Abstract
Background:
Postoperative delirium is common among older surgical patients and may be associated with anesthetic management during the perioperative period. The aim of this study was to assess whether intravenous midazolam, a short-acting benzodiazepine used frequently as a premedication, increased the incidence of postoperative delirium.
Methods:
Analyses of existing data were conducted using a database created from three prospective studies in patients 65 years of age or older who underwent elective major non-cardiac surgery. Postoperative delirium occurring on the first postoperative day was measured using the Confusion Assessment Method. We assessed the association between the use- or non-use of premedication with midazolam and postoperative delirium using a chi-square test, using propensity- scores to match up to three midazolam patients for each control patient that did not receive midazolam.
Results:
A total of 1,266 patients were included in this study. Intravenous midazolam was administered as a premedication in 909 patients (72%), and 357 patients did not receive midazolam. Those who did and did not receive midazolam significantly differed in age, Charlson Co-Morbidity Scores, preoperative cognitive status, preoperative use of benzodiazepines, type of surgery, and year of surgery. Propensity score matching for these variables and American Society of Anesthesiology classification scores, resulted in propensity score matched samples with one to three patients who used midazolam N=749) for each patient who did not receive midazolam (N= 357). After propensity score matching, all standardized differences in preoperative patient characteristics ranged from −0.07 to 0.06, indicating good balance on baseline variables between the two exposure groups. No association was found between premedication with midazolam and incident delirium on the morning of the first postoperative day in the matched dataset, with odds ratio (95% confidence interval) of 0.91 (0.65 – 1.29), P=0.67.
Conclusions:
Premedication using midazolam was not associated with higher incidence of delirium on the first postoperative day in older patients undergoing major non-cardiac surgery.
Introduction
Benzodiazepines are frequently used clinically to relieve anxiety and insomnia, but may also cause delirium in susceptible population, such as older adults after major surgery. Postoperative delirium is a common geriatric syndrome which occurs in older patients after surgery.1,2 Delirium in hospitalized patients is associated with increased length of stay and hospital costs.3,4 In a prior study of patients who were older than 50 years of age undergoing elective non-cardiac surgeries, postoperative use of longer acting benzodiazepine (chlordiazepoxide, diazepam, and flurazepam) was shown to be associated with higher risk of postoperative delirium than short-acting agents (oxazepam, lorazepam, triazolam, midazolam, and temazepam).5 Midazolam, as a short-acting benzodiazepine with an elimination half-life of 1.5–2.5 hours, is commonly used as a premedication.6,7 However, the half-life of midazolam can be doubled in older adults.8 An important clinical question is whether premedication with midazolam is associated with postoperative delirium and whether its use should be limited in older patients at risk for postoperative delirium.
Benzodiazepines such as midazolam are commonly administered as a premedication immediately prior to surgery to serve as an anxiolytic. However, whether the use of single dose of short-acting benzodiazepine is associated with delirium in older patients particularly in those 65 years of age or older is a clinical area that has not been clarified. One prior study suggested that preoperative chronic use of benzodiazepines was associated with postoperative confusion.9 Others found that new use of lorazepam in critically ill patients was associated with delirium.10 In mechanically ventilated patients in the intensive care unit, midazolam-treated patients experienced more delirium than patients treated with dexmedetomidine.11 The above studies referred to patients who were already at risk for delirium due to patients being long-term benzodiazepine users, or who were critically ill and mechanically ventilated. However, whether premedication with a short acting benzodiazepine such as midazolam has similar adverse cognitive effects in older surgical patients who do not have these predisposing risk for postoperative delirium is unknown.
A recent meta-analysis reported that there are few high quality studies quantifying the direct association between preoperative medication use and postoperative delirium.12 Accordingly, the aim of this study was to assess the effect of intravenous midazolam as a premedication on the incidence of postoperative delirium in older adults. We hypothesize that the use of midazolam as a premedication is not associated with postoperative delirium in older patients undergoing elective surgical procedures. We combined three prospective studies in order to investigate whether premedication with midazolam is associated with postoperative delirium and adjusted for confounding using propensity score matching.
Materials and Methods
This is a secondary analysis of prospectively conducted studies, as we followed all patients prospectively for the occurrence of the primary outcome. Data were from three prospective studies at the University of California San Francisco (UCSF) Medical Center (San Francisco, California) between June 2001 and August 2014. These studies received approval from the institutional review board. Written informed consent was obtained from each patient for each study. All these studies were related to a general goal of investigating the pathophysiology of postoperative delirium (Study 1: Randomized trial of general anesthesia with or without nitrous oxide; Study 2: Prospective cohort of postoperative cognitive function in older surgical adults; Study 3: Randomized trial of perioperative administration of gabapentin or placebo).13–15 This manuscript reports results according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.16
Criteria for patient selection
Patients from all three studies were included if they were aged 65 to 85 years of age, and scheduled for elective major non-cardiac surgery at UCSF medical center. Patients were recruited if they were fluent in English and were expected to stay in the hospital for at least 48 hours after surgery. Patients were excluded if they could not provide written informed consent, or undergoing brain or cardiac surgery.
Preoperative assessment
The preoperative assessment was performed by trained research assistants in the preoperative anesthesia clinic, typically within one week of surgery. Patients were interviewed and their medical history of coexisting diseases, and chronic preoperative use of benzodiazepine were measured. The severity of preoperative co-existent conditions was determined using the Charlson comorbidity index.17 Physical status and anesthesia risk were measured by the American Society of Anesthesiologists classification (ASA).18 Preoperative cognitive status was measured using the Telephone Interview for Cognitive Status (TICS)19, an 11-item screening test (maximum score = 41 points), that was adapted from Mini-Mental State Examination.
Clinical management
Midazolam as a premedication was given as the patient is taken to the operating room, and typically within 15 minutes of anesthetic induction. The use of midazolam as a premedication and chronically preoperative use of benzodiazepines were abstracted from the anesthesia records. As this was not a randomized study, the use of midazolam was not standardized but rather determined by the treating anesthesiologists. The type and duration of surgery was recorded. As the patient recruitment dated from 2001 to 2014, the potential changing medical practice over time may affect clinical practices, we stratified patients into two groups based on the decade when they were recruited.
Outcome Assessment
The primary outcome was postoperative delirium on the first postoperative day. In secondary analysis, we also included the measurement of delirium on the second postoperative day. Structured interviews were conducted by trained research assistants on both weekdays and weekends between the hours of 9 am to 12 pm to determine the occurrence of postoperative delirium using the Confusion Assessment Method (CAM).20 The CAM was developed as a screening instrument to detect delirium based on DSM-III-R criteria for the use of non-psychiatric clinicians with a high sensitivity (94–100%) and specificity (90–95%).20 Postoperative delirium was measured on the first two postoperative days for two of the three studies and the third study measured delirium on the first three postoperative days. We chose two to three days as the cut-off as the majority of our patients were discharged by the early postoperative period.
Statistical Analysis
Bi-variate analyses were computed to compare the characteristics of patients who received midazolam as a premedication vs. those who did not receive treatment. Independent samples t-tests or Mann Whitney U tests were used to compare the two groups for continuous valued variables. Chi-square tests were used to compare the groups on categorical variables.
To reduce potential bias due to preoperative patient characteristics associated with being treated with midazolam, we used propensity score matching to derive two groups that did not differ on average in preoperative characteristics; one group used midazolam and the other did not. Propensity scores reflect the probability of receiving midazolam based on preoperative characteristics. We matched up to three midazolam patients with each no midazolam patient. To maximize the number of matches for each midazolam patient we used a Statistical analysis system (SAS) macro that first assigned matches to those midazolam patients with the fewest possible matches, then proceeded with midazolam patients with more possible matches.21 The matching is based on a preset maximum allowable difference. We used a common value of 0.2 of the standard deviation of the logit of the propensity scores.22
We computed weighted matched standardized differences (the difference in means or proportions divided by the pooled standard deviation) and weighted matched variance ratios for each covariate to assess the performance of propensity score matching.23 The weight for the patients in the no midazolam group was set to 1, and the weight for the patients in midazolam group was the inverse of the number of patients in the midazolam group (one to three) that matched to a patient in no midazolam group. The weighting ensured differences between the two groups were not overestimated when more than one patient in midazolam group was matched to a patient in the no midazolam group. We present the difference in means between those in the no midazolam (control) group and those in the midazolam group. We sought to ensure weighted matched standardized difference between groups to be smaller than 0.1.24
Our study included 357 patients who received midazolam preoperatively, and a weighted sample of 357 who did not receive midazolam. Our prior study found 20% of standard care elective surgery patients had incident delirium.25 We determined that the current sample size is sufficiently large to detect a difference in incident delirium of 0.25 between those receiving midazolam versus 0.20 who did not receive midazolam, or an Odds Ratio (OR) of 1.33. We determined the sample allows detection of an OR of 1.33 with a power of 0.92 when alpha = 0.05.
Based on the propensity-matched groups, incidence of delirium and OR with 95% confidence intervals were calculated for all subjects. All analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, NC).
Results
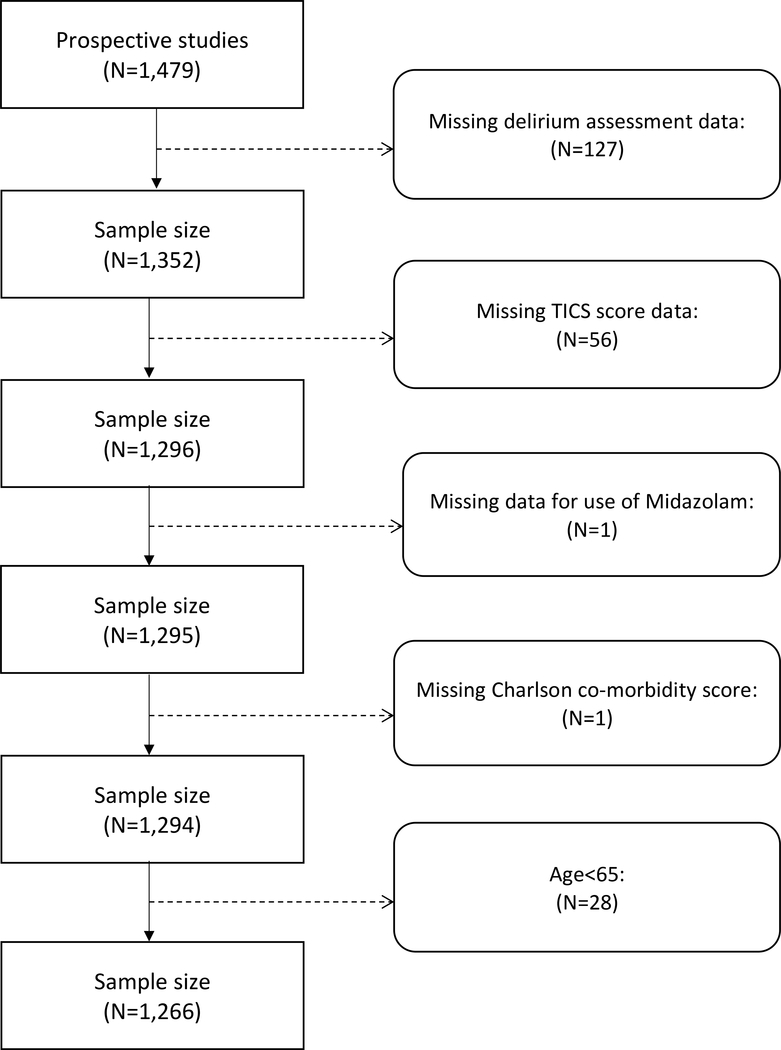
The flow chart for inclusion of patients in the analytic sample is shown in Figure 1. Of 1,479 patients with data, 127 were excluded due to incomplete postoperative delirium assessment. The reasons for the missing delirium data included patients’ refusal to assessment, stupor or non-responsive, or postoperative tracheal intubation. An additional 56 were excluded due to missing TICS scores, and another subject was excluded due to missing midazolam data. One subject had missing data for the Charlson co-morbidity score and 28 subjects were younger than 65 years old; these patients were also excluded. The final analytic sample included 1,266 patients.
Figure 1.
Flow Chart for Inclusion of Patients in the Analytic Sample
Sensitivity analysis
Comparison of the patients with missing delirium data vs. those with data showed that there was no difference in their mean age, and other co-variates of postoperative delirium, such as gender, ASA classification, surgical site, Charlson Comorbidity score, dates of surgery, or preoperative use of benzodiazepines, surgical site or year of surgery (supplemental table).
Patient Characteristics
The clinical characteristics of patients with or without the use of preoperative midazolam as a premedication are shown in Table 1. Patients who were administered midazolam were younger, had lower Charlson co-morbidity scores, higher preoperative TICS total scores, and were more likely to have used benzodiazepines prior to the scheduled surgery. There was also a difference in the administration of midazolam between the different surgical types and the dates of surgery. The dose of midazolam that was given as a premedication was 1.99 ± 1.03 (mean ± standard deviation) mg.
Table 1.
Preoperative Characteristics of All Patients
| Use of midazolam | No midazolam | p-value | Standardized Difference* | |
|---|---|---|---|---|
| n (%) | 909 (71.8%) | 357 (28.2%) | ||
|
| ||||
| Age (yr), mean ± SD | 72.2 ± 5.6 | 74.5 ± 6.6 | < 0.01 | 0.38 |
|
| ||||
| Female, n (%) | 474 (52.2%) | 169(47.3%) | 0.12 | −0.10 |
|
| ||||
| Charlson, mean ± SD | 0.9 ± 1.5 | 1.2 ± 1.4 | < 0.01 | 0.17 |
|
| ||||
| ASA Classification, n (%) | ||||
| I – II | 497 (54.7%) | 191 (53.5%) | 0.71 | −0.02 |
| III – V | 412 (45.3%) | 166 (46.5%) | ||
|
| ||||
| TICS score, mean ± SD | 33.8 ± 3.5 | 32.7 ± 4.2 | < 0.01 | −0.28 |
|
| ||||
| Preoperative use of benzodiazepine, n (%) | 112 (12.3%) | 30 (8.4%) | 0.05 | 0.13 |
|
| ||||
| Surgical site, n (%) | ||||
| Hip | 221(24.3%) | 77 (21.6%) | < 0.01 | 0.20 |
| Knee | 204 (22.4%) | 55(15.4%) | ||
| Spine | 324 (35.6%) | 137(36.4%) | ||
| Other | 160 (17.6%) | 88 (24.7%) | ||
|
| ||||
| Year, n (%) | ||||
| < 2010 | 449 (66.9%) | 222 (33.1%) | < 0.01 | 0.26 |
| ≥ 2010 | 460 (77.3%) | 135 (22.7%) | ||
Abbreviations: ASA, American Society of Anesthesiology; SD, standard deviation; TICS, Telephone Interview for Cognitive Status.
Standardized Difference: the difference in means or proportions divided by the pooled standard deviation
Propensity Score Matching
Prior to propensity score matching, standardized differences in preoperative characteristics ranged between −0.28 to 0.38. The largest standardized differences were for age (0.38), Charlson co-morbidity score (0.17), preoperative cognitive score (−0.28), surgical site (0.20) and year of surgery (0.26). After propensity score matching, all standardized differences were less than |0.10|, indicating the groups were well matched (Table 2). Specifically, the standardized differences between groups ranged from −0.07 to 0.06.
Table 2.
Preoperative Characteristics for the Propensity Score Matched Cohorts
| Use of midazolam (weighted) | No midazolam | p-value | Standardized Difference* | |
|---|---|---|---|---|
| n | 357 | 357 | ||
|
| ||||
| Age (yr), mean ± SD | 74.1±4.2 | 74.5±6.6 | 0.35 | 0.06 |
|
| ||||
| Female, n (%) | 182.2 (51.0%) | 169 (47.3%) | 0.36 | −0.07 |
|
| ||||
| Charlson, mean ± SD | 1.2±1.2 | 1.2±1.4 | 0.63 | −0.03 |
|
| ||||
| ASA Classification, n (%) | ||||
| I – II | 190.5 (53.4%) | 191 (53.5%) | 1.00 | 0.00 |
| III – V | 166.5 (46.6%) | 166 (46.5%) | ||
|
| ||||
| TICS score, mean ± SD | 32.9±2.6 | 32.7±4.2 | 0.52 | −0.04 |
|
| ||||
| Preoperative use of benzodiazepine, n (%) | 30.8 (8.6%) | 30 (8.4%) | 1.00 | 0.01 |
|
| ||||
| Surgical site, n (%) | ||||
| Hip | 62.3 (17.5%) | 77 (21.6%) | 0.48 | −0.03 |
| Knee | 65 (18.2%) | 55 (15.4%) | ||
| Spine | 137.7 (38.6%) | 137 (36.4%) | ||
| Other | 92 (25.8%) | 88 (24.7%) | ||
|
| ||||
| Year, n (%) | ||||
| < 2010 | 223.3 (62.6%) | 222 (62.2%) | 0.98 | −0.01 |
| ≥ 2010 | 132.7 (37.4%) | 135 (37.8%) | ||
Abbreviations: ASA, American Society of Anesthesiology; SD, standard deviation; TICS, Telephone Interview for Cognitive Status.
Standardized Difference: the difference in means or proportions divided by the pooled standard deviation
Incidence of Postoperative Delirium
The incidence of postoperative delirium did not differ significantly between the propensity score matched groups: 23.30% in the midazolam group developed delirium the first day after surgery compared to 24.93% for the group that did not receive midazolam [OR = 0.91; 95% CI = (0.65 – 1.29); P=0.67]. The result was similar when including delirium assessment on the second postoperative day; or between hypo- or hyper-active delirium subtypes. In additional secondary data analysis, when excluding patients who used benzodiazepines on a chronic basis, the incidence of delirium on the first postoperative day also did not differ between the groups who were given midazolam (22.9%) vs. those who did not receive the drug (25.4%), OR 0.88 (95% CI 0.61–1.25).
Discussion
In this study, we found that intravenous midazolam given as a premedication immediately prior to surgery was not associated with higher risk of postoperative delirium.
Comparison with previous studies
No study has examined the use of premedication of midazolam and its association with postoperative delirium in older surgical patients. Studies that have examined the use of benzodiazepines have mainly focused on new use in the intensive care unit or postoperative exposure.5,10 In the study by Pandharipande et al., new use of lorazepam was associated with delirium in the critical care unit. In the study by Marcantonio et al., postoperative exposure to meperidine and benzodiazepines was found to be associated with postoperative delirium. In the study by Chatwat et al., perioperative exposure to benzodiazepine was reported to be a risk factor for postoperative delirium in patients who were admitted to surgical intensive care unit within 7 days after surgery.21 However, they did not report on the types of benzodiazepine used or whether they were used before or after surgery. All of the studies above did not evaluate the association between midazolam used as a premedication and postoperative delirium. One study by Kudoh et al. reported that “postoperative confusion” increased in older patients who had preoperative use of benzodiazepine.9 In the 57 patients (17%) who reported taking benzodiazepines, most of them used triazolam (53%) or ethyl loflazepate (26%). No one had preoperative exposure to midazolam. Of importance, only long-term users (daily use for more than 1 year) were found to have higher incidence of postoperative confusion. In our cohort, preoperative use of benzodiazepine was used in the computation of the propensity scores and consequently did not differ between the propensity matched groups.
Patients who received midazolam as a premedication in our study were younger, with fewer medical comorbidities and had more depressive symptoms preoperatively. This potential bias of not administering midazolam based on age is likely secondary to the anticipated negative effects of benzodiazepine as a premedication on cognition in older patients.22,23
Potential limitations
There are several potential limitations in our study. First, the use of premedication of benzodiazepine or the administration of postoperative benzodiazepine was not randomized. We adjusted for this potential confounding factor using propensity score matching method. However, despite this method, there may be other factors that cannot been controlled adequately such as the depth of anesthesia during surgery, which may affect the incidence of postoperative delirium. Second, we focused on measuring postoperative delirium once on the first few postoperative days. As a result, we may have missed patients who developed postoperative delirium that was not assessed during the interview because of the fluctuating nature of delirium, or delirium which occur in the later postoperative period. However, given the short distribution half-life of midazolam, it is unlikely that the drug will have substantial effect on cognition beyond the first day after surgery. Finally, our studies focused on patients who underwent elective non-cardiac, non-cranial surgery and the results may not be directly generalized to patients undergoing emergency or other types of surgery.
Currently, a pragmatic, multicenter, cluster crossover trial is ongoing to evaluate the association between intraoperative benzodiazepine and postoperative delirium in cardiac surgical patients (ClinicalTrials.gov Identifier: NCT03928236). A recently completed multicenter, randomized trial investigated the impact of premedication with midazolam on outcome of older surgical patients scheduled for non-cardiac surgery (ClinicalTrials.gov Identifier: NCT03052660).24 We need to wait for the current prospective studies to make a broader generalization about whether benzodiazepines can be used without concern for postoperative delirium.
Conclusion
In summary, our study showed that premedication using midazolam was not associated with higher incidence of delirium in the early postoperative period. These results suggest that the pathophysiology of postoperative delirium is likely complex and not as a direct result from exposure to a small dose of premedication with midazolam.
Supplementary Material
Key Points Summary.
Question:
Does premedication with midazolam increase the risk of postoperative delirium in older adults?
Findings:
Premedication using midazolam was not associated with higher incidence of delirium on the first postoperative day in older patients undergoing major non-cardiac surgery.
Meaning:
The pathophysiology of postoperative delirium is likely complex and not as a direct result from exposure to a small dose of premedication with midazolam.
Acknowledgments:
We acknowledge the Perioperative Medicine Research Group in their assistance in patient recruitment, interviews and data collection. Perioperative Medicine Research group - principal investigator Jacqueline M. Leung, M.D., M.P.H. Research associates Christopher Tang, B.A., Devon Pleasants, B.S., Sanam Tabatabai, B.S., Danielle Tran B.S., Stacey Chang B.A., Gabriela Meckler B.A., Stacey Newman B.A., Tiffany Tsai M.D., Vanessa Voss M.D., and Emily Youngblom B.A.
Funding Disclosure: This study was funded in part by National Institutes of Health grants R21AG048456 (Leung) and 1R01NR017622-01A1 (Leung)
Glossary of Terms
- ASA
American Society of Anesthesiologists
- CAM
Confusion Assessment Method
- CI
Confidence interval
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- NCT
National clinical trial
- OR
Odds ratio
- SAS
Statistical analysis system
- STROBE
Strengthening The Reporting of Observational studies in Epidemiology
- UCSF
University of California, San Francisco
- TICS
Telephone interview of cognitive status
Footnotes
Conflict of interest: None.
References:
- 1.Santana Santos F, Wahlund LO, Varli F, et al. Incidence, clinical features and subtypes of delirium in elderly patients treated for hip fractures. Dement Geriatr Cogn Disord. 2005;20:231–237. [DOI] [PubMed] [Google Scholar]
- 2.Kalisvaart KJ, Vreeswijk R, de Jonghe JF, et al. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc. 2006;54:817–822. [DOI] [PubMed] [Google Scholar]
- 3.Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320:578–582. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RI, Cameron DJ, Fahs MC. A prospective study of delirium and prolonged hospital stay. Exploratory study. Arch Gen Psychiatry. 1988;45:937–940. [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–1522. [PubMed] [Google Scholar]
- 6.Kanto JH. Midazolam: the first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy. 1985;5:138–155. [DOI] [PubMed] [Google Scholar]
- 7.Kain ZN, Mayes LC, Bell C, et al. Premedication in the United States: a status report. Anesth Analg. 1997;84:427–432. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht S, Ihmsen H, Hering W, et al. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999;65:630–639. [DOI] [PubMed] [Google Scholar]
- 9.Kudoh A, Takase H, Takahira Y, Takazawa T. Postoperative confusion increases in elderly long-term benzodiazepine users. Anesth Analg. 2004;99:1674–1678. [DOI] [PubMed] [Google Scholar]
- 10.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. [DOI] [PubMed] [Google Scholar]
- 11.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. [DOI] [PubMed] [Google Scholar]
- 12.Kassie GM, Nguyen TA, Kalisch Ellett LM, Pratt NL, Roughead EE. Preoperative medication use and postoperative delirium: a systematic review. BMC Geriatr. 2017;17:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung JM, Sands LP, Vaurio LE, Wang Y. Nitrous oxide does not change the incidence of postoperative delirium or cognitive decline in elderly surgical patients. Br J Anaesth. 2006;96:754–760. [DOI] [PubMed] [Google Scholar]
- 14.Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry. 2013;21:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung JM, Sands LP, Chen N, et al. Perioperative Gabapentin Does Not Reduce Postoperative Delirium in Older Surgical Patients: A Randomized Clinical Trial. Anesthesiology. 2017;127:633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 18.Wolters U, Wolf T, Stutzer H, Schroder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217–222. [DOI] [PubMed] [Google Scholar]
- 19.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 20.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. [DOI] [PubMed] [Google Scholar]
- 21.Murphy B, Fraeman KH,Walham E. SAS® Macro to Implement Optimal N:1 Propensity Score Matching with a Maximum Radius. Paper 812–2017 https://support.sas.com/resources/papers/proceedings17/0812-2017.pdf
- 22.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte PJ, Mascha EJ. Propensity Score Methods: Theory and Practice for Anesthesia Research. Anesthesia Analgesia. 2018;127(4);1074–84. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statist. Med. 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang CJ, Jin Z, Sands LP, et al. ADAPT-2: A randomized clinical trial to reduce intraoperative EEG suppression in older surgical patients undergoing major noncardiac surgery. Anesth Analg. 2020;131(4);1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaiwat O, Chanidnuan M, Pancharoen W, et al. Postoperative delirium in critically ill surgical patients: incidence, risk factors, and predictive scores. BMC Anesthesiol. 2019;19:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun GC, Hsu MC, Chia YY, Chen PY, Shaw FZ. Effects of age and gender on intravenous midazolam premedication: a randomized double-blind study. Br J Anaesth. 2008;101:632–639. [DOI] [PubMed] [Google Scholar]
- 28.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowark A, Rossaint R, Keszei AP, et al. Impact of PReOperative Midazolam on OuTcome of Elderly patients (I-PROMOTE): study protocol for a multicentre randomised controlled trial. Trials. 2019;20:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.