Abstract
BACKGROUND/OBJECTIVES
Limited knowledge exists regarding sex differences in prescribing potentially inappropriate medications (PIM) for various multimorbidity patterns. This study sought to determine sex differences in PIM prescribing in older adults with cardiovascular-metabolic patterns.
DESIGN
Retrospective cohort study.
SETTING
Health and Retirement Study (HRS) 2004-2014 interview data, linked to HRS-Medicare claims data annualized for 2005-2014.
STUDY SAMPLE
6,341 Health and Retirement Study (HRS) participants ≥65 y/o with two and more chronic conditions.
MEASUREMENTS
PIM events were calculated using 2015 American Geriatrics Society Beers Criteria. Multimorbidity patterns included: ‘cardiovascular-metabolic only’, ‘cardiovascular-metabolic plus other physical conditions’, ‘cardiovascular-metabolic plus mental conditions’, and ‘no cardiovascular-metabolic disease’ patterns. Logistic regression models were used to determine the association between PIM and sex, including interaction between sex and multimorbidity categories in the model, for PIM overall and for each PIM drug class.
RESULTS
Women were prescribed PIMs more often than men (39.4% vs 32.8%). Overall, women had increased odds of PIM (Adj. OR = 1.30, 95% CI: 1.16–1.46). Women had higher odds of PIM than men with cardiovascular-metabolic plus physical patterns (Adj.OR=1.25, 95% CI: 1.07-1.45) and cardiovascular-metabolic plus mental patterns (Adj.OR=1.25, 95% CI: 1.06-1.48), and there were no sex differences in adults with a cardiovascular-metabolic only patterns (Adj.OR=1.13, 95% CI: 0.79-1.62). Women had greater odds of being prescribed the following PIMs: anticholinergics, antidepressants, antispasmodics, benzodiazepines, skeletal muscle relaxants, and had lower odds of being prescribed pain drugs and sulfonylureas compared to men.
CONCLUSION
This study evaluated sex differences in PIM prescribing among adults with complex cardiovascular-metabolic multimorbidity patterns. The effect of sex varied across multimorbidity patterns and by different PIM drug classes. This study identified important opportunities for future interventions to improve medication prescribing among older adults at risk for PIM.
Keywords: potentially inappropriate medications, sex differences, multimorbidity patterns, older adults
INTRODUCTION
Over 70% of older adults living with two or more chronic conditions—or multimorbidity1, 2—often have complex medication regimens, greater healthcare utilization,3, 4 and incur significantly higher healthcare costs.5 Providers have the difficult task making informed clinical decisions around prescribed medications to manage multimorbidity. While women, on average, have more chronic conditions than men6, 7 and likely use more medications,8, 9 prior studies are inconsistent regarding sex differences in medication prescribing patterns.8, 10, 11 Uncovering the multimorbidity and medication prescribing patterns associated with adverse and avoidable outcomes will help inform efforts to provide care that is timely, appropriate, and minimizes costs associated with ineffective health care use.
Depending on chronic disease burden, high polypharmacy levels may not equate to poor quality care. Instead, a more useful clinical concept is the use of potentially inappropriate medications (PIMs) —defined as “a drug in which the risk of an adverse event outweighs its clinical benefit, particularly when there is a safer or more effective alternate therapy for the same condition.” 12 PIM prescribing is more common among women13 and older patients with cardiovascular conditions,14 especially in the presence of multimorbidity.15 While cardiovascular disease is more prevalent among men, women have worse cardiovascular-related health outcomes.16 A number of biological factors contribute to this difference:17 however, disparities in cardiovascular care and lack of appropriate treatment of cardiovascular disease in women compared to men18 may be contributing factors. Individuals with multimorbidity are often excluded from clinical trials, and older women are under-represented in clinical trials, especially for cardiovascular disease treatment.19
There is a gap in understanding the role of sex on the complexity of medication use in the context of multimorbidity, particularly for cardiovascular-metabolic conditions. Identifying sex differences in PIM prescribing in older adults with multimorbidity is critical to promote a sex-appropriate, tailored approach to medical care. Therefore, the purpose of this study was to determine sex differences in PIM prescribing across various cardiovascular-metabolic multimorbidity patterns among older adults. We hypothesized that women with complex patterns of cardiovascular-metabolic plus other conditions have greater odds for PIM prescribing compared to men. In addition, we hypothesized that women have greater odds of being prescribed certain PIMs compared to men.
METHODS
Study design and data source
This study is a secondary analysis of the Health and Retirement Study (HRS) data, linked to Medicare claims. The HRS is an ongoing, nationally-representative longitudinal study of community-dwelling middle-aged and older adults in the US.20 For this study, we analyzed HRS interview data from 2004-2014, and linked annualized HRS-Medicare claims from 2005-2014. The study protocol was approved by Oregon Health & Science University Institutional Review Board.
Study Sample
The study sample included HRS participants aged 65 and over who agreed to link their interview responses with Medicare claims data (n=24,066 participants, Figure 1). We excluded participants without multimorbidity (i.e. < 2 chronic conditions) at baseline, and those with missing race/ethnicity data, and those who were identified as Asian, American Indian, or “Other” race due to small number of participants in those categories, as their inclusion would provide insufficient power to analyze these groups. The final analytic sample consisted of 6,341 participants.
Figure 1: Flow chart of study population selection.
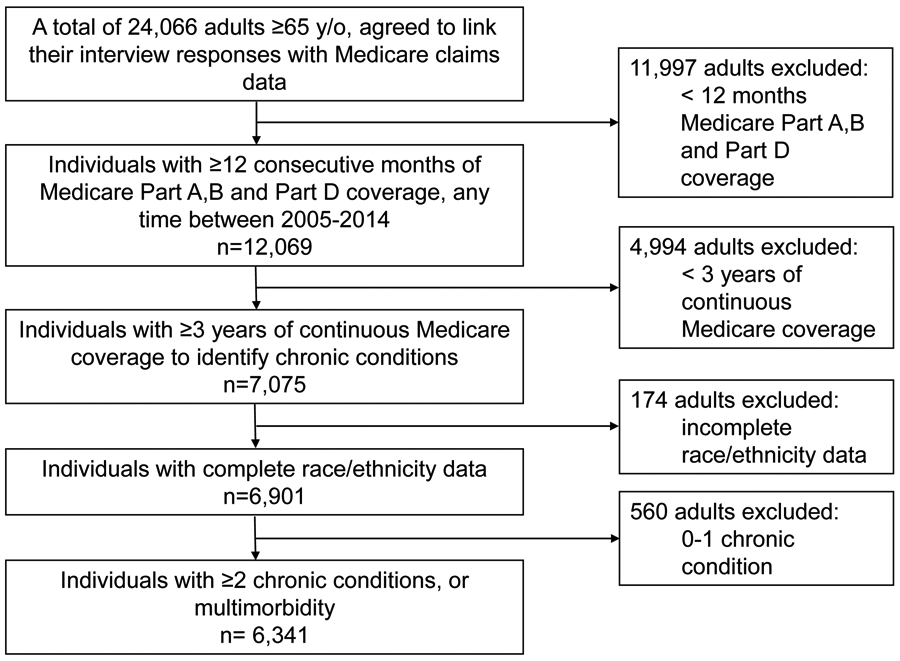
As Medicare claims data were annualized, and follow-up spanned a 10-year period, inclusion of participant information was assessed for each year of Medicare enrollment. For a year to be included in our study, we required participants to have 12 consecutive months of Medicare Parts A, B and Part D coverage, any time between 2005- 2014. A number of our chronic conditions of interest, as defined by the CMS Chronic Conditions Data Warehouse, required at least three years of Medicare coverage in order to assign a diagnosis (e.g. dementia).25
Measures
Dependent variable - PIM prescribing
We identified PIMs according to the 2015 Beers Criteria,21 and focused on the list of medications to avoid in adults 65 years and older with no exceptions.22 Briefly, we used National Drug Codes (NDC) associated with Medicare Part D claims to assign each medication a drug class and a generic drug name (approximately 1.2% of drugs remained unknown after this process); we used generic drug names to identify PIMs. Of the 138 single-formulation drugs on the list, we were unable to determine the appropriateness for 57 drugs, as their appropriateness was dependent on clinical factors that could not be determined from Medicare claims data (e.g. insulin dosed on a sliding scale). The final list of medications is provided in Supplementary Table S1. For each patient and for each year, we calculated the total number of PIMs prescribed. We then constructed our dichotomous primary dependent variable (at least one PIM versus no PIM).23 We further created a series of dichotomous variables indicating prescription of specific PIM drug class (e.g. prescription of anticholinergics vs. no prescription of anticholinergics).
Independent Variables
Sex.
Male sex, as self-reported in HRS, was our main dichotomous independent variable of interest (male vs. female).
Chronic conditions.
We identified participants’ annual diagnosis status for twenty chronic conditions prioritized for measuring multimorbidity based on criteria for prevalence, chronicity, and being amenable to public health or clinical intervention.24 To identify a chronic diagnosis, we used CMS Chronic Conditions Data Warehouse algorithms to indicate a clinical diagnosis using Medicare claims data.25 The 20 chronic conditions included: arthritis, asthma, autism spectrum disorder, cancer, cardiac arrhythmias, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), congestive heart failure, coronary artery disease, dementia (including Alzheimer’s and other senile dementias), depression, diabetes, hepatitis, hyperlipidemia, hypertension, human immunodeficiency virus (HIV), osteoporosis, schizophrenia, stroke, substance use disorders (drug and alcohol). We utilized a conceptual framework of multimorbidity that defines chronicity of health conditions. 24, 26 Based on this framework, once a diagnosis was indicated by the CMS Chronic Conditions Data Warehouse algorithm, we assumed a participant continued to have that disease at all subsequent years they were under observation.
As all participants in our study had multimorbidity, we were able to classify individuals into mutually-exclusive multimorbidity categories comprised of: 1) cardiovascular-metabolic only (including any combination of: cardiac arrhythmias, congestive heart failure, coronary artery disease, diabetes, hyperlipidemia, hypertension, or stroke); 2) cardiovascular-metabolic plus discordant physical (including combinations of ≥1 cardiometabolic disease and ≥1 discordant physical: arthritis, asthma, cancer, CKD, COPD, hepatitis, HIV, or osteoporosis); 3) cardiovascular-metabolic plus mental health (including combinations of ≥1 cardiometabolic disease and ≥1 mental: autism spectrum disorder, dementia, depression, schizophrenia, or substance abuse disorders; with or without a physical disease) and, 4) no cardiovascular-metabolic disease (arthritis, asthma, cancer, CKD, COPD, hepatitis, HIV, osteoporosis, autism spectrum disorder, dementia, depression, schizophrenia, or substance abuse disorders).
Covariates
Covariates shown to affect the likelihood of PIM prescribing included number of medications used and health services utilization indicators (annual, based on Medicare data), sociodemographic factors and health and lifestyle characteristics (biannual, based on recent HRS survey wave).22, 27 Total number of medications was calculated as simultaneous use of medications for ≥90 days in a given calendar year and constructed as a count variable. Polypharmacy was defined as simultaneous use of ≥5 such medications and constructed as a dichotomous variable. Health services utilization covariates included annual number of all-cause ED visits, annual number of all-cause hospitalizations, and annual number of physician visits. Sociodemographic variables included: race/ethnicity (non-Hispanic White vs. Hispanic, and non-Hispanic Black), years of education, self-reported net worth (in US$), marital status (single vs. coupled), and geographical region based on U.S. Census Divisions (New England vs., Midwest, South, and West). Age (years) was calculated as of January 1 of each year using Medicare claims data. Health characteristics included body mass index (BMI) categorized as underweight/normal (BMI≤25) vs. overweight (BMI=25-30), and obese (BMI>30). Additional health characteristics were current tobacco use (yes/no), current alcohol use (yes/no).
Missing data
While HRS participants complete surveys biannually, we structured the Medicare administrative claims data annually. To map HRS survey responses onto the Medicare data, we carried forward survey responses to the HRS off-years. Additionally, if a respondent did not participate in a HRS wave due to death or other reason, we carried forward or pulled back information from an adjacent wave (one wave previous or subsequent) to obtain covariate variables.
Statistical Analysis
We used descriptive methods to examine baseline characteristics, baseline chronic condition distributions, and baseline prescription of PIM drug classes in the study population, overall and by sex. We calculated frequencies and percents for categorical variables, and standard deviations for continuous variables; respectively, chi-squared and T-tests were used to test for differences in baseline characteristics according to sex.
To assess the association between prescription of PIM and sex, we created a series of nested logistic regression models. Our first model was the unconditional association between PIM and sex. The second model included covariates for sustained medication use and healthcare utilization. Our third logistic regression model additionally included demographic and health characteristics. We also determined if the association between PIM and sex varied by multimorbidity category. To do this, we added an interaction between sex and multimorbidity category into our aforementioned models. In all models, we used a cluster robust variance estimator to account for the multiple measurements of participants over the study period.
We further explored the association between sex and prescription of each PIM drug class. As detailed above, we created a series of nested logistic regression models, with and without an interaction between sex and multimorbidity category. We present odds ratios (ORs) and associated 95% confidence intervals (CIs) for all models. Analyses were performed in Stata/MP, version 13.1 (College Station, TX: StataCorp LP).
RESULTS
Overall, the study sample included 6,341 participants, who had a mean age of 75.7 years (SD 8.1) at baseline and included 64.4% women (Table 1). Women more often reported lower net worth and were less likely to be partnered than men. At baseline, more men reported current alcohol use and being overweight or obese.
Table 1.
Characteristics of Study Population by Sex.
| Characteristic | Overall (n=6,341) |
Women (n=4,084) |
Men (n=2,257) |
p-value |
|---|---|---|---|---|
| Demographic | ||||
| Age at first interview, mean (SD) | 75.7 (8.1) | 76.1 (8.4) | 75.1 (7.5) | <0.001 |
| Age category at first interview, n (%) | <0.001 | |||
| 65-74 | 3317 (52.3) | 2078 (50.9) | 1239 (54.9) | |
| 75-84 | 1929 (30.4) | 1225 (30.0) | 704 (31.2) | |
| 85+ | 1095 (17.3) | 781 (19.1) | 314 (13.9) | |
| Age at last interview, mean (SD) | 79.4 (8.2) | 79.9 (8.5) | 78.5 (7.5) | <0.001 |
| Age category at last interview, n (%) | <0.001 | |||
| 65-74 | 2083 (32.8) | 1284 (31.4) | 799 (35.4) | |
| 75-84 | 2471 (38.9) | 1538 (37.6) | 933 (41.3) | |
| 85+ | 1787 (28.1) | 1262 (31.0) | 525 (23.3) | |
| Education level, mean (SD) | 11.7 (3.6) | 11.5 (3.3) | 11.9 (3.9) | 0.001 |
| Race/ethnicity group, n (%) | <0.001 | |||
| NH White | 4908 (77.4) | 3106 (76.1) | 1802 (79.8) | |
| NH Black | 906 (14.3) | 637 (15.6) | 269 (11.9) | |
| Hispanic | 527 (8.3) | 341 (8.3) | 186 (8.2) | |
| Wealth (net worth), mean (SD)1 | $491,581.9 ($1,412,825.9) | $391,879.4 ($1,179,712.7) | $675,151.4 ($1,748,725.4) | <0.001 |
| Married or partnered, n (%)1 | 3010 (50.5) | 1476 (38.3) | 1534 (73.1) | <0.001 |
| Geographical regions, n (%)1 | 0.812 | |||
| Northeast | 848 (15.1) | 557 (15.3) | 291 (14.7) | |
| Midwest | 1454 (25.9) | 952 (26.1) | 502 (25.4) | |
| South | 2593 (46.1) | 1669 (45.8) | 924 (46.7) | |
| West | 724 (12.9) | 464 (12.7) | 260 (13.2) | |
| Health/clinical | ||||
| BMI, n (%)1 | <0.001 | |||
| <25.0 | 1828 (34.3) | 1291 (37.8) | 537 (28.1) | |
| 25.0–29.9 | 1922 (36.1) | 1069 (31.3) | 853 (44.6) | |
| ≥30.0 | 1576 (29.6) | 1055 (30.9) | 521 (27.3) | |
| Current smoker, n (%)1 | 512 (9.2) | 302 (8.3) | 210 (10.7) | 0.003 |
| Current alcohol use, n (%)1 | 2146 (38.2) | 1172 (32.2) | 974 (49.3) | <0.001 |
| Multimorbidity clusters, n (%) | <0.001 | |||
| CVM2 only | 800 (12.6) | 389 (9.5) | 411 (18.2) | |
| CVM & no MHC & 1+ DD | 2880 (45.4) | 1791 (43.9) | 1089 (48.2) | |
| CVM & 1+ MHC | 2571 (40.5) | 1838 (45) | 733 (32.5) | |
| No CVM | 90 (1.4) | 66 (1.6) | 24 (1.1) | |
| Health care utilization | ||||
| Number of all-cause ED visits, mean (SD) | 0.8 (1.4) | 0.8 (1.4) | 0.8 (1.5) | 0.921 |
| Number of all-cause hospitalizations, mean (SD) | 0.5 (1) | 0.5 (1) | 0.5 (1.1) | 0.054 |
| Number of physician visits, mean (SD) | 11.6 (9.2) | 11.5 (9) | 11.7 (9.7) | 0.427 |
| Other | ||||
| Count of full years of enrollment, mean (SD) | 4.5 (2.9) | 4.7 (2.9) | 4.3 (2.8) | <0.001 |
| Ever proxy interview, n (%)1 | 1010 (18.7) | 656 (18.7) | 354 (18.6) | 0.916 |
| Deceased, n (%) | 2949 (46.5) | 1869 (45.8) | 1080 (47.9) | 0.111 |
| Medication use | ||||
| Total number of medications used (for ≥90 days during year), mean (SD) | 5.8 (4.5) | 5.9 (4.6) | 5.5 (4.4) | 0.004 |
| Polypharmacy (≥5 simultaneously used medications), n (%)3 | 2619 (41.3) | 1737 (42.5) | 882 (39.1) | 0.007 |
| Number of PIMs, mean (SD)4 | 0.9 (1.7) | 0.9 (1.8) | 0.7 (1.5) | <0.001 |
| Individuals with ≥1 PIM, n (%) | 2351 (37.1) | 1611 (39.4) | 740 (32.8) | <0.001 |
| Prevalence of ≥1 PIM by multimorbidity cluster, n (%) | <0.001 | |||
| CVM2 only | 208 (26) | 104 (26.7) | 104 (25.3) | |
| CVM & no MHC & 1+ DD | 912 (31.7) | 610 (34.1) | 302 (27.7) | |
| CVM & 1+ MHC | 1199 (46.6) | 875 (47.6) | 324 (44.2) | |
| No CVM | 32 (35.6) | 22 (33.3) | 10 (41.7) | |
Note: Calculations were based on baseline year for each individual.
Missing values due to non-participation in HRS at baseline wave. Missing net worth, n=444 female, n=280 male; coupled, n=226 female, n=159 male; region, n=442 female, n=280 male; BMI, n=669 female, n=346 male; smoking status, n=465 female, n=299 males; alcohol use, n=444 female, n=281 male.
CVM only – concordant cardiovascular-metabolic only pattern; CVM & no MHC & 1+ DD – cardiovascular-metabolic plus discordant physical conditions pattern; CVM & 1+ MHC- DD – cardiovascular-metabolic plus mental health conditions pattern; No CVM – no cardiovascular-metabolic patterns.
Only medications used for ≥90 days were considered in the definition of Polypharmacy
No minimum days used for PIM determination
Abbreviations: BMI (body mass index) was calculated according to the established formula (BMI = weight [pounds] x 703 / height^2 [inches]) using participants’ first self-reported height (HRS does not record height at each interview) and self-reported weight at each interview.
PIM-potentially inappropriate medication.
Almost all individuals in the study sample (data not shown) had at least one cardiovascular-metabolic condition at baseline (98.6%), with the most prevalent conditions for both sexes being hypertension (89.7%), hyperlipidemia (80%) and diabetes (41.2%). At baseline, a higher percentage of men had a cardiovascular-metabolic only pattern (Table 1), as well as a cardiovascular-metabolic plus discordant physical pattern. Women had a higher prevalence of a cardiovascular-metabolic plus mental health pattern.
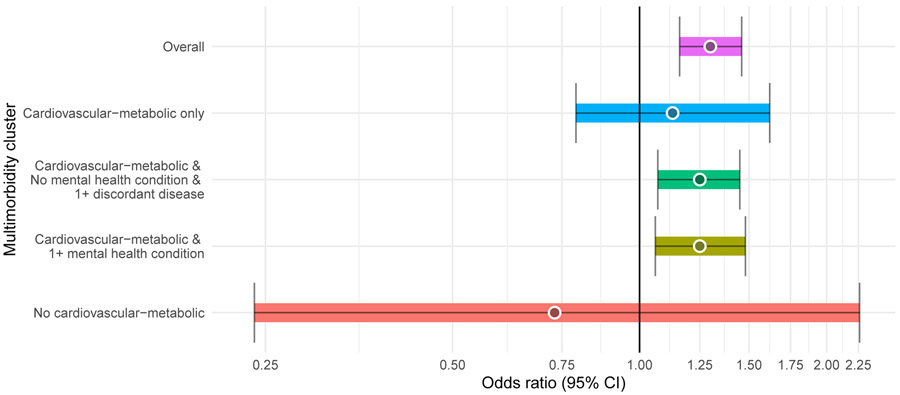
Women were prescribed PIMs more often than men (32.8% of men and 39.4% of women) and women were prescribed a higher number of PIMs at baseline year (Table 1). Overall, PIM prescribing declined with age: from 39.2% in 65-74 y/o group to 33.8% in 85 years and older group. However, there were zero-to-minimal shifts in PIM prescribing by age across several PIM drug classes (Supplementary Table S2). After controlling for covariates, women had increased odds of PIMs (OR= 1.30, 95% CI: 1.16-1.46; Table 2) regardless of multimorbidity patterns. Women with cardiovascular-metabolic plus discordant physical patterns had higher odds of PIMs than men in the same multimorbidity category (OR=1.25, 95% CI: 1.07-1.45; Figure 2). Likewise, women who had cardiovascular-metabolic plus mental health patterns had higher odds of PIMs than their male peers (OR=1.25, 95% CI: 1.06-1.48). Participants who had a cardiovascular-metabolic only patterns did not exhibit sex differences (OR=1.13, 95% CI: 0.79-1.62).
Table 2.
Unadjusted and Adjusted Odds Ratios for Prescribing at Least One Potentially Inappropriate Medication.
| Variable | Model: Sex | Model: Multimorbidity cluster by Sex Interaction |
||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Medicare- Adjusted OR (95% CI) |
Fully- Adjusted OR (95% CI)1 |
Unadjusted OR (95% CI) |
Medicare- Adjusted OR (95% CI) |
Fully- Adjusted OR (95% CI)1 |
|
| Sex | ||||||
| Men | ref | ref | ref | - | - | - |
| Women | 1.38 (1.26, 1.52) | 1.38 (1.26, 1.52) | 1.30 (1.16, 1.46) | - | - | - |
| Multimorbidity clusters 2 by Sex interaction | ||||||
| CVM only | ||||||
| Men (n=1,357) | - | - | - | ref | ref | ref |
| Women (n=1,114) | - | - | - | 1.09 (0.80, 1.48) | 1.11 (0.82, 1.51) | 1.13 (0.79, 1.62) |
| CVM & no MHC & 1+ DD | ||||||
| Men (n=4,752) | - | - | - | ref | ref | ref |
| Women (N=8,127) | - | - | - | 1.29 (1.13, 1.47) | 1.33 (1.17, 1.52) | 1.25 (1.07, 1.45) |
| CVM & 1+ MHC | ||||||
| Men (n=3,579) | - | - | - | ref | ref | ref |
| Women (n=9,605) | - | - | - | 1.25 (1.10, 1.42) | 1.31 (1.14, 1.49) | 1.25 (1.06, 1.48) |
| No CVM | ||||||
| Men (n=45) | - | - | - | ref | ref | ref |
| Women (n=212) | - | - | - | 0.58 (0.22, 1.56) | 0.63 (0.22, 1.75) | 0.73 (0.24, 2.26) |
| Control variables | ||||||
| Medication use | ||||||
| Total number of medications used (for ≥90 days during year) | 1.07 (1.06, 1.08) | 1.07 (1.06, 1.08) | 1.07 (1.06, 1.08) | 1.06 (1.05, 1.07) | ||
| Health care utilization | ||||||
| Number of all-cause ED visits | ||||||
| 0 | ref | ref | ref | ref | ||
| 1 | 1.24 (1.15, 1.34) | 1.24 (1.14, 1.36) | 1.20 (1.11, 1.30) | 1.21 (1.11, 1.32) | ||
| ≥2 | 1.58 (1.43, 1.75) | 1.58 (1.40, 1.78) | 1.47 (1.32, 1.63) | 1.47 (1.31, 1.66) | ||
| Number of all-cause hospitalizations | ||||||
| 0 | ref | ref | ref | ref | ||
| 1 | 1.06 (0.98, 1.15) | 1.08 (0.98, 1.18) | 1.04 (0.96, 1.12) | 1.06 (0.97, 1.17) | ||
| ≥2 | 0.96 (0.85, 1.09) | 0.98 (0.85, 1.14) | 0.93 (0.82, 1.06) | 0.96 (0.83, 1.12) | ||
| Number of physician visits | 1.03 (1.02, 1.03) | 1.03 (1.02, 1.04) | 1.02 (1.02, 1.03) | 1.03 (1.02, 1.03) | ||
| Demographic/SES | ||||||
| Age, years | ||||||
| 65-74 | ref | ref | ||||
| 75-84 | 0.77 (0.70, 0.85) | 0.74 (0.67, 0.82) | ||||
| 85+ | 0.58 (0.51, 0.67) | 0.55 (0.47, 0.63) | ||||
| Education, years | 0.96 (0.95, 0.98) | 0.97 (0.95, 0.98) | ||||
| Race/Ethnicity group | ||||||
| NH white | ref | ref | ||||
| NH black | 0.88 (0.74, 1.04) | 0.89 (0.75, 1.05) | ||||
| Hispanic | 1.05 (0.86, 1.28) | 1.02 (0.84, 1.25) | ||||
| Marital status, Coupled | 0.86 (0.77, 0.96) | 0.89 (0.80, 1.00) | ||||
| Geographical regions | ||||||
| Northeast | ref | ref | ||||
| Midwest | 1.07 (0.91, 1.27) | 1.08 (0.92, 1.28) | ||||
| South | 1.37 (1.18, 1.58) | 1.37 (1.19, 1.59) | ||||
| West | 1.32 (1.09, 1.59) | 1.30 (1.08, 1.57) | ||||
| Health/clinical characteristics | ||||||
| BMI | ||||||
| <25.0 | ref | ref | ||||
| 25.0–29.9 | 0.93 (0.83, 1.05) | 0.95 (0.85, 1.06) | ||||
| ≥30.0 | 0.90 (0.80, 1.03) | 0.93 (0.82, 1.05) | ||||
| Current smoker | 0.96 (0.81, 1.13) | 0.92 (0.78, 1.09) | ||||
| Alcohol use | 0.93 (0.85, 1.03) | 0.96 (0.87, 1.06) | ||||
Note: Multicollinearity was assessed, and to reduce variance inflation, we Bayesian Information Criterion (BIC) was used to discern the best fitting model; this led to the removal of polypharmacy and net worth from adjusted models. Model errors were corrected for potential repeated measures of a beneficiary using cluster robust standard errors.
Number of observations was reduced to 21,130; female n=13,915; male n=7,215
CVM only – concordant cardiovascular-metabolic only pattern; CVM & no MHC & 1+ DD – cardiovascular-metabolic plus discordant physical conditions pattern; CVM & 1+ MHC- DD – cardiovascular-metabolic plus mental health conditions pattern; No CVM – no cardiovascular-metabolic patterns.
Postestimation predicted values from logistic regressions are reported for interaction term.
Values in bold are statistically significant at p = 0.05.
Figure 2: Potentially inappropirate medication (PIM): OR, female vs. male beneficiaries.
Logistic regression model with the interaction term between sex and multimorbidity patterns was conducted to estimate sex differences in PIM prescribing. Model was adjusted for: number of medications used ≥90 days, number of all-cause ED visits, number of all-cause hospitalizations, number of physician visits and count of chronic disease, age, years of education, race/ethnicity, marital status, region, body mass index, current smoking status, and current alcohol use. Statistically significant at p = 0.05
The most commonly-prescribed PIM drug classes were anticholinergics, antidepressants, benzodiazepines, and skeletal muscle relaxants (Table 3). Women had greater odds of being prescribed the following PIMs: anticholinergics, antidepressants, antispasmodics, benzodiazepines, and skeletal muscle relaxants (Table 3). Women also had lower odds of being prescribed pain drugs and sulfonylureas. Differences in prescription of PIM drug classes by sex and multimorbidity categories are shown in Supplementary Table S3.
Table 3.
Prevalence of Potentially Inappropriate Drug Classes, Unadjusted and Adjusted Sex Differences in Odds of prescribing at Least One Potentially Inappropriate Drug Class.
| PIM category | Prevalence of PIM1, % | Unadjusted OR (women vs men (ref.) |
Fully- Adjusted OR women vs men (ref.)2 |
||||
|---|---|---|---|---|---|---|---|
| Overall | Women | Men | Women vs men, % |
p-value | |||
| Any PIM | 37.1 | 39.4 | 32.8 | +6.6 | <0.001 | 1.38 (1.26, 1.52) | 1.30 (1.16, 1.46) |
| PIM category | |||||||
| Anticholinergics | 10.2 | 11.5 | 7.9 | +3.6 | <0.001 | 1.55 (1.36, 1.78) | 1.51 (1.28, 1.78) |
| Antidepressants | 6.3 | 7.6 | 4.0 | +3.6 | <0.001 | 2.35 (1.86, 2.97) | 2.17 (1.64, 2.87) |
| Antispasmodics | 4.0 | 4.7 | 2.6 | +2.1 | <0.001 | 1.60 (1.26, 2.02) | 1.53 (1.12, 2.08) |
| Antithrombotics | 1.2 | 1.2 | 1.2 | 0 | 0.872 | 0.97 (0.62, 1.52) | 0.71 (0.41, 1.22) |
| Benzodiazepines | 10.6 | 10.9 | 9.9 | +1.0 | 0.217 | 1.24 (1.08, 1.43) | 1.33 (1.12, 1.59) |
| Cardiovascular | 2.7 | 2.9 | 2.4 | +0.5 | 0.226 | 1.35 (0.95, 1.90) | 1.16 (0.79, 1.69) |
| Megestrol | 1.8 | 1.7 | 1.9 | −0.2 | 0.499 | 1.02 (0.80, 1.31) | 0.88 (0.61, 1.26) |
| Pain drugs | 2.8 | 2.5 | 3.3 | −0.8 | 0.049 | 0.63 (0.52, 0.77) | 0.64 (0.50, 0.82) |
| Skeletal muscle relaxants | 6.4 | 7.4 | 4.7 | +2.7 | <0.001 | 1.58 (1.34, 1.85) | 1.57 (1.29, 1.90) |
| Sulfonylureas | 3.5 | 3.1 | 4.3 | −1.2 | 0.021 | 0.69 (0.52, 0.92) | 0.54 (0.38, 0.76) |
Note: Drug types with >1% prevalence of use were considered for these analyses.
PIM-potentially inappropriate medication.
The logistic regression models were conducted for each PIM class separately. Models were adjusted for: number of medications used >=90 days, number of all-cause ED visits, number of all-cause hospitalizations, number of physician visits and count of chronic disease, age group, years of education, race/ethnicity, marital status, region, BMI, current smoking status, and current alcohol use. Statistically significant at p = 0.05. Values in bold are statistically significant at p = 0.05.
DISCUSSION
This study sought to characterize sex differences in the association between specific multimorbidity categories and PIM prescribing in a national sample of community-dwelling older adults in the United States. Overall, the prevalence of PIM prescribing in this study was similar to what has been previously reported in the literature.22, 28-30 We found women had higher odds of PIM prescribing. Previous studies reported a similar association16, 29, 31; however, a few studies have reported that men are at higher odds of being prescribed a PIM.32, 33 After taking health status into consideration and comparing risk of PIM prescribing across prevalent multimorbidity combinations, we observed that older women with cardiovascular-metabolic plus discordant physical patterns and cardiovascular-metabolic plus mental health patterns were at approximately 25% greater odds of receiving PIM compared to men with the same patterns, although the absolute differences in PIM prevalence were 6.4% and 3.4% respectively. Further, there were no sex differences in PIM prescribing among individuals with cardiovascular-metabolic only patterns.
There is no clear evidence how chronic diseases, whether concordant or discordant, affect treatment of patients with cardiovascular-metabolic conditions. While concordant diseases share parts of common pathophysiologic processes and treatment approaches with an index or set of diseases, discordant diseases do not have these pathophysiologic commonalities nor necessarily overlap in medication treatment regimens. Previous studies reported that patients with diabetes and concordant comorbidities were more adherent to treatment regimes,34, 35 diabetes-discordant comorbidities negatively affected diabetes self-care behaviors e.g. dieting,34 and patients with discordant mental health comorbidity reported lower scores of various dimensions of coordinated care.36 Regardless, the presence of concordant or discordant conditions may require management that increases the risk of polypharmacy and adverse drug events.37, 38 Still, there is little known about how underlying comorbid conditions affect the risk of PIM prescribing. In our sample, women and men had relatively similar numbers of chronic conditions in each pattern, but there were sex differences in the prevalence of multimorbidity patterns: cardiovascular-metabolic only patterns were more common among men, and cardiovascular-metabolic plus mental health patterns were more common among women. While a number of studies recognize biological sex differences in cardiovascular medication treatment, 39,40 and women are often undertreated17, we did not observe significant differences in PIM prescribing in individuals with cardiovascular-metabolic only patterns. These patterns had lower prevalence of PIMs compared to the other multimorbidity groups for both sexes. Indeed, many of the most common medications used to treat psychiatric conditions and pain are PIMs, whereas medications most often used to treat cardiovascular-metabolic disorders are infrequently PIMs. It is likely that concordant conditions may require fewer providers to manage them, and for patients with a usual source of care, their providers may have more opportunities to evaluate prescribing regimens.
In more complex multimorbidity patterns with discordant physical or mental health conditions, there was a higher risk for PIMs in women compared to men. This may be explained by different health-seeking behavior and patterns of healthcare services use. A previous study reported that, on average, women use more healthcare services than men and may be at higher risk of fragmented care, especially in the presence of mental health disorders.41 Further, prior research reported that women are more likely to be treated for mental health conditions than men,11 which may partially explain our observations. However, medication evaluation and timely deprescribing of PIMs should be the standard of practice to decrease PIMs in both sexes.
To further evaluate sex differences in PIM prescribing we evaluated classes of PIMs. We found women had higher odds of being prescribing anticholinergics, antidepressants, antispasmodics, benzodiazepines, and skeletal muscle relaxants (absolute differences in PIMs prevalence varied between 1.0% and 3.6%), while pain drugs and sulfonylureas had only slightly higher prescribing rates in men (absolute differences in PIMs prevalence varied between 0.8% and 1.2%). Thus, risk of PIM was associated with the presence of mental health or discordant physical conditions, rather than cardiovascular conditions alone. Women are reported to use sleep-inducing drugs, antidepressants, and analgesics more frequently, possibly due to higher rates of mental health conditions.42 Previous studies reported sex differences in prescription patterns: men were less frequently prescribed antidepressants than women, possibly signaling less preference for pharmacotherapies in treating depression, 11 and women had higher risk for PIM, especially for benzodiazepines and other hypnotics, tertiary tricyclic antidepressants and for non-selective NSAIDs, muscle relaxants, first-generation antihistamines and nitrofurantoin.29 Social factors such as age, marital status, education and cultural differences also influence patient attitudes toward these medications. For example, difference in antidepressant use may be explained by patients’ own preferences and willingness to ask for, or agree to take them; and by provider’s perception of risk and benefits regarding their prescription.43 Future research is needed to further evaluate the impact of provider factors on sex differences in PIM prescribing.
Policy/Practice implementation
Our results suggest that older adults have high level of exposure to PIM. There is a need for more interventions to change prescriber behavior to further reduce PIMs prescribing. Polypharmacy is the strongest predictor of PIM use, thus to improve quality and safety of medication use, prescribing strategies are needed to reduce certain types of PIM use, especially focusing on common PIM classes such as anticholinergic and psychotropic medications.28
Medications from the Beer’s Criteria list might be safely deprescribed to reduce risks of complications among elder population, especially in women.44, 45 There is a demonstrated need for provider education about deprescribing. Further, there is a need for greater awareness and understanding of the risks of PIM for older adults, and a need for better implementation and integration of deprescribing into primary care clinical workflow. Our study findings highlight the need for increased awareness of PIM prescribing in complex care cases, such as older adults with cardiovascular-metabolic multimorbidity combinations further complicated by the presence of discordant physical or mental health conditions. These findings emphasize the opportunity for improving coordination of care and communication between providers, including utilization of a single pharmacy to maintain accurate medication lists and enable safety checks on a regular basis; using EMR-enabled clinical decision support systems and responding to PIM alerts, referring patients for comprehensive medication reviews to identify PIMs that otherwise would not be recognized, and facilitating interdisciplinary teams with pharmacists.45
Our study further evaluated variation in PIM prescribing, overall and for individual drug classes across different multimorbidity groups. We also identified target populations for future care management programs focusing on prevalent PIM classes and patients with certain multimorbidity patterns.
Strengths and Limitations
This study used linked HRS and Medicare claims data from the study sample which can be extrapolated to national-representative population. These are robust, high-quality multiple year data, where self-reported information was complemented by claims records to minimize recall bias in reporting chronic conditions, medication use, and health services utilization. No studies to our knowledge have compared risk of PIM in concordant and discordant multimorbidity patterns in population with cardiovascular-metabolic conditions, which enabled better understanding of sex difference in PIM prescribing.
While use of HRS data have many advantages, this study has some limitations. We were not able to assess whether participants used supplemental health insurance with drug coverage or over-the-counter medication use (e.g. mineral oil), thus we may be underestimating PIM prevalence. However, the majority of over-the-counter PIMs were already excluded from the selection list. We were unable to assess if there was closer monitoring of health conditions during PIM prescribing, e.g. kidney function checks, to mitigate risks of adverse events. This may be important to contextualize why providers prescribed certain medications including PIMs, which may be justified in certain cases when clinical benefits outweigh the risk. We have applied 2015 AGS Beers criteria to determine PIMs, thus we may have overestimated the prevalence of certain PIMs which were not included in the criteria list in earlier years.
In conclusion, this study evaluated sex difference in PIM prescribing among individuals with cardiovascular-metabolic conditions. Older women with cardiovascular-metabolic plus discordant physical conditions and cardiovascular-metabolic plus mental health conditions were at only slightly higher risk of receiving PIM. There were no sex differences in PIM prescribing among individuals with cardiovascular-metabolic only patterns. In addition, there was variation in the effect of sex in prescribing different PIM drug classes. Thus, this study identified important opportunities for future interventions to eliminate PIM prescribing among older adults.
Supplementary Material
Supplementary Table S1: List of the Selected Potentially Inappropriate Medications.
Supplementary Table S2: Prevalence of Different Classes of Potentially Inappropriate Medications by Age Groups.
Supplementary Table S3: Differences in Prescribing of Potentially Inappropriate Drug Classes by Sex and Multimorbidity patterns.
Key Points.
There were no sex differences in PIM prescribing in the cardiovascular-metabolic only patterns.
Women with cardiovascular-metabolic and unrelated physical or mental health comorbidities had higher odds of PIM than men.
Men had slightly higher odds of prescribing certain PIM drug classes than women.
Why does this matter?
PIM prevalence remains high in women and men. There are sex differences in prescribed PIM in cardiovascular-metabolic plus discordant physical and mental health patterns. The study highlights the need to further improve medication prescribing, and identifies target groups for future interventions.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (R01AG055681; R01AG055681-02S2 to ARQ). Award revised to include co-funding from Office of Research on Women’s Health (ORWH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors have declared no conflicts of interest for this article.
REFERENCES
- 1.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. September2011;10(4):430–9. doi: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
- 3.Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. January2011;61(582):e12–21. doi: 10.3399/bjgp11X548929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. November112002;162(20):2269–76. doi: 10.1001/archinte.162.20.2269 [DOI] [PubMed] [Google Scholar]
- 5.Naessens JM, Stroebel RJ, Finnie DM, et al. Effect of multiple chronic conditions among working-age adults. Am J Manag Care. February2011;17(2):118–22. [PubMed] [Google Scholar]
- 6.Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7):e102149. doi: 10.1371/journal.pone.0102149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agur K, McLean G, Hunt K, Guthrie B, Mercer SW. How Does Sex Influence Multimorbidity? Secondary Analysis of a Large Nationally Representative Dataset. Int J Environ Res Public Health. March312016;13(4):391. doi: 10.3390/ijerph13040391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santalucia P, Franchi C, Djade CD, et al. Gender difference in drug use in hospitalized elderly patients. Eur J Intern Med. September2015;26(7):483–90. doi: 10.1016/j.ejim.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 9.Venturini CD, Engroff P, Ely LS, et al. Gender differences, polypharmacy, and potential pharmacological interactions in the elderly. Clinics (Sao Paulo). 2011;66(11):1867–72. doi: 10.1590/s1807-59322011001100004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loikas D, Wettermark B, von Euler M, Bergman U, Schenck-Gustafsson K. Differences in drug utilisation between men and women: a cross-sectional analysis of all dispensed drugs in Sweden. BMJ Open. 2013;3(5):e002378. doi: 10.1136/bmjopen-2012-002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thunander Sundbom L, Bingefors K, Hedborg K, Isacson D. Are men under-treated and women over-treated with antidepressants? Findings from a cross-sectional survey in Sweden. BJPsych Bull. June2017;41(3):145–150. doi: 10.1192/pb.bp.116.054270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corsonello A, Pranno L, Garasto S, Fabietti P, Bustacchini S, Lattanzio F. Potentially inappropriate medication in elderly hospitalized patients. Drugs Aging. December2009;26Suppl 1:31–9. doi: 10.2165/11534640-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 13.Rodenburg EM, Stricker BH, Visser LE. Sex differences in cardiovascular drug-induced adverse reactions causing hospital admissions. Br J Clin Pharmacol. December2012;74(6):1045–52. doi: 10.1111/j.1365-2125.2012.04310.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh-Taha M, Dimassi H. Potentially inappropriate home medications among older patients with cardiovascular disease admitted to a cardiology service in USA. BMC Cardiovasc Disord. July172017;17(1):189. doi: 10.1186/s12872-017-0623-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. May282019;73(20):2584–2595. doi: 10.1016/j.jacc.2019.03.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bierman AS, Pugh MJ, Dhalla I, et al. Sex differences in inappropriate prescribing among elderly veterans. Am J Geriatr Pharmacother. June2007;5(2):147–61. doi: 10.1016/j.amjopharm.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Woodward M Cardiovascular Disease and the Female Disadvantage. Int J Environ Res Public Health. April12019;16(7)doi: 10.3390/ijerph16071165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. November82011;124(19):2145–54. doi: 10.1161/circulationaha.110.968792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitale C, Fini M, Spoletini I, Lainscak M, Seferovic P, Rosano GM. Under-representation of elderly and women in clinical trials. Int J Cardiol. April12017;232:216–221. doi: 10.1016/j.ijcard.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 20.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol. April2014;43(2):576–85. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. November2015;63(11):2227–46. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 22.Davidoff AJ, Miller GE, Sarpong EM, Yang E, Brandt N, Fick DM. Prevalence of potentially inappropriate medication use in older adults using the 2012 Beers criteria. J Am Geriatr Soc. March2015;63(3):486–500. doi: 10.1111/jgs.13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez T, Moriarty F, Wallace E, McDowell R, Redmond P, Fahey T. Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. Bmj. November142018;363:k4524. doi: 10.1136/bmj.k4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. April252013;10:E66. doi: 10.5888/pcd10.120239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CMS Chronic Conditions Data Warehouse (CCW). CCW condition algorithms. www2.ccwdata.org
- 26.Parekh AK, Goodman RA, Gordon C, Koh HK. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. Jul-Aug 2011;126(4):460–71. doi: 10.1177/003335491112600403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GE, Sarpong EM, Davidoff AJ, Yang EY, Brandt NJ, Fick DM. Determinants of Potentially Inappropriate Medication Use among Community-Dwelling Older Adults. Health Serv Res. August2017;52(4):1534–1549. doi: 10.1111/1475-6773.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jirón M, Pate V, Hanson LC, Lund JL, Jonsson Funk M, Stürmer T. Trends in Prevalence and Determinants of Potentially Inappropriate Prescribing in the United States: 2007 to 2012. J Am Geriatr Soc. April2016;64(4):788–97. doi: 10.1111/jgs.14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan SG, Weymann D, Pratt B, et al. Sex differences in the risk of receiving potentially inappropriate prescriptions among older adults. Age Ageing. July2016;45(4):535–42. doi: 10.1093/ageing/afw074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark CM, Shaver AL, Aurelio LA, et al. Potentially Inappropriate Medications Are Associated with Increased Healthcare Utilization and Costs. J Am Geriatr Soc. August52020;doi: 10.1111/jgs.16743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnell K, Weitoft GR, Fastbom J. Sex differences in inappropriate drug use: a register-based study of over 600,000 older people. Ann Pharmacother. July2009;43(7):1233–8. doi: 10.1345/aph.1M147 [DOI] [PubMed] [Google Scholar]
- 32.Santos AP, da Silva DT, dos Santos Júnior GA, et al. Evaluation of the heterogeneity of studies estimating the association between risk factors and the use of potentially inappropriate drug therapy for the elderly: a systematic review with meta-analysis. Eur J Clin Pharmacol. September2015;71(9):1037–50. doi: 10.1007/s00228-015-1891-2 [DOI] [PubMed] [Google Scholar]
- 33.Bradley MC, Fahey T, Cahir C, et al. Potentially inappropriate prescribing and cost outcomes for older people: a cross-sectional study using the Northern Ireland Enhanced Prescribing Database. Eur J Clin Pharmacol. October2012;68(10):1425–33. doi: 10.1007/s00228-012-1249-y [DOI] [PubMed] [Google Scholar]
- 34.Aga F, Dunbar SB, Kebede T, Gary RA. The role of concordant and discordant comorbidities on performance of self-care behaviors in adults with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes. 2019;12:333–356. doi: 10.2147/dmso.s186758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnan EM, Palta M, Johnson HM, Bartels CM, Schumacher JR, Smith MA. The impact of a patient's concordant and discordant chronic conditions on diabetes care quality measures. J Diabetes Complications. March2015;29(2):288–94. doi: 10.1016/j.jdiacomp.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benzer JK, Singer SJ, Mohr DC, et al. Survey of Patient-Centered Coordination of Care for Diabetes with Cardiovascular and Mental Health Comorbidities in the Department of Veterans Affairs. J Gen Intern Med. May2019;34(Suppl 1):43–49. doi: 10.1007/s11606-019-04979-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luijks HD, Lagro-Janssen ALM, van Weel C. Multimorbidity and the primary healthcare perspective. J Comorb. 2016:46–49. vol. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients' treatment priorities and self-management? J Gen Intern Med. December2007;22(12):1635–40. doi: 10.1007/s11606-007-0313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeland U, Regitz-Zagrosek V. Sex and gender differences in cardiovascular drug therapy. Handb Exp Pharmacol. 2012;(214):211–36. doi: 10.1007/978-3-642-30726-3_11 [DOI] [PubMed] [Google Scholar]
- 40.Zhao M, Woodward M, Vaartjes I, et al. Sex Differences in Cardiovascular Medication Prescription in Primary Care: A Systematic Review and Meta-Analysis. J Am Heart Assoc. June22020;9(11):e014742. doi: 10.1161/jaha.119.014742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morden NE, Mistler LA, Weeks WB, Bartels SJ. Health care for patients with serious mental illness: family medicine's role. J Am Board Fam Med. Mar-Apr 2009;22(2):187–95. doi: 10.3122/jabfm.2009.02.080059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toepfer S, Bolbrinker J, König M, Steinhagen-Thiessen E, Kreutz R, Demuth I. Potentially inappropriate medication in older participants of the Berlin Aging Study II (BASE-II) - Sex differences and associations with morbidity and medication use. PLoS One. 2019;14(12):e0226511. doi: 10.1371/journal.pone.0226511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weisse CS, Sorum PC, Sanders KN, Syat BL. Do gender and race affect decisions about pain management? J Gen Intern Med. April2001;16(4):211–7. doi: 10.1046/j.1525-1497.2001.016004211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams S, Miller G, Khoury R, Grossberg GT. Rational deprescribing in the elderly. Ann Clin Psychiatry. May2019;31(2):144–152. [PubMed] [Google Scholar]
- 45.Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. Challenges and Enablers of Deprescribing: A General Practitioner Perspective. PLoS One. 2016;11(4):e0151066. doi: 10.1371/journal.pone.0151066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: List of the Selected Potentially Inappropriate Medications.
Supplementary Table S2: Prevalence of Different Classes of Potentially Inappropriate Medications by Age Groups.
Supplementary Table S3: Differences in Prescribing of Potentially Inappropriate Drug Classes by Sex and Multimorbidity patterns.