Abstract
Objectives:
To characterize the relationship between cochlear duct length (CDL) and initial hearing preservation among cochlear implant (CI) recipients of a fully-inserted 31.5 mm flexible lateral wall electrode array.
Study Design:
Retrospective review.
Setting:
Tertiary academic referral center.
Patients:
Adult CI recipients who presented preoperatively with unaided hearing detection thresholds of ≤65 dB HL at 125 Hz and underwent cochlear implantation with a 31.5 mm flexible lateral wall array.
Intervention:
Cochlear implantation with a hearing preservation surgical approach.
Main Outcome Measures:
Computed tomography was reviewed to determine CDL. Hearing preservation was characterized by the shift in low-frequency pure-tone average (LFPTA; 125, 250 and 500 Hz), and shift in individual unaided hearing detection thresholds at 125, 250, and 500 Hz.
Results:
Nineteen patients met the criteria for inclusion. The mean CDL was 34.2 mm (range: 30.8–36.5 mm). Recipients experienced a mean LFPTA shift of 27.6 dB HL (range: 10–50 dB HL). Significant, negative correlations were observed between CDL and smaller threshold shifts at individual frequencies and LFPTA (p≤0.048).
Conclusion:
A longer CDL is associated with greater likelihood of preserving low frequency hearing with long arrays. Low-frequency hearing preservation is feasible with fully-inserted long flexible arrays within the initial months after cochlear implantation. Preoperative measurement of CDL may facilitate a more individualized approach in array selection to permit optimal cochlear coverage while enhancing hearing preservation outcomes.
Keywords: Cochlear implant, electric-acoustic stimulation, FlexSOFT, low-frequency
INTRODUCTION
The candidacy criteria for cochlear implantation includes patients with acoustic hearing detection thresholds in the normal low-frequency range sloping to the moderate-to-profound range at higher frequencies, and limited speech perception with appropriately-fit amplification. Advances in electrode array design and surgical technique have improved the ability to preserve low-frequency acoustic hearing postoperatively; however, hearing preservation rates remain variable, both initially and with long-term device use (1–6). Selection of an array that supports hearing preservation and optimal performance with the device is of interest, as cochlear implant (CI) recipients demonstrate superior speech perception in noise, improved spatial hearing, and significant subjective benefit when listening with electric-acoustic stimulation (EAS) as compared to a CI-alone (7–12).
It is widely accepted that shorter lateral wall arrays increase the likelihood of hearing preservation with reduced intracochlear trauma (1,6,13). If residual hearing is lost postoperatively, longer array recipients typically experience better speech perception with a CI-alone device as compared to shorter array recipients (3,14,15). One potential mechanism to explain the benefit conferred by deeply inserted arrays may be closer alignment between the default electric frequency information and the tonotopic organization of the cochlea when listening with a CI-alone device (16). As such, preoperative array selection must consider the risk-benefit ratio of preserving acoustic hearing with shorter arrays and maximizing cochlear coverage with a longer array to optimize performance if acoustic hearing is lost. This is particularly challenging in patients who are borderline EAS candidates (e.g., moderate to moderately-severe low-frequency detection thresholds), as even a modest threshold shift can preclude the ability to fit the acoustic component.
With recent advances in array design, low-frequency hearing preservation has been demonstrated in recipients of a long (31.5 mm) flexible array (17,18). The ability to preserve hearing with a long flexible array offers an appealing option for borderline EAS candidates, as the benefits of listening with an EAS device would be supported in many cases while also providing maximal benefit listening with a CI-alone device if hearing is lost. However, substantial variability in cochlear duct length (CDL) (16,19–22) can introduce large differences (~200°) in the angular insertion depth (AID) of the most apical electrode contact among CI recipients of a fully-inserted 31.5 mm array (16), with a shorter CDL resulting in a deeper angular insertion. As deeper insertions generally carry greater risk for loss of acoustic hearing (3,6), the degree of hearing preservation among CI recipients of long arrays may be dependent upon CDL. Considering this, preoperative selection of an array length based upon measurement of CDL could offer an individualized approach to reduce trauma to the cochlear apex while still ensuring optimal outcomes with a CI-alone device if acoustic hearing is lost. The current study aimed to characterize the relationship between CDL and initial low-frequency hearing preservation among adult CI recipients of a fully-inserted 31.5 mm flexible array.
METHODS
Subjects
The study-site Institutional Review Board approved a retrospective review of the unaided hearing detection thresholds of adult CI recipients. Data were reviewed for patients who presented with preoperative unaided hearing detection thresholds of ≤65 dB HL at 125 Hz and underwent cochlear implantation with a MED-EL GmbH (Innsbruck, Austria) FlexSOFT electrode array. The 65 dB HL criterion was selected since the MED-EL clinical mapping software uses this criterion for activation of the acoustic component for an EAS processor. The FlexSOFT array is 31.5 mm in length from the tip of the array to the hub with 12 electrode contacts evenly spaced over 26.4 mm. The five most apical contacts are unpaired to provide a more flexible design as compared to previous generations of 31.5 mm arrays. Postoperative assessment of unaided hearing detection thresholds is standard of care at the study site for CI recipients who presented preoperatively with low-frequency thresholds of ≤65 dB HL (see Dillon et al. (23)).
Surgical Description
Cochlear implantation was performed by one of three surgeons at the tertiary academic center. All patients received intraoperative, intravenous and intratympanic dexamethasone, and underwent a standard posterior tympanotomy approach with a round window insertion. Array placement was confirmed with intraoperative plain film radiography, which is standard of care at the study site. Angular insertion depth of the most apical and basal electrode contacts were determined with intraoperative x-ray (24) or postoperative computed tomography (CT) (25). For subjects with pre- or postoperative CT, CDL at the organ of Corti was determined with the elliptic-circular approximation method (26) using OTOPLAN (25), which is not currently labeled for use in the US. Briefly, this method incorporates the cochlear diameter (A-value) and width (B-value), measured in cochlear view, to calculate the basal turn length, which is then used in the derivation of CDL. These values were used to assess the relationship between CDL and the initial shift in unaided hearing detection thresholds.
Unaided Hearing Detection
Low-frequency unaided hearing detection thresholds were assessed behaviorally in a soundproof booth with stimuli presented via insert phones. In cases of no response, a value of 95, 105, and 115 dB HL was recorded at 125, 250, and 500 Hz, respectively. These values correspond to 5 dB HL higher than the maximum output of the audiometer at each individual frequency. Audiometric data were obtained from the cochlear implantation candidacy evaluation (i.e., preoperative interval), an initial interval, and the most recent interval to assess stability in hearing preservation over time. Hearing preservation was characterized by the 1) shift in the low-frequency pure-tone average (LFPTA; 125, 250, and 500 Hz), as well as the shift in unaided hearing detection thresholds at individual frequencies, and 2) maintenance of functional hearing at individual frequencies, defined as ≤80 dB HL, which is the maximum output of the acoustic component of the EAS device.
Statistical Analysis
Preoperative and initial postoperative unaided hearing detection thresholds at individual frequencies (125, 250, and 500 Hz) and preoperative and initial postoperative LFPTAs were analyzed using a paired samples t-test. Initial postoperative unaided hearing detection thresholds were compared to thresholds obtained at the most recent test interval using a paired samples t-test to evaluate stability of hearing preservation over time. Pearson correlation was used to evaluate the relationship between CDL and the initial shift in unaided hearing detection thresholds. Multiple linear regression was performed to model the relationship between AID of the most apical electrode contact, CDL, and AID of the most basal electrode contact. Statistical analyses were performed with SPSS version 26 (IBM Corp, Armonk, New York). Significance was defined as α < 0.05.
RESULTS
Demographics
Nineteen CI recipients (8 female) met the inclusion criteria. Subject demographics are listed in Table 1, including unaided hearing detection thresholds at each interval. The mean age at implantation was 67.4 years (SD, 16.4 yr; range, 23–85 yr). The etiology was unknown in the majority of cases (n=15), followed by noise induced hearing loss (n=3) and neurofibromatosis type 1 (n=1). The average reported duration of deafness was 14.1 years (SD, 8.7 yr; range, 1–30 yr). The mean CDL was 34.2 mm (SD, 1.7 mm; range, 30.8–36.5 mm). A full insertion (defined as all 12 electrode contacts being intracochlear) was achieved in all cases, with a mean AID of 623° (SD, 49.1°; range, 533–700°) for the most apical electrode contact.
TABLE 1.
Subject demographics and preoperative and postoperative hearing levels (dB HL). Subjects are ordered by cochlear duct length.
| Case | Age (years) | Ear | Sex | Reported Deafness Duration‡ | Etiology | CDL (mm) | AID (°) | Post-Op Interval (Months) | Pre-Operative (dB HL) | Post-Operative (dB HL) | Threshold Shift (dB HL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| 125 Hz | 250 Hz | 500 Hz | LFPTA | 125 Hz | 250 Hz | 500 Hz | LFPTA | 125 Hz | 250 Hz | 500 Hz | LFPTA | |||||||||
| 1 | 79 | L | M | 15 | Unknown | 30.8 | 650 | 1 | 55 | 60 | 75 | 63 | 65 | 95 | 115 | 91 | 10 | 35 | 40 | 28 |
| 2 | 71 | R | F | 19 | Unknown | 31.8 | 670 | 2 | 60 | 60 | 60 | 60 | 95 | 105 | 115 | 105 | 35 | 45 | 55 | 45 |
| 3 | 84 | R | F | 23 | Unknown | 32.0 | 668 | 1 | 30 | 25 | 35 | 30 | 70 | 80 | 90 | 80 | 40 | 55 | 55 | 50 |
| 4 | 78 | L | F | 8 | Unknown | 32.4 | 641 | 5 | 45 | 50 | 80 | 58 | 70 | 95 | 110 | 91 | 25 | 45 | 30 | 33 |
| 5 | 79 | L | F | 10 | Unknown | 33.1 | 700 | 1 | 30 | 35 | 80 | 48 | 80 | 95 | 110 | 95 | 50 | 60 | 30 | 47 |
| 6 | 65 | R | F | 30 | Unknown | 33.2 | 675 | 1 | 55 | 90 | 100 | 82 | 80 | 105 | 115 | 100 | 25 | 15 | 15 | 18 |
| 7 | 79 | L | M | 15 | NIHL | 33.3 | 626 | 3 | 50 | 55 | 60 | 55 | 95 | 105 | 105 | 101 | 45 | 50 | 45 | 46 |
| 8 | 59 | L | F | 1 | Unknown | 33.7 | 700 | 1 | 50 | 50 | 65 | 55 | 75 | 90 | 115 | 93 | 25 | 40 | 50 | 38 |
| 9 | 23 | L | M | 20 | Unknown | 34.1 | 602 | 5 | 45 | 75 | 95 | 72 | 80 | 100 | 105 | 95 | 35 | 25 | 10 | 23 |
| 10 | 75 | L | M | 16 | Unknown | 34.2 | 575 | 1 | 50 | 65 | 70 | 62 | 80 | 100 | 105 | 95 | 30 | 35 | 35 | 33 |
| 11 | 48 | R | M | 1 | Unknown | 34.7 | 646 | 2 | 45 | 55 | 90 | 63 | 60 | 80 | 85 | 75 | 15 | 25 | -5 | 12 |
| 12 | 69 | R | M | 15 | NIHL | 35.1 | 636 | 3 | 65 | 70 | 75 | 70 | 80 | 90 | 105 | 91 | 15 | 20 | 30 | 21 |
| 13 | 85 | L | M | 20 | Unknown | 35.3 | 622 | 2 | 60 | 70 | 70 | 67 | 65 | 85 | 85 | 78 | 5 | 15 | 15 | 11 |
| 14 | 80 | L | M | 15 | Unknown | 35.3 | 546 | 1 | 60 | 75 | 80 | 72 | 80 | 100 | 115 | 98 | 20 | 25 | 35 | 26 |
| 15 | 65 | R | M | 27 | NF1 | 35.5 | 581 | 2 | 60 | 80 | 95 | 78 | 65 | 95 | 105 | 88 | 5 | 15 | 10 | 10 |
| 16 | 62 | R | F | 20 | Unknown | 35.8 | 533 | 4 | 50 | 55 | 60 | 55 | 50 | 75 | 90 | 71 | 0 | 20 | 30 | 16 |
| 17 | 80 | L | M | 6 | NIHL | 36.1 | 590 | 1 | 60 | 65 | 65 | 63 | 85 | 90 | 90 | 88 | 25 | 25 | 25 | 25 |
| 18 | 38 | L | F | 5 | Unknown | 36.3 | 594 | 1 | 65 | 70 | 70 | 70 | 70 | 90 | 95 | 86 | 5 | 20 | 25 | 16 |
| 19 | 62 | L | M | 1 | Unknown | 36.5 | 574 | 2 | 45 | 70 | 80 | 65 | 65 | 95 | 115 | 91 | 20 | 25 | 35 | 26 |
| Mean | 67.4 | 14.1 | 34.2 | 623 | 2.1 | 51.6 | 61.8 | 73.9 | 62.5 | 74.2 | 93.2 | 103.7 | 90.1 | 22.6 | 31.3 | 29.7 | 27.6 | |||
| SD | 16.4 | 8.7 | 1.7 | 49.1 | 1.4 | 10.1 | 15.4 | 15.4 | 11.5 | 11.5 | 8.7 | 11.0 | 9.0 | 14.3 | 14.1 | 15.9 | 12.8 | |||
AID, angular insertion depth; CDL, cochlear duct length; NIHL, noise induced hearing loss; NF1, neurofibromatosis type 1; LFPTA, low-frequency pure-tone average at 125, 250, and 500 Hz.
. Reported duration of deafness considered severe-profound in years.
Hearing Preservation
Individual and mean unaided hearing detection threshold shifts at each frequency within the initial months following cochlear implantation are shown in Table 1. The preoperative mean unaided hearing detection thresholds at 125, 250, and 500 Hz were 51.6, 61.8, and 73.9 dB HL, respectively. The initial interval when unaided hearing detection thresholds were measured ranged from 1 to 5 months, with a mean of 2.1 months (SD, 1.4 mo). The postoperative mean unaided hearing detection thresholds at 125, 250, and 500 Hz were 74.2, 93.2, and 103.7 dB HL, respectively. There was a significant change in unaided hearing detection thresholds at each frequency (22.6, 31.3, and 29.7 dB HL at 125, 250, and 500 Hz, respectively) between the preoperative and initial test interval (p<0.001). Subjects presented with a mean LFPTA of 62.5 dB HL (SD, 11.5 dB HL; range, 30–82 dB HL) at the preoperative interval and a mean LFPTA of 90.1 dB HL (SD, 9.0 dB HL; range, 71–105 dB HL) at the initial postoperative interval. As observed with individual thresholds, there was a significant difference between the mean preoperative and postoperative LFPTA (27.6 dB HL; t(18)=9.39, p<0.001). Sixteen subjects (84.2%) maintained functional hearing (≤80 dB HL) at one or more individual frequencies, with 4 (21.1%) maintaining functional hearing when measured by the LFPTA. The mean unaided hearing detection threshold shifts at each frequency obtained at the most recent test interval (mean, 9.7 mo; SD, 3.5) are shown in Table 2. Notably, there was no significant difference between thresholds during the initial post-activation period and most recent test interval (p≥0.249), indicating stable hearing preservation over the reviewed time period.
TABLE 2.
Postoperative hearing levels (dB HL) obtained at the most recent test interval.
| Case | Post-Op Interval (Months) | Post-Operative (dB HL) | |||
|---|---|---|---|---|---|
|
| |||||
| 125 Hz | 250 Hz | 500 Hz | LFPTA | ||
| 1 | 13 | 80 | 105 | 115 | 100 |
| 2 | 17 | 95 | 105 | 115 | 105 |
| 3 | 7 | 65 | 75 | 85 | 75 |
| 4 | 8 | 70 | 90 | 110 | 90 |
| 5 | 9 | 80 | 90 | 105 | 92 |
| 6 | 12 | 80 | 100 | 115 | 98 |
| 7 | 9 | 85 | 100 | 100 | 95 |
| 8 | 12 | 95 | 105 | 115 | 105 |
| 9 | 13 | 80 | 100 | 110 | 97 |
| 10 | 6 | 90 | 105 | 110 | 102 |
| 11 | 9 | 65 | 85 | 100 | 83 |
| 12 | 10 | 80 | 90 | 105 | 92 |
| 13 | 7 | 60 | 75 | 80 | 72 |
| 14 | 9 | 80 | 105 | 115 | 100 |
| 15 | 5 | 80 | 100 | 115 | 98 |
| 16 | 7 | 50 | 70 | 105 | 75 |
| 17 | 14 | 85 | 90 | 85 | 87 |
| 18 | 4 | 70 | 90 | 85 | 82 |
| 19 | 14 | 60 | 90 | 115 | 88 |
| Mean | 9.7 | 76.3 | 93.2 | 104.5 | 91.3 |
| SD | 3.5 | 12.2 | 11.1 | 12.1 | 10.2 |
LFPTA, low-frequency pure-tone average at 125, 250, and 500 Hz.
Figure 1 plots the initial shift in unaided hearing detection thresholds at individual frequencies and LFPTA as a function of CDL. A significant negative correlation was found (p<0.05 for each comparison, individual r values displayed in Fig. 1), with subjects with a longer CDL experiencing a smaller shift in unaided hearing detection thresholds. This pattern of significance remained unchanged for partial correlations between LFPTA shift and CDL while controlling for age at implantation or duration of hearing loss (p≤0.011). Figure 2 plots the initial shift in unaided hearing detection thresholds at individual frequencies and LFPTA as a function of AID of the most apical electrode contact. No significant correlations were observed between AID of the most apical electrode contact and the shift in unaided hearing detection thresholds at 125 (r=0.42, p=0.073), 250 (r=0.44, p=0.060), 500 Hz (r=0.18, p=0.451), or LFPTA (r=0.40, p=0.089).
FIG 1. Longer CDL is associated with greater hearing preservation with a long 31.5 mm array.
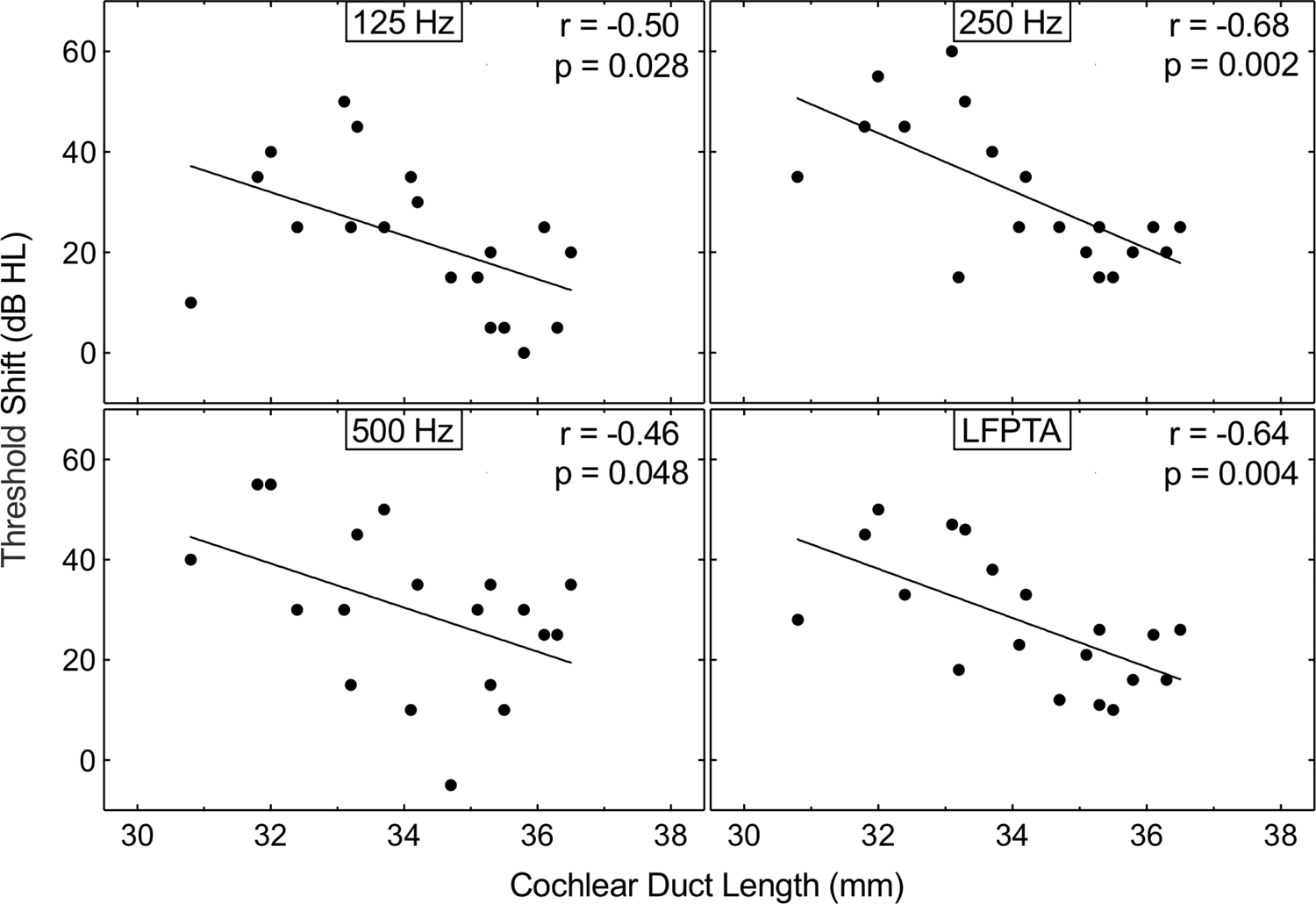
Shift in unaided hearing detection thresholds at 125, 250, and 500 Hz, and LFPTA as a function of CDL. Text at the top right of each panel indicates the correlation illustrated with line fits. CDL, cochlear duct length; LFPTA, low-frequency pure-tone average.
FIG 2. A shallow AID is not significantly associated with lower threshold shifts at 125, 250 or 500 Hz, after insertion of a 31.5 mm array.
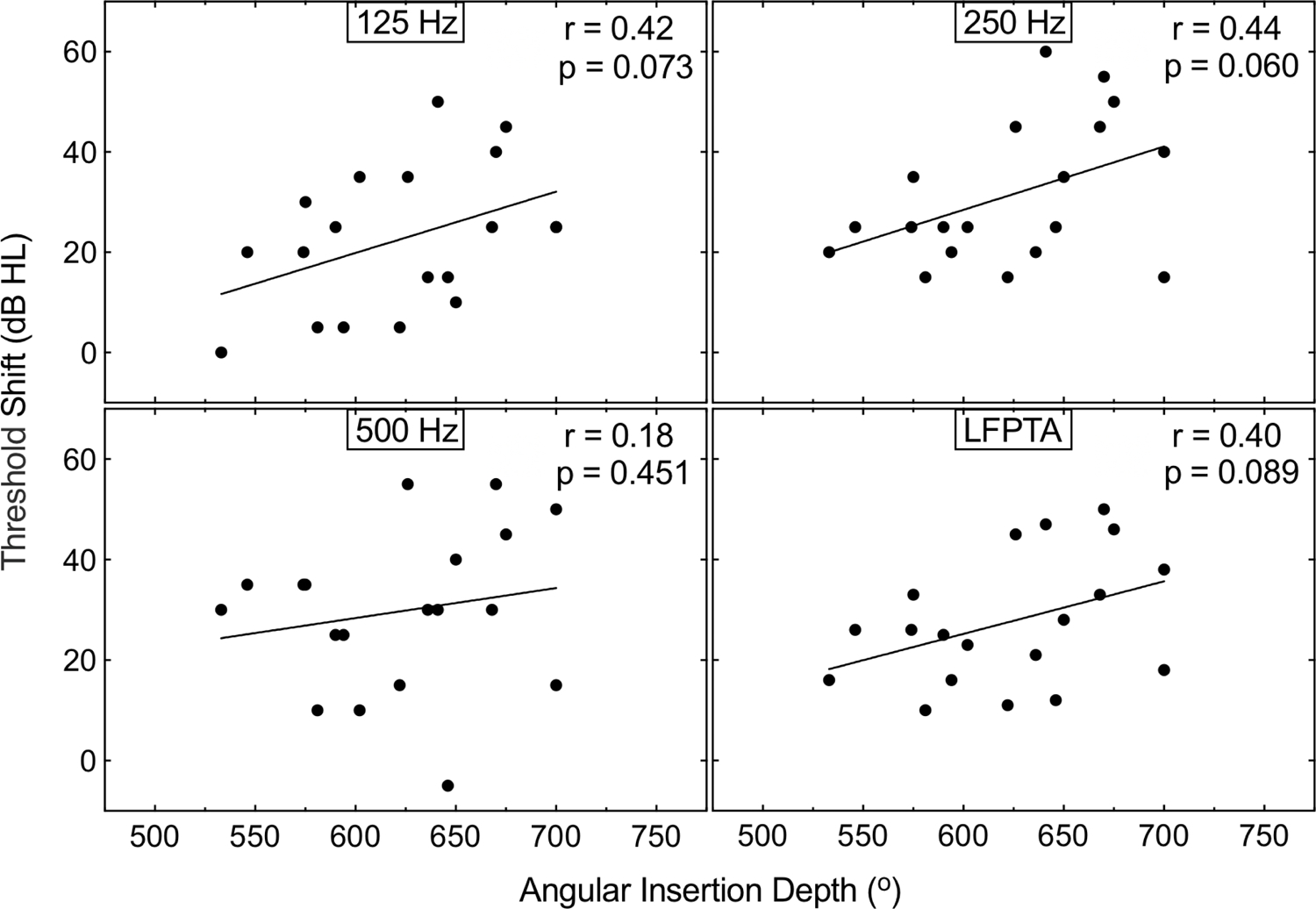
Shift in unaided hearing detection thresholds at 125, 250, and 500 Hz, and LFPTA as a function of AID. Text at the top right of each panel indicates the correlation illustrated with line fits. AID, angular insertion depth; LFPTA: low-frequency pure-tone average.
There was a significant correlation between CDL and AID of the most apical electrode contact (r2=0.47, p=0.001), with longer CDLs associated with shallower insertion depths. A multiple linear regression model including CDL and AID of the most basal electrode contact was able to account for the majority of variance in AID of the most apical electrode contact (r2=0.89, p<0.001). This indicates the importance of accounting for basal insertion depth when assessing variance in apical AID.
DISCUSSION
Hearing preservation with longer lateral wall arrays has been shown previously, albeit at lower rates when compared to shorter arrays (17,18,23). As such, clinicians are currently faced with the challenge of selecting the ideal array length in hearing preservation cases to optimize outcomes. This task requires critical consideration in weighing the risk of losing acoustic hearing against the benefit of greater cochlear coverage with longer arrays. The present study provides additional information regarding the feasibility of low-frequency hearing preservation with a long (31.5 mm) flexible lateral wall array and offers insight into the utility of preoperative CDL measurement for array length selection.
Comparing hearing preservation rates with 31.5 mm arrays remains a difficult task with differing methodologies and definitions employed across studies. Prior work by Dillon et al. (23) investigated hearing preservation rates with an earlier generation 31.5 mm array. Five of the 25 subjects demonstrated an aidable (≤80 dB HL) acoustic threshold at 125 Hz at initial activation. Four additional subjects demonstrated an improvement in unaided detection thresholds at a post-activation interval. By the 12-month post-activation interval, six subjects maintained acoustic thresholds that warranted the fitting of EAS. The higher rates of hearing preservation at 125 Hz observed in the present study (n=16, 84%, Table 1) are likely attributable to the flexible design of the newer array and routine use of hearing preservation techniques. However, it is possible that improvements in hearing preservation could also be attributed to lower unaided detection thresholds at 125 Hz (mean=52 dB HL vs 62 dB HL). Helbig et al. (17) reported similar shifts in unaided low-frequency hearing detection thresholds among long flexible lateral wall array recipients, yet it is difficult to make a direct comparison as the study sample included both partial and full insertions. Nonetheless, it is worth noting that their data demonstrated stable hearing preservation with longer arrays over time, with a mean postoperative test interval of 14.8 months, which is corroborated by our finding that hearing preservation remained stable over later intervals (mean, 9.7 months).
While important to characterize hearing preservation with longer flexible arrays, it is imperative to identify the subset of patients that may gain the greatest benefit from this approach. Recent work has focused upon customized approaches in the array selection process, such that CDL can be estimated with preoperative CT (27–29) or magnetic resonance imaging (30,31). Inter-individual differences in CDL result in substantial variability in the AID of the most apical electrode contact (16), as evidenced by the broad range (533–700°) observed in the present cohort of fully-inserted 31.5 mm arrays. In the current sample, a longer CDL was associated with smaller shifts in low-frequency unaided hearing detection thresholds. It is worth noting that all patients with a CDL >35 mm experienced postoperative threshold shifts <30 dB HL at both 125 Hz and 250 Hz (Fig 1). Conversely, the majority of patients with CDL ≤35 mm experienced threshold shifts greater than 30 dB HL. These findings suggest that a shorter array may be better suited for a borderline EAS candidate with a CDL ≤35 mm; however, determination of a CDL criterion for array selection was not the primary aim of the present study. Interestingly, threshold shifts were better predicted by CDL than AID of the most apical electrode contact. While deeper insertion depths have been associated with greater loss of acoustic hearing (3,6), this finding could suggest that the measurement of cochlear diameter (A-value) and width (B-value) used to determine CDL may indirectly capture additional morphologic features (e.g., cochlear volume and scala tympani dimensions). Larger cochlear dimensions could be associated with reduced intracochlear trauma (32,33). Considering this, preoperative assessment of cochlear morphology may support an individualized approach in array selection that ensures sufficient cochlear coverage while minimizing trauma. In the future, larger data sets may allow for a model-based approach to array selection, which would consider the degree of low-frequency acoustic hearing, CDL, anticipated AID, predicted threshold shifts, and benefit gained with EAS as compared to a CI-alone device.
A limitation of the current study is the retrospective design and thus limited performance data for the sample. Only 5 of the subjects in the sample were fit with EAS. The clinical follow-up protocol includes the assessment of speech perception with the familiar device/settings; therefore, there are no comparison data with the CI-alone for these EAS users. For the remaining 14 subjects, fitting of EAS was either not discussed by the clinician or the subject elected not to be fit with EAS due to associated out-of-pocket costs (e.g., earmold impression, earmold fitting, and acoustic fitting and verification). Rejection of EAS has been reported in CI recipients of shorter arrays (i.e., <28 mm), with rationales including preference for an off-the-ear device, borderline acoustic hearing thresholds, and discomfort (34). There is a need for prospective assessment of speech perception for CI recipients of long arrays when listening with EAS versus the CI-alone to determine the associated performance benefits and assess how much acoustic hearing is needed to observe a benefit. Additionally, optimal mapping procedures should be considered for this patient population, as CI recipients of shorter arrays (i.e., <28 mm) have demonstrated poorer performance due to peripheral masking of the acoustic and electric output (35). An ongoing prospective study is investigating EAS mapping procedures that account for electrode array placement, including CI recipients of long arrays with postoperative hearing preservation, and the associated speech perception outcomes.
CONCLUSION
Low-frequency hearing preservation is feasible with fully-inserted long flexible arrays, with 84% of patients maintaining functional hearing (≤80 dB HL) at one or more individual frequencies within the initial months after cochlear implantation. Better hearing preservation was associated with a longer CDL. Preoperative measurement of CDL may facilitate a more individualized approach to array selection by identifying cases when a shorter array provides sufficient cochlear coverage without jeopardizing the potential benefit of EAS device use. Investigations are ongoing to assess the long-term low-frequency hearing preservation rates with long flexible arrays and compare hearing preservation rates between CI recipients with arrays at different AIDs.
Acknowledgments
Financial Disclosures/Conflicts of Interest:
This project was funded in part by the NIH through NIDCD (T32 DC005360). KDB serves on the surgical advisory board for MED-EL Corporation. HCP serves as a consultant for MED-EL Corporation and BPO serves as a consultant for Advanced Bionics Corporation. MTD and MAR and are supported by a research grant from MED-EL Corporation. EH, MWC, ALB, SAD, AY, KQ, and MMD declare that involvement in research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Wanna GB, O’Connell BP, Francis DO et al. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope 2018;128:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gantz BJ, Hansen MR, Turner CW et al. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol 2009;14Suppl 1:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connell BP, Hunter JB, Haynes DS et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope 2017;127:2352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jurawitz MC, Buchner A, Harpel T et al. Hearing preservation outcomes with different cochlear implant electrodes: Nucleus(R) Hybrid-L24 and Nucleus Freedom CI422. Audiol Neurootol 2014;19:293–309. [DOI] [PubMed] [Google Scholar]
- 5.Gstoettner WK, Helbig S, Maier N et al. Ipsilateral electric acoustic stimulation of the auditory system: results of long-term hearing preservation. Audiol Neurootol 2006;11Suppl 1:49–56. [DOI] [PubMed] [Google Scholar]
- 6.Suhling MC, Majdani O, Salcher R et al. The Impact of Electrode Array Length on Hearing Preservation in Cochlear Implantation. Otol Neurotol 2016;37:1006–15. [DOI] [PubMed] [Google Scholar]
- 7.Pillsbury HC 3rd, Dillon MT, Buchman CA et al. Multicenter US Clinical Trial With an Electric-Acoustic Stimulation (EAS) System in Adults: Final Outcomes. Otol Neurotol 2018;39:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon MT, Buss E, Adunka OF et al. Influence of Test Condition on Speech Perception With Electric-Acoustic Stimulation. Am J Audiol 2015;24:520–8. [DOI] [PubMed] [Google Scholar]
- 9.Gifford RH, Dorman MF, Skarzynski H et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear 2013;34:413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helbig S, Van de Heyning P, Kiefer J et al. Combined electric acoustic stimulation with the PULSARCI(100) implant system using the FLEX(EAS) electrode array. Acta Otolaryngol 2011;131:585–95. [DOI] [PubMed] [Google Scholar]
- 11.Dunn CC, Perreau A, Gantz B et al. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol 2010;21:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gantz BJ, Turner C, Gfeller KE et al. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope 2005;115:796–802. [DOI] [PubMed] [Google Scholar]
- 13.Gantz BJ, Dunn C, Oleson J et al. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope 2016;126:962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchman CA, Dillon MT, King ER et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol 2014;35:1773–9. [DOI] [PubMed] [Google Scholar]
- 15.Buchner A, Illg A, Majdani O et al. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One 2017;12:e0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canfarotta MW, Dillon MT, Buss E et al. Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear Epub ahead of print; doi: 10.1097/AUD.0000000000000864. [DOI] [PMC free article] [PubMed]
- 17.Helbig S, Baumann U, Hey C et al. Hearing preservation after complete cochlear coverage in cochlear implantation with the free-fitting FLEXSOFT electrode carrier. Otol Neurotol 2011;32:973–9. [DOI] [PubMed] [Google Scholar]
- 18.Mick P, Amoodi H, Shipp D et al. Hearing preservation with full insertion of the FLEXsoft electrode. Otol Neurotol 2014;35:e40–4. [DOI] [PubMed] [Google Scholar]
- 19.Hardy M The length of the organ of corti in man. Am J Anat 1938;63:291–311. [Google Scholar]
- 20.Wurfel W, Lanfermann H, Lenarz T et al. Cochlear length determination using Cone Beam Computed Tomography in a clinical setting. Hear Res 2014;316:65–72. [DOI] [PubMed] [Google Scholar]
- 21.Meng J, Li S, Zhang F et al. Cochlear Size and Shape Variability and Implications in Cochlear Implantation Surgery. Otol Neurotol 2016;37:1307–13. [DOI] [PubMed] [Google Scholar]
- 22.Koch RW, Ladak HM, Elfarnawany M et al. Measuring Cochlear Duct Length – a historical analysis of methods and results. J Otolaryngol Head Neck Surg 2017;46:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillon MT, Buss E, O’Connell BP et al. Low-Frequency Hearing Preservation With Long Electrode Arrays: Inclusion of Unaided Hearing Threshold Assessment in the Postoperative Test Battery. Am J Audiol 2019:1–5. [DOI] [PMC free article] [PubMed]
- 24.Giardina CK, Canfarotta MW, Thompson NT et al. Assessing Cochlear Implant Insertion Angle from an Intraoperative X-ray Using a Rotating 3-D Helical Scala Tympani Model. Otol Neurotol 2020; In Press. [DOI] [PMC free article] [PubMed]
- 25.Canfarotta MW, Dillon MT, Buss E et al. Validating a New Tablet-based Tool in the Determination of Cochlear Implant Angular Insertion Depth. Otol Neurotol 2019;40:1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schurzig D, Timm ME, Batsoulis C et al. A Novel Method for Clincial Cochlear Duct Length Estimation toward Patient-Specific Cochlear Implant Selection. OTO Open 2018:1–8. [DOI] [PMC free article] [PubMed]
- 27.Mistrik P, Jolly C. Optimal electrode length to match patient specific cochlear anatomy. Eur Ann Otorhinolaryngol Head Neck Dis 2016;133Suppl 1:S68–71. [DOI] [PubMed] [Google Scholar]
- 28.Timm ME, Majdani O, Weller T et al. Patient specific selection of lateral wall cochlear implant electrodes based on anatomical indication ranges. PLoS One 2018;13:e0206435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas A, Cakir A, Hunter JB et al. Automatic Cochlear Duct Length Estimation for Selection of Cochlear Implant Electrode Arrays. Otol Neurotol 2017;38:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nash N, Otero S, Lavy J. Use of MRI to determine cochlear duct length in patients undergoing cochlear implantation. Cochlear Implants Int;20(2)57–61. [DOI] [PubMed] [Google Scholar]
- 31.George-Jones NA, Tolisano AM, Kutz JW et al. Comparing Cochlear Duct Lengths Between CT and MR Images Using an Otological Surgical Planning Software. Otol Neurotol 2020;Epub ahead of print. DOI: 10.1097/MAO.0000000000002777 [DOI] [PubMed]
- 32.Avci E, Nauwelaers T, Lenarz T et al. Variations in microanatomy of the human cochlea. J Comp Neurol, 2014;522:3245–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi A, Arai Y, Sakuma N et al. Cochlear volume as a predictive factor for residual-hearing preservation after conventional cochlear implantation. Acta Oto-Laryngologica, 2018;138:345–350. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer ER, Waltzman SB, Landsberger DM et al. Acceptance and Benefits of Electro-Acoustic Stimulation for Conventional-Length Electrode Arrays. Audiol Neurootol 2020:1–10. [DOI] [PubMed]
- 35.Imsiecke M, Kruger B, Buchner A et al. Interaction Between Electric and Acoustic Stimulation Influences Speech Perception in Ipsilateral EAS Users. Ear Hear 2020;41:868–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


