Abstract
Objective:
Asthma is a common chronic disease that disproportionately affects children and minorities, particularly African Americans. Inhaled bronchodilators are first-line treatment for asthma exacerbations, but individual bronchodilator response (BDR) varies by race and ethnicity. Studies have attempted to examine the genetic underpinnings of African American BDR, but many did not include young children and/or were not conducted when subjects were experiencing an actual asthma exacerbation. Therefore, this pilot study tested candidate single nucleotide polymorphisms’ (SNPs’) association with pediatric African American BDR during an acute asthma exacerbation.
Methods:
This was a prospective study of pediatric asthma patients ages 2 – 18 years seeking care in the ED for an asthma exacerbation. We measured BDR before and after each inhaled bronchodilator treatment using both the Pediatric Asthma Severity Score (PASS) asthma severity score and spirometry, and divided patients’ BDR into equal terciles (low, medium, high). We collected genomic deoxyribonucleic acid (DNA) and examined whether 21 candidate SNPs from a review of the literature were associated with BDR using crude odds ratios and adjusted analysis (incorporating age, gender, obesity, and home tobacco smoke exposure).
Results:
The final sample population was 53 children, with an average age of 7.2 years. The average initial PASS score (on a scale of ascending severity from 0–6) was 2.5 (standard deviation (SD) 1.6), and average initial forced expiratory volume in 1 second (FEV1) / forced vital capacity (FVC) as a percent of predicted was 75.8% (SD 10.3%). After adjusting for body mass index (BMI), age category, gender, and smoke exposure, rs912142 was associated with decreased odds of having low BDR (odds ratio (OR) 0.20, 95% confidence interval (CI) 0.02–0.92), and rs7081864 and rs7903366 were associated with decreased odds of having high BDR (OR 0.097, 95% CI 0.009–0.62)
Conclusions:
In a study measuring BDR in African American children during an acute asthma exacerbation, we found 3 SNPs significantly associated with BDR that provide bidirectional information regarding a child’s potential response to emergency asthma exacerbation treatment. Once validated in larger studies and implemented into practice, such information could guide pharmacogenomic evidence-based emergency asthma treatment that improves patient outcomes.
Keywords: Pediatric, Asthma, Bronchodilator Response, Pharmacogenomics, Emergency Department
Introduction
Asthma is the most common chronic disease of childhood in the United States (US).1–5 An estimated 7 million children in the US suffer from asthma, over half of whom experience at least one self-reported exacerbation every year, resulting in over 6 million outpatient primary care visits and nearly 1 million emergency department (ED) visits annually.1–6 Asthma disproportionately affects minority populations, and the estimated prevalence in the African American population is 13%.2–5 Furthermore, African Americans experience asthma exacerbations at a higher frequency and greater severity, resulting in nearly five-fold higher mortality than whites.7–10
The first-line and mainstay of emergency treatment for an asthma exacerbation is inhaled short-acting beta agonist bronchodilators (typically albuterol).11 Yet 1 in 5 pediatric asthma patients do not respond to bronchodilators, and clinical and environmental data alone do not explain bronchodilator response (BDR) variation amongst patients.12 Prior studies estimate that BDR variation due to genetics ranges from 47–92%,13–15 and have found that BDR varies significantly by race/ethnicity.8,9,16 Moreover, African Americans have worse BDR than those of European descent, even when controlling for asthma severity.17 In an outpatient study that administered maximum doses of bronchodilators, African Americans had a 10% lower BDR than whites.17 Yet national guidelines recommend certain doses and frequencies of bronchodilators without taking BDR variation into account.11 Since bronchodilators can have adverse side effects (e.g., tachycardia, tremors, and even life-threatening hypokalemia and arrhythmias), the current “one size fits all” approach to emergency asthma treatment with bronchodilators is inadequate and potentially harmful.
To date, few studies have leveraged pharmacogenomics to close the knowledge gap on individualized emergency asthma treatments with bronchodilators.18,19 Additionally, many BDR pharmacogenomics studies did not include a significant number of minority patients.20,21 Of the studies that did examine African American BDR response, BDR was measured when the asthma patients were not experiencing an acute exacerbation.22–24 Those studies also excluded younger patients ages 2–7 years who often have more severe asthma, and administered lower doses of albuterol than recommended by guidelines.20–24 Those studies did produce candidate single nucleotide polymorphisms (SNPs) that may explain variation in BDR amongst African Americans.22–24 However, those SNPs must be tested in the context of emergency treatment for an asthma exacerbation, when the albuterol dose-response curve has shifted to the right, and when inflammation, epigenetic, and other confounders may change what genes are truly associated with BDR.25–28 Therefore, this pilot study’s objective was to explore whether candidate SNPs proposed by previous studies are associated with pediatric African American BDR during an acute asthma exacerbation.
Methods
Study Design, Setting, & Patient Population
This is a prospective pilot study of African American pediatric patients treated in the ED for an acute asthma exacerbation. This study was conducted in the University of Florida Health Jacksonville’s (UFHJ) Pediatric ED, which sees approximately 15,000 encounters annually, of which 10% are for asthma. UFHJ treats a mostly inner-city population and serves as Northeast Florida’s regional safety-net institution. This study was approved by the UF Institutional Review Board. Eligible patients were ages 2–18 years, self or guardian-identified as African American, English-speaking, and who presented to the ED with an acute asthma exacerbation. We excluded patients younger than 2 (to avoid confounding with bronchiolitis), known pregnant patients, patients with cystic fibrosis or bronchopulmonary dysplasia, those in law enforcement custody or wards of the state, and those for whom we were unable to contact a legal guardian (if under 18 years of age). Additionally, eligible patients were enrolled between the hours of 8 am and 11 pm when ED research coordinators were available.
Asthma Exacerbation Treatment
Our facility, the UFHJ Pediatric ED, uses a clinical pathway to guide asthma treatment. Patients typically receive between 1–3 treatments of albuterol, each combined with 0.5 mg ipratropium bromide and delivered 20 minutes apart (total number of treatments at the attending physician’s discretion). Albuterol is dosed at 2.5 mg for patients less than 10 kg, and 5 mg for patients 10 kg or greater. The pathway advises administration of systemic corticosteroids (oral prednisone, oral prednisolone, dexamethasone, or solumedrol) within 60 minutes of ED arrival. Other adjunctive therapies (e.g., magnesium sulfate) are at the discretion of the treating physician and are recommended based on the Pediatric Asthma Severity Score (PASS).29
BDR Measurements
Standard ED nursing practice is to assess and document the patient’s PASS score prior to the first bronchodilator treatment and after every bronchodilator treatment. PASS is a numeric asthma severity scoring system validated for use during acute exacerbations that incorporates degree/severity of wheezing, work of breathing, and prolongation of expiration.29 Additionally, we also measured BDR using hand-held spirometers (Spirobank II, Medical International Research, New Berlin, Wisconsin, USA, compatible with 2019 American Thoracic Society (ATS) spirometry guidelines)30 for patients ages 7 years and older who could cooperate with spirometry. Following the Spirobank II manufacturer instructions and 2019 ATS guidelines, patients performed spirometry in-between bronchodilator treatments instructed by either a respiratory therapist or a pediatric ED nurse trained in spirometry. Patients wore nose clips during spirometry. We obtained the forced expiratory volume in 1 second (FEV1) / forced vital capacity (FVC) as a percent of the patient’s predicted value from the Spirobank’s predicted sets in hand-held mode, which uses the Knudson equation for pediatric patients. As with the PASS score, FEV1/FVC as a percent of predicted value was measured before and after each bronchodilator treatment. BDR was calculated as the change in the PASS score and/or spirometry from before the first bronchodilator treatment to after the last bronchodilator treatment, (e.g., BDR = ΔPASS = PASSfinal – PASSinitial). We categorized BDR into equal terciles (low, medium, high) based on the sample population distribution. We then created modified BDR categories (low, medium, high) that adjusted for the PASSinitial (or FEV/FVCinitial) absolute value and the number of bronchodilator treatments given.
SNPs & deoxyribonucleic acid (DNA) Sequencing
Based on a search of published, peer-reviewed literature, we identified 21 SNPs potentially associated with BDR (Table 1). Most were associated with BDR in African American and/or pediatric asthma patients. We chose to include some studies of mostly white children in case the small or null sample size of African American patients failed to pick up a significant association.
Table 1:
Candidate SNPs Associated with BDR
| Reference Paper | SNP | Chromosome | Position | Allele | Gene | Putative Mechanism (if known) | |
|---|---|---|---|---|---|---|---|
| Mutant or risk allele | Reference | ||||||
| 122 | rs28450894 | 4 | 103453535 | T | C | NFKB1 | Bronchial smooth muscle regulation |
|
| |||||||
| 23 | rs9507294 | 13 | 24823347 | C | T | SPATA13-AS1 | Anti-sense RNA - may regulate smooth muscle contraction |
| rs912142 | 13 | 24827500 | G | A | SPATA13-AS1 | ||
| rs2248119 | 13 | 24827094 | A | G | SPATA13-AS1 | ||
| rs9551086 | 13 | 24830330 | T | C | SPATA13-AS1 | ||
| rs9553225 | 13 | 24823006 | A | G | SPATA13-AS1 | ||
|
| |||||||
|
20, 21 |
rs1042713 | 5 | 148206440 | A | G | ADRB2 | Beta-2 adrenergic receptor |
| 24 | rs73650726 | 9 | 85152666 | G | A | ^ | |
| rs7903366 | 10 | 53689774 | T | C | PRKG1 | Related to NO signaling pathways | |
| rs7070958 | 10 | 53691116 | G | A | PRKG1 | ||
| rs7081864 | 10 | 53690331 | A | G | PRKG1 | ||
|
| |||||||
| 25 | rs13090972 | 3 | 89076896 | G | T | Near EPHA3 | Cell adhesion, calcium regulation, NO synthesis |
| rs958144 | 3 | 88994672 | T | C | Near EPHA3 | ||
| rs7041938 | 9 | 113091523 | G | T | TXNDC8 | ||
|
| |||||||
| 26 | Gln27Glu/ rs1042714 | 5 | 148206473 | G | C | ADRB2 | |
| rs2781659 | 6 | 131891820 | G | A | ARG1 | ||
| rs892940 | 3 | 24538838 | A | G | THRB | ||
|
| |||||||
| 27 | rs295137 | 2 | 201150040 | T | C | SPATS2L | ↑ protein levels for albuterol receptor; |
| rs7037276 | 9 | 6247430 | T | C | IL33 | ||
| rs1342326 | 9 | 6190076 | A | C | IL33 | ||
|
| |||||||
| 28 | rs2626393 | 2 | 52965931 | C | T | Near ASB3 | Muscle cell differentiation |
Does not map to gene
DNA Collection & Sequencing
Two buccal swabs were collected per patient, one from each buccal mucosa. Genomic DNA was extracted using Isohelix SK-1S Buccal-Prep Plus DNA Isolation Kits (Boca Scientific, Dedham, Massachusetts, USA). Pure genomic DNA was then quantified using the Qubit 4.0 (Thermo Fisher Scientific, Waltham, MA) and normalized to a total starting input of 20 ng across all samples. Sequencing library preparation was carried out using the AmpliSeq assay for custom panels by Illumina (San Diego, CA) following the manufacturer’s protocol for the custom designed assay. Custom probe sets were prepared in Illumina’s Design Studio™ for gene targeting which included multiple primer pools adequate to cover all SNPs of interest and 97.2% of the exonic bases of the genes of interest with an average amplicon length of 240 base pairs (bp). The amplified genes were sequenced on the NextSeq 550Dx with paired ends reads covering 151 bp for each paired end orientation with an average of 1000x coverage across all genes. SNP calls over the targeted genes were carried out using Illumina’s DNA Amplicon application in BaseSpace and SNP positions of interest were provided for the variant calling analysis. The application produced 2 sets of variant call format files (VCF) which included the genome VCF where bases in exons of the genes of interest were called, as well as a genotype VCF which included only the SNPs of interest in the genes. Exons (and surrounding intronic regions for intronic SNPs) were sequenced for future study and potential identification of novel SNPs, though only previously reported SNPs were analyzed in the current pilot study. We used Python 3.7.7 and the library cyvcf2, version 0.20.1, to parse the VCF files for analysis.
Data Analysis
We computed SNPs crude odds ratios (OR) using R version 3.6.1 and the library EpiStats version 1.4.1. We then adjusted the ORs for age category (2–9 versus 10–18 years old), gender, overweight body mass index (BMI) (BMI > 25 or not), and home tobacco smoke exposure using logistic regression in R. We present results as ORs with 95% confidence intervals (CI). Statistical significance was set at a p value < 0.05. As this was an exploratory pilot study, we did not perform an a priori sample size calculation, but rather intend to use our results to inform future larger studies of the same patient population in the ED setting.
Results
Clinical
We enrolled a total of 54 African American patients ages 2–18 years from October 11, 2019 to March 7, 2020. Enrollment was stopped on March 7, 2020 due to the COVID-19 pandemic. Of those 54 patients, PASS scores were completed for 51, spirometry was performed in 15, and a single patient had neither PASS scores nor spirometry and was excluded. Therefore, for our final sample of 53 patients, we primarily performed BDR analysis with PASS scores and confirmed results with spirometry when available. Clinical patient characteristics are listed in Table 2. On average, patients had 1.4 ED visits for asthma exacerbations in the past year, but recorded an overall total of only 8 hospitalizations for asthma over the same period of time (1 patient with 2 of those 8 hospitalizations). Only 2 patients had a history of prior intubation.
Table 2:
Patient Characteristics
| Total N = 53 | |
|---|---|
| Male Gender | 33 (65%) |
|
| |
| Age - years (average) | 7.2 (SD 4) |
|
| |
| Non-Hispanic Ethnicity | 53 (100%) |
|
| |
| Medicaid Insurance | 53 (100%) |
|
| |
| BMI | |
| Underweight | 26 (49%) |
| Normal | 12 (22.6%) |
| Overweight | 7 (13.2%) |
| Obese | 2 (3.8%) |
|
| |
| Second-hand smoke exposure | 26 (49.1%) |
| Lives with current smoker | 16 (30.2%) |
|
| |
| Average number ED bronchodilator treatments | 2.6 (SD 1) |
|
| |
| Average ED Length-of-Stay (hours) | 3.2 (SD 1.4) |
|
| |
| ED Disposition | |
| Discharge | 42 (79%) |
| Admit General Ward | 9 (17%) |
| Admit Intensive Care | 2 (4%) |
SD = standard deviation
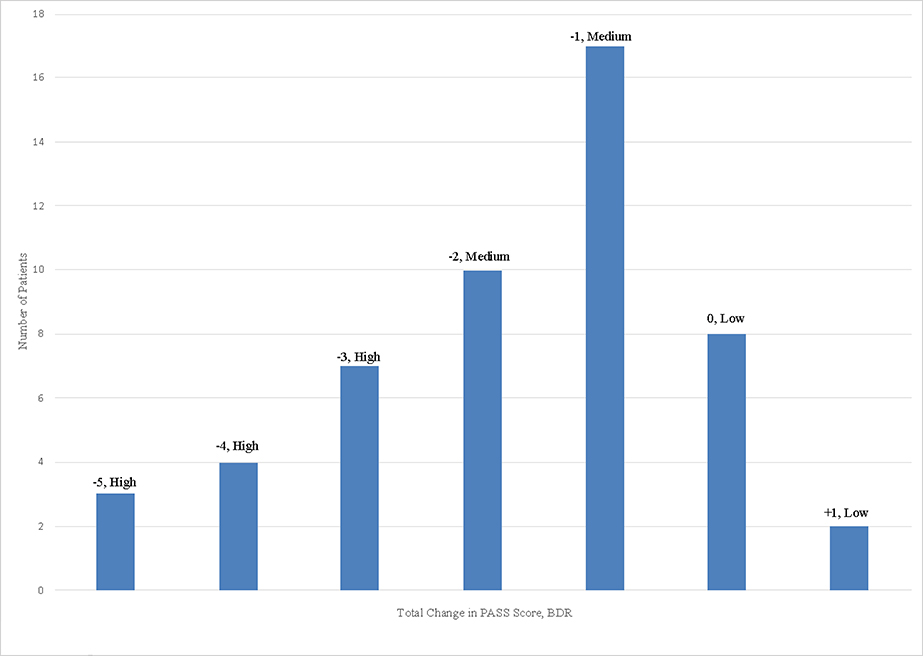
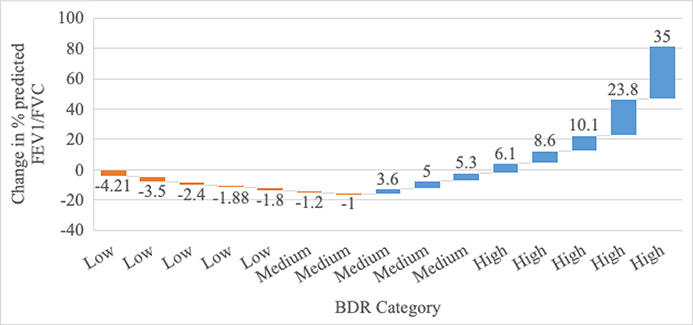
The average initial PASS score (on a scale of ascending severity from 0–6) was 2.5 (standard deviation (SD) 1.6) for 51 patients with complete PASS data, and average initial FEV1/FVC as a percent of predicted was 75.8% (SD 10.3%) for 15 patients with complete spirometry data. Changes in PASS scores and spirometry from the initial to the final bronchodilator treatment are displayed in Figure 1a & 1b, respectively.
Figure 1a: BDR Categories measured by change in PASS (N=51 patients).
Figure 1b: BDR Categories measured by change in percent FEV1/FVC predicted (N=15 patients).
Each bar’s number annotation represents one patient’s change in spirometry from initial to final value (Y axis value). Each bar reflects a single patient.
Pharmacogenomic Interrogation
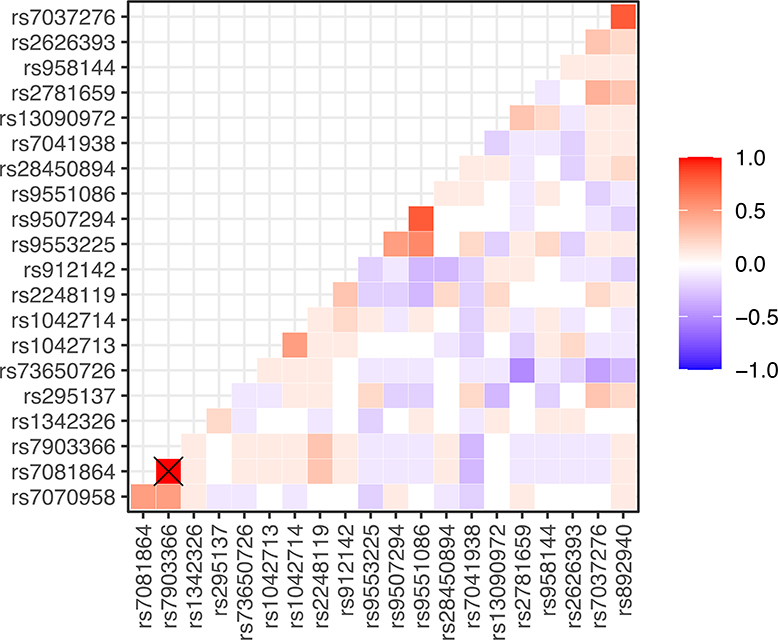
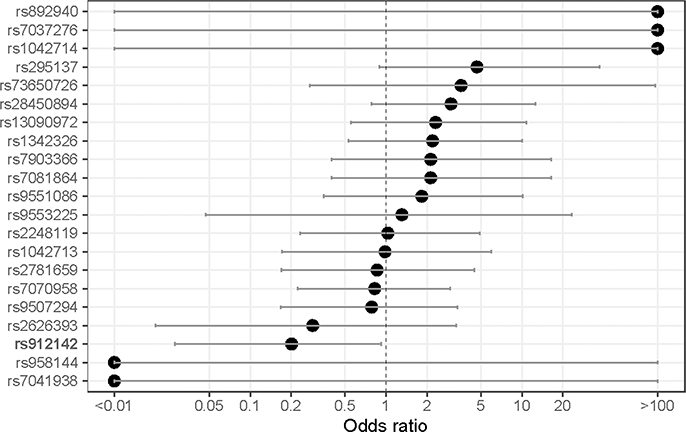
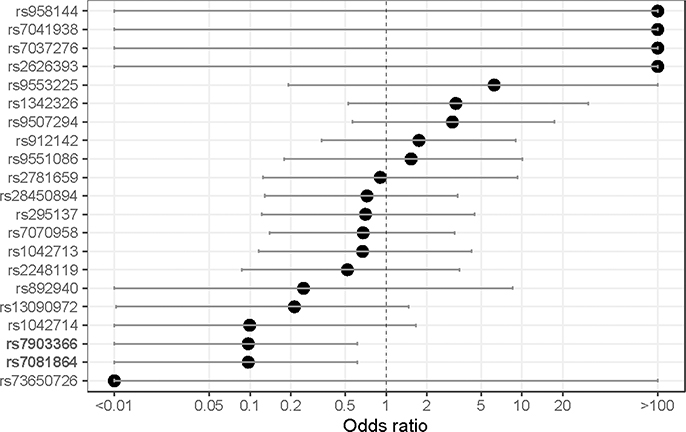
The prevalence, associated gene, reference, and alternate allele of the SNPs tested in our sample population are displayed in Table 3, along with allele frequencies of African and American populations from the 1000 Genomes Project. Most, but not all, allele frequencies broadly aligned with the African 1000 Genomes Project-reported frequency. Of note, mutant alleles in rs7081864 and rs7903366 were in complete linkage disequilibrium (i.e., co-occurrent in all study patients) (Figure 2). When considering crude odd-ratios, those two SNPs - rs7081864 and rs7903366, had a significant negative association with the high BDR category (OR 0.91, 95% CI 0.02–0.15 for both), and no SNPs were significant for any direction of association with the low BDR category (Supplemental Figures 1 & 2). After adjusting for BMI, age category, gender, and smoke exposure, rs912142 was associated with decreased odds of having low BDR (OR 0.20, 95% CI 0.02–0.92), and rs7081864 and rs7903366 again were associated with decreased odds of having high BDR (OR 0.097, 95% CI 0.009–0.62) (Figures 3a & 3b). Repeating those analyses using spirometry derived BDR (N=15 patients) did not identify any SNPs with significant associations with BDR upon crude or adjusted analysis (Supplemental Figures 3-5).
Table 3:
Prevalence of SNP mutant alleles in study sample population, and compared to prevalence from the 1000 Genomes Project
| SNP | Study Count | Study Prevalence | 1K Genomes AF for AFR* | 1K Genomes AF for AMR* |
|---|---|---|---|---|
| rs7037276 | 52 | 96.3% | 94% | 95% |
| rs1042714 | 51 | 94.4% | 86% | 76% |
| rs892940 | 51 | 94.4% | 82% | 50% |
| rs2626393 | 49 | 90.7% | 74% | 58% |
| rs2781659 | 45 | 83.3% | 71% | 55% |
| rs7081864 | 45 | 83.3% | 67% | 33% |
| rs7903366 | 45 | 83.3% | 66% | 33% |
| rs2248119 | 44 | 81.5% | 49% | 22% |
| rs1042713 | 43 | 79.6% | 52% | 46% |
| rs295137 | 41 | 75.9% | 51% | 28% |
| rs1342326 | 32 | 59.3% | 35% | 14% |
| rs7070958 | 29 | 53.7% | 67% | 33% |
| rs28450894 | 16 | 29.6% | 16% | 4% |
| rs912142 | 16 | 29.6% | 52% | 22% |
| rs13090972 | 14 | 25.9% | 20% | 38% |
| rs9507294 | 13 | 24.1% | 10% | 62% |
| rs9551086 | 9 | 16.7% | 5% | 58% |
| rs7041938 | 4 | 7.4% | 4% | 16% |
| rs9553225 | 4 | 7.4% | 2% | 46% |
| rs958144 | 4 | 7.4% | 2% | 33% |
| rs73650726 | 3 | 5.6% | 9% | 1% |
Highlighted SNPs discussed in the text
1K Genomes = 1000 Genomes Project: https://www.internationalgenome.org/1000-genomes-project-publications
AFR = African, AMR = American
Figure 2:
Correlation between SNPs in our sample population. The intersection of the two co-occurrent SNPs are marked with a cross (rs7081864 and rs7903366).
‘X’ denotes value of 1
Figure 3a:
Adjusted odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a low BDR (i.e., residing in the lowest adjusted ΔPASS category).
Note: SNPs with statistical significance in bold.
Figure 3b:
Adjusted odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a high BDR (i.e., residing in the highest adjusted ΔPASS category). Adjustments performed for age category, gender, overweight BMI, and home tobacco exposure.
Note: SNPs with statistical significance in bold.
Discussion
To our knowledge, this is one of the first studies to measure BDR in African American children during an acute asthma exacerbation in the emergency department. Further to this, we included much younger ages than previous studies.11,20–24 Importantly, we replicated the ability of 3 SNPs’ significant association with BDR (rs912142, rs7081864, and rs7903366).23,24 While our small pilot study sample size may have precluded reproducing other’s results referenced in Table 1, this study uniquely tested BDR when it matters most, during an acute asthma exacerbation. As such, those 3 SNPs provide important bidirectional information about a patient’s potential BDR, and those results can be used to move forward in the process of eventually implementing predictive pharmacogenomics into clinical practice.
With regards to predicting suboptimal response to treatment, we found that rs7081864 and rs7903366 were significantly negatively associated with having a high BDR category in an adjusted analysis (i.e., could predict low BDR). That confirms results from a larger study by Spear, et al, of African Americans and Latinos.24 Both SNPs are located in PRKG1, which is related to nitric oxide pathways and cGMP signaling, which affects smooth muscle relaxation. PRKG1 is expressed in lung tissues, and therefore having mutant alleles present in both SNPs is a mechanistically plausible way to lower BDR, since bronchial smooth muscle relaxation is a major component of bronchodilation.
Notably, Spear, et al also identified rs73650726 as the unique significant predictor for low BDR in African Americans with asthma.24 In this pilot study, rs73650726 had an OR of 3.57 for low BDR, but with a population of only three subjects, the result did not reach statistical significance. Additionally, the rs73650726 had a very low frequency of mutant alleles in our study population, and also a very low prevalence in the African and American populations in the 1000 Genomes Project (Table 3). The Spear study also found rs7070958 to be significant in a study involving Latinos and African Americans.24 In our study rs7070958 had a prevalence of 53.7% but a non-significant OR of only 0.82. However, apart from our smaller sample size, the Spear study measured BDR by withholding bronchodilators from asthma patients who were otherwise well and then performing pulmonary function testing, rather than measuring response to bronchodilators during an acute asthma attack.24 The Spear study could not reproduce their results in 3 other multiracial groups indicating that BDR might be best predicted by population-specific studies and looking at high prevalent-SNPs shared amongst racial/ethnic populations.24 That too supports our strategy to initially focus on the African American pediatric population.
Clinically, how is knowing the risk of low BDR in an individual patient helpful? For emergency care, pharmacogenomic testing would need to be performed pre-emptively, as ED treatment decisions are made in minutes. However, if such testing were performed by primary care or asthma specialty providers, the result could be populated in the patient’s electronic health record (EHR). If a patient experiences an exacerbation severe enough to seek ED care, the emergency provider could see an electronic health record (EHR) alert when ordering bronchodilators and systemic corticosteroids. Depending on that patient’s pharmacogenomic profile and severity of the exacerbation, future evidence-based guidelines could advise earlier administration of adjunctive therapies such as magnesium or terbutaline. Other supportive respiratory interventions such as high flow nasal cannula could also be employed earlier, rather than catching up to repeated cycles of bronchoconstriction, mucous plugging, and airway inflammation. Additionally, knowing a patient likely will not respond well to conventional therapy may also avoid unnecessary chest radiography (often ordered to rule out a complicating pneumonia) and its attendant radiation if a provider is aware that a patient may not quickly “turnaround.” Unnecessary use of chest radiographs in the ED for pediatric patients is a major quality of care issue and the subject of national efforts to decrease the unnecessary radiation exposure in children.31 Finally, in the primary care preventative setting, knowing a patient may have significant issues in the event of an asthma exacerbation may guide stepping-up inhaled corticosteroid controller therapy regimens.11
We also found that rs912142 predicted a more robust response to bronchodilators (mutant allele significantly negatively associated with low BDR). That confirms results from Padhukasahasram et al., who conducted a sizable genome-wide association study of BDR in a multiracial population of patients aged 12 to 56 years.23 Of the SNPs tested in that study, rs912142 had the largest effect size. Interestingly, in that study, rs912142 had a negative association with BDR in African Americans without asthma, but a positive association with the BDR of African Americans with asthma, and a positive association with the BDR of Europeans both with and without asthma. rs912142 is located in the gene SPATA13-AS1, which codes for an anti-sense ribonucleic acid (RNA) that is thought to regulate smooth muscle contraction and is expressed in brain and lung tissues. Therefore, our results predicting high BDR have mechanistic plausibility, and a very similar pathophysiologic pathway to our results predicting low BDR.
Understanding which pediatric patients will not respond well to conventional treatment is essential, but knowing which patients are likely to have robust responses is also useful. With adequate validation and assurance of accurate prediction of high BDR, clinicians can trust and give time for bronchodilators and systemic corticosteroids to take effect, rather than employing adjunctive therapies with adverse systemic side effects (e.g., terbutaline – elevation of cardiac enzymes, epinephrine – tachycardia, systemic vasoconstriction, magnesium – hypotension, headache). Conversely, if a patient thought to have a high BDR does not respond well to initial therapy, that could guide informed use of chest radiography or viral testing (e.g., respiratory syncytial virus, influenza, COVID-19) to investigate for other causes of a more severe asthma exacerbation.
Lastly, for new and significant pharmacogenomic knowledge to improve actual patient outcomes, it must be implemented into clinical practice. Although our results require validation in studies with larger sample sizes, it is never too early to plan for implementation and even design future studies “for dissemination.”18 Clinical implementation of pharmacogenomic-guided emergent therapy will undoubtedly require multidisciplinary efforts from emergency providers, pharmacogenomic experts, information technologists, and importantly patients’ and caregivers’ input.19 Given the nature of emergency medicine, translational qualitative and pragmatic study designs should be considered to run in parallel with the genomic studies needed to confirm our and others’ results.
Limitations:
This study has limitations that merit consideration. First and foremost, it is a pilot study with a small sample size at a single institution. However, our study population comprises pediatric patients residing in an inner-city environment with a high asthma prevalence. To study that younger population, we substituted the PASS for spirometry, the typical gold standard for BDR. Although PASS is validated for use in the emergency setting,29 it is a subjective score compared to spirometry, and future studies should consider alternative methods for systematically evaluating BDR in a younger population in the ED.32 Of note, we did not perform principal component analysis or estimation of global ancestry for the self-identified African American patients included in this study. Therefore, there could be a degree of admixture between African American and European ancestry that may have influenced our results.
Conclusion
In a study measuring BDR in African American children during an acute asthma exacerbation, we found 3 SNPs significantly associated with BDR that provide bidirectional information regarding a child’s potential response to emergency asthma exacerbation treatment. We plan to expand this sample size for ample statistical power to validate those SNPs in future studies for African American pediatric populations and explore potential SNP-SNP and gene-environment interactions. The process of planning to implement pharmacogenomic findings relating to BDR into emergency care should also begin to ensure that results translate into clinical practice in order to improve outcomes for patients.
Supplementary Material
Supplemental Figure 1: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a high BDR (i.e., residing in the highest adjusted ΔPASS category)
Supplemental Figure 2: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a low BDR (i.e., residing in the lowest adjusted ΔPASS category)
Supplemental Figure 3: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a low BDR (i.e., residing in the lowest adjusted ΔFEV/FVC % predicted category)
Supplemental Figure 4: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a high BDR (i.e., residing in the lowest adjusted ΔFEV/FVC % predicted category)
Supplemental Figure 5: Adjusted odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a low BDR (i.e., residing in the lowest adjusted ΔFEV/FVC % predicted category)
Acknowledgements:
The authors acknowledge Alexander Parker, PhD, and Kimberly Vigal, MHA for their programmatic support through the UF Jacksonville College of Medicine Office of Research Affairs. The authors acknowledge the assistance of research coordinators from the UF Jacksonville Department of Emergency Medicine and staff from the UF Health Jacksonville Pediatric Emergency Department.
Funding:
Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1 TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Asthma Facts: CDC’s National Asthma Control Program Grantees. 2013Available at https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf.Accessed September 26, 2018.
- 2.Centers for Disease Control and Prevention. 2015 National Health Interview Survey Data. Available at: https://www.cdc.gov/asthma/most_recent_data.htm.Accessed September 26, 2018.
- 3.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 4.Newacheck PW, Halfon N. Prevalence, impact, and trends in childhood disability due to asthma. Arch Pediatr Adolesc Med. 2000;154(3):287–93. [DOI] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;12(32):1–14. [PubMed] [Google Scholar]
- 6.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123Suppl 3:S131–45. [DOI] [PubMed] [Google Scholar]
- 7.Akimbami L “Asthma prevalence, health care use and mortality: United States, 2003–05.” 2015. Available from: http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed April 13, 2019.
- 8.Naqvi M, Thyne S, Choudhry S, et al. Ethnic-specific differences in bronchodilator responsiveness among African Americans, Puerto Ricans, and Mexicans with asthma. J Asthma. 2007;44(8):639–48. [DOI] [PubMed] [Google Scholar]
- 9.Burchard EG, Avila PC, Nazario S, et al. “Lower bronchodilator responsiveness in Puerto Rican than in Mexican subjects with asthma.” Am J Respir Crit Care. 2005; 171(6):563–70. [DOI] [PubMed] [Google Scholar]
- 10.Gorina Y “QuickStats: asthma death rates, by race and age group – United States, 2007–2009. MMWR. Centers for Disease Control and Prevention. 2012. [Google Scholar]
- 11.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 12.Sneller H, Carroll CL, Welch K, Sturm J. “Differentiating non-responders from responders in children with moderate and severe asthma exacerbations.” J Asthma. DOI: 10.1080/02770903.2019.1579343. [DOI] [PubMed] [Google Scholar]
- 13.McGeachie MJ, Stahl EA, Himes BE, et al. “Polygenic heritability estimates in pharmacogenetics: focus on asthma and related phenotypes.” Pharmacogenet Genomics. 2013;23:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieminem MM, Kaprio J, Koskenvuo M. “A population-based study of bronchial asthma in adult twin pairs.” Chest. 1991;100:70–75. [DOI] [PubMed] [Google Scholar]
- 15.Fagnani C, Annesi-Maesano I, Brescianini S, et al. “Heritability and shared genetic effects of asthma and hay fever: an Italian study of young twins.” Twin Res Hum Genet. 2008;11:121–31. [DOI] [PubMed] [Google Scholar]
- 16.Drake KA, Torgerson DG, Gignoux CR, et al. “A genome-wide association study of bronchodilator response in Latinos implicates rare variants.” J Allergy Clin Immunol. 2014;133(2):370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blake KV, Madabushi R, Derendorf H, Lima J. “Population pharmacodynamic model of bronchodilator response to inhaled albuterol in children and adults with asthma.” Chest. 2008;134(5):981–989. [DOI] [PubMed] [Google Scholar]
- 18.Engelgau MM, Khoury MJ, Roper RA, Curry JS, Mensah GA. “Predictive analytics: Helping guide the implementation research agenda at the National Heart, Lung, and Blood Institute.” Glob Heart. 2019;14(1):75–79. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno T, Dong M, Taylor ZL, Ramsey LB, Vinks VA. “Clinical implementation of pharmacogenetics and model-informed precision dosing to improve patient care.” Br J Clin Pharmacol. June 2020. DOI: 10.1111/bcp.14426. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Ducharme FM, Zemek R, Gravel J, et al. “Determinants of oral corticosteroid responsiveness in wheezing asthmatic youth (DOORWAY): protocol for a prospective multicentre cohort study of children with acute moderate-to-severe asthma exacerbations.” BMJ Open. 2014;4:e004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez FD, Graves PE, Baldini M, et al. “Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing.” J Clin Invest. 1997;100:3184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mak ACY, White MJ, Eckalbar WL, et al. “Whole-genome sequencing of pharamacogenetic drug response in racially diverse children with asthma.” Am J Respir Crit Care Med. 2018;197(12):1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padhukasahasram BK, Yang JJ, Levin AM, et al. “Gene-based association identifies SPATA13-AS1 as a pharmacogenomic predictor of inhaled short-acting beta-agonist response in multiple population groups.” Pharmacogenomics J. 2014;14(4):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear ML, Hu D, Pino-Yanes M, et al. “A genome-wide association and admixture mapping study of bronchodilator drug response in African Americans with asthma.” Pharmacogenomics J. 2018. DOI: 10.1038/s41397-018-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ierodiakonou D, Coull BA, Zanobetti A, Postma DS, Boezen HM, Vonk JM, et al. Pathway analysis of a genome-wide gene by air pollution interaction study in asthmatic children. Journal of Exposure Science and Environmental Epidemiology. 2019;29:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scaparrotta A, Franzago M, Marcovecchio ML, Di Pillo S, Chiarelli F, Mohn A, Stuppia L. Role of THRB, ARG1, and ADRB2 Genetic Variants on Bronchodilators Response in Asthmatic Children. J Aerosol Med Pulm Drug Deliv. 2019;32(3):164–173. [DOI] [PubMed] [Google Scholar]
- 27.Tse SM, Krajinovic M, Chauhan BF, Zemek R, Gravel J, Chalut D, et al. Genetic determinants of acute asthma therapy response in children with moderate-to-severe asthma exacerbations. Pediatr Pulmonol. 2019;54(4):378–385. [DOI] [PubMed] [Google Scholar]
- 28.Sordillo JE, McGeachie M, Lutz SM, Lasky-Su J, Tantisira K, Tsai CH, et al. Longitudinal analysis of bronchodilator response in asthmatics and effect modification of age-related trends by genotype. Pediatr Pulmonol. 2019;54(2):158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelick MH, Stevens MW, Schultz TR, Scribano PV. “Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma.” Acad Emerg Med. 2004;11(1):10–8. [DOI] [PubMed] [Google Scholar]
- 30.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update: An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watnick C, Arnold DH, Latuska R, O’Connor M, Johnson DP. “Initiative to reduce chest x-ray use for pediatric patients with acute asthma exacerbations. Pediatrics. Jan 2018, 141 (1 MeetingAbstract) 147; DOI: 10.1542/peds.141.1_MeetingAbstract.147 [DOI] [Google Scholar]
- 32.Ayuk AC, Uwaezouke SN, Ndukwu CI, Ndu IK, Iloh KK, Okoli CV. “Spirometry in Asthma Care: A Review of the Trends and Challenges in Pediatric Practice.” Clinical Medicine Insights: Pediatrics. 2017;11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a high BDR (i.e., residing in the highest adjusted ΔPASS category)
Supplemental Figure 2: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a low BDR (i.e., residing in the lowest adjusted ΔPASS category)
Supplemental Figure 3: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a low BDR (i.e., residing in the lowest adjusted ΔFEV/FVC % predicted category)
Supplemental Figure 4: Crude odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a high BDR (i.e., residing in the lowest adjusted ΔFEV/FVC % predicted category)
Supplemental Figure 5: Adjusted odds-ratio (x-axis) for association between having alternative allele in SNPs of interest and patient having a low BDR (i.e., residing in the lowest adjusted ΔFEV/FVC % predicted category)