratory syndrome- associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannigama D.L., Amarasiri M., Hurst C., Phattharapornjaroen P., Abe S., Hongsing P., Rad S.M.A.H., Pearson L., Saethang T., Luk-in S., Kueakulpattana N., Storer R.J., Ounjai P., Jacquet A., Leelahavanichkul A., Chatsuwan T. Tracking COVID-19 with wastewater to understand asymptomatic transmission. Int. J. Infect. Dis. 2021;108:296–299. doi: 10.1016/j.ijid.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K., Vander Laan J., LaCross N. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775:145790. doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2003. Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS)https://www.who.int/csr/sars/en/WHOconsensus.pdf accessed. [Google Scholar]
- WHO . 2006. Guidelines for the Safe Use of Wastewater, Excreta and Greywater.https://www.who.int/water_sanitation_health/publications/gsuweg4/en/ accessed. [Google Scholar]
- WHO . 2020. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020.https://www.who.int/director-general/speeches/detail/who-director- general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 accessed. [Google Scholar]
- Wigginton K.R., Kohn T. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr Opin Virol. 2012;2:84–89. doi: 10.1016/j.coviro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci.: Water Res. Technol. 2015;1:735–746. http://doi:10.1039/C5EW00125K [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. http://doi:10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/mSystems.00614-20. http://doi:10.1128/mSystems.00614-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Euro Surveill. 2020;25:2000776. doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., Zhao J., Huang J., Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. http://doi:10.3201/eid2608.200681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS- CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. http://doi:10.1038/s41591-020-0817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.-M., Xu L.-M., Xu P.-C., Wang X.C. Elimination of viruses from domestic wastewater: requirements and technologies. World J. Microbiol. Biotechnol. 2016;32:69. doi: 10.1007/s11274-016-2018-3. http://doi:10.1007/s11274-016-2018-3 [DOI] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X., Wang X., Liu Y., Li G., Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyara A.M., Heinonen-Tanski H., Veijalainen A.-M., Torvinen E. UV-LEDs efficiently inactivate DNA and RNA coliphages. Water. 2017;9 http://doi:10.3390/w9010046 [Google Scholar]